α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation
Abstract
1. Introduction
2. Results
2.1. LPS Activation of Human THP-1 Monocytic Cells Is Dependent on α4 nAChRs
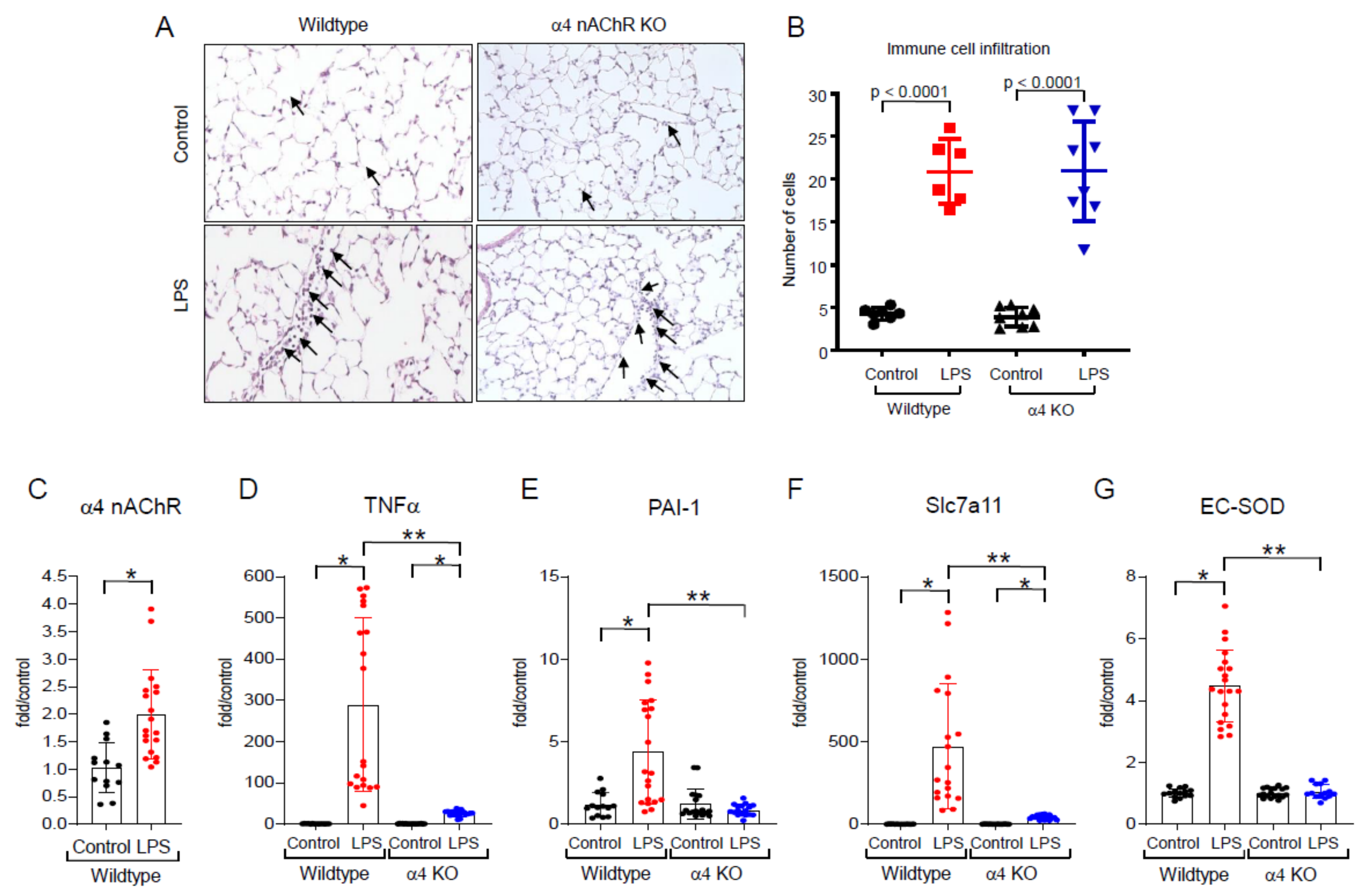
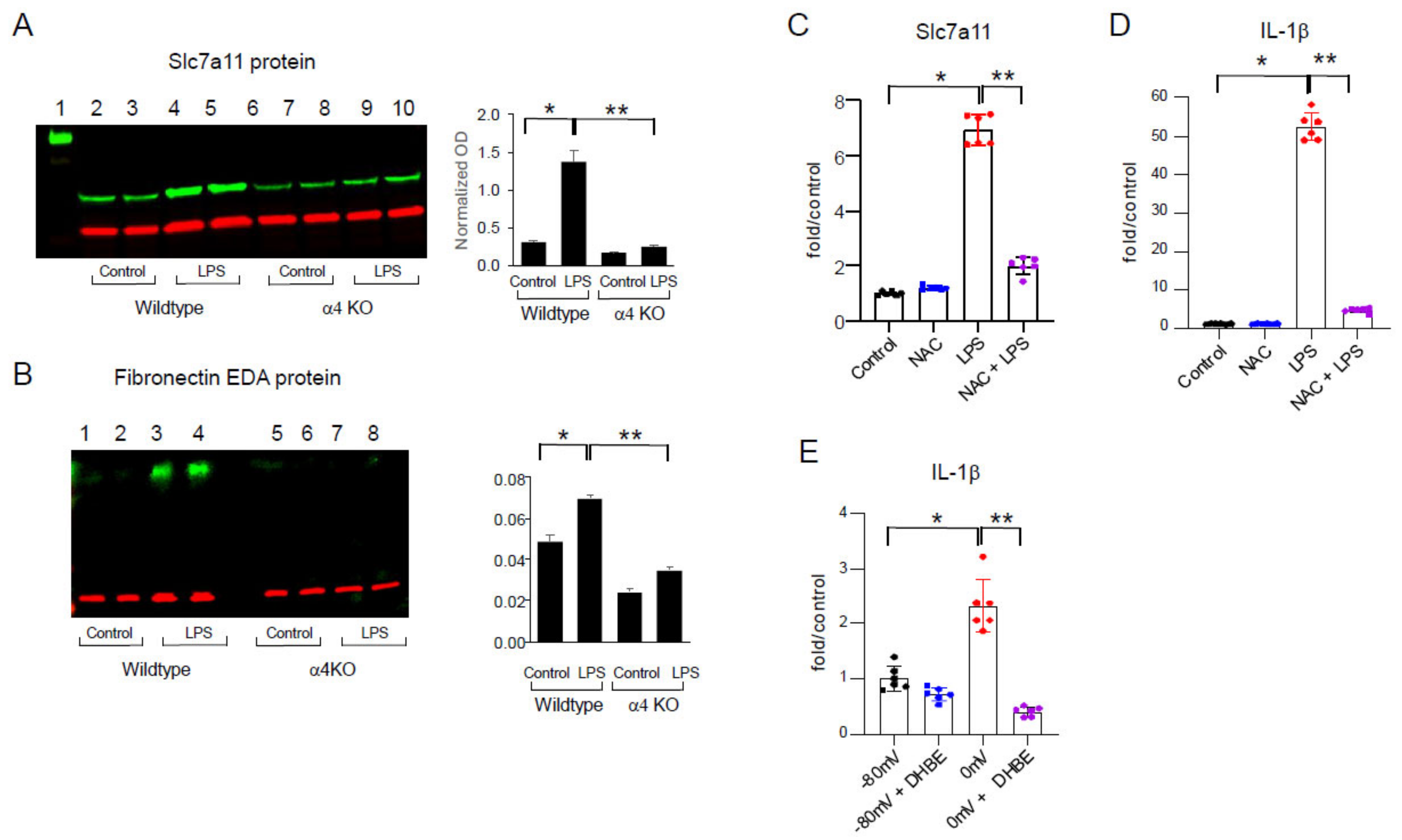
2.2. Effects of LPS In Vivo
2.3. LPS-Induced Redox Stress Results in α4 nAChR Activation and Signaling
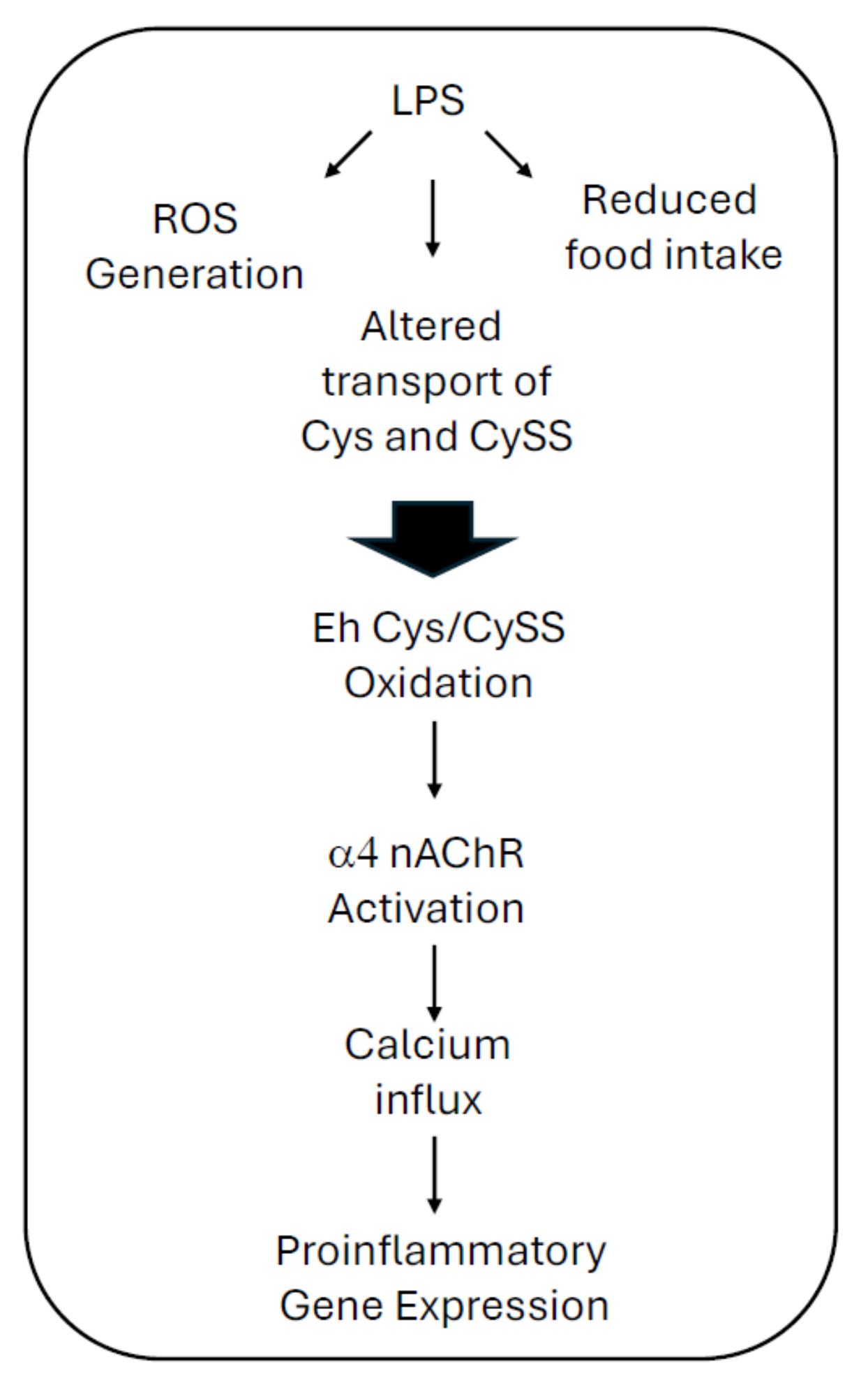
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Generation of α4 nAChR Knockout Mice
4.4. LPS-Induced Lung Injury
4.5. Isolation and Measurement of mRNA by qPCR
4.6. Western Blot
4.7. Histology and Myeloperoxidase Staining
4.8. Calcium Influx Assay
4.9. Analysis of Data and Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deitschmann, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernand, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock. JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, K.; Daniels, R.; Kissoon, N.; Machado, F.R.; Schachter, R.D.; Finfer, S. Recognizing sepsis as a global health priority—A WHO Resolution. N. Engl. J. Med. 2017, 377, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-Y.; Hong, S.-B. Sepsis and acute respiratory distress syndrome: Recent update. Tuberc. Respir. Dis. 2016, 79, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Domscheit, H.; Hegeman, M.A.; Carvalho, N.; Spieth, P.M. Molecular dynamics of lipopysaccharide-induced lung injury in rodents. Front. Physiol. 2020, 11, 36. [Google Scholar] [CrossRef]
- Skireck, T.; Cavaillon, J.-M. Inner sensors of endotoxin—Implications for sepsis research and therapy. FEMS Microbiol. Rev. 2019, 43, 239–256. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 499, 819–826. [Google Scholar] [CrossRef]
- McCloskey, R.V.; Starube, R.C.; Sanders, C.; Smith, S.M.; Smith, C.R. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double blind, placebo-controlled trial. CHESS Trial Study Group. Ann. Intern. Med. 1994, 388, 394–397. [Google Scholar]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS-TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.-P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef]
- Iyer, S.S.; Accardi, C.J.; Ziegler, T.R.; Blanco, R.A.; Ritzenthaler, J.D.; Rojas, M.; Roman, J.; Jones, D.P. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS ONE 2009, 4, e5017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iyer, S.S.; Jones, D.P.; Brigham, K.L.; Rojas, M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2009, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Liang, Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic. Biol. Med. 2009, 47, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Lennicke, C.; Cocheme, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3703. [Google Scholar] [CrossRef]
- Nkabyo, Y.S.; Young-Mi, G.; Ziegler, T.R.; Jones, D.P. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G70–G78. [Google Scholar] [CrossRef]
- Young-Mi, G.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar]
- Ramirez, A.; Ramadan, B.; Ritzenthaler, J.D.; Rivera, H.N.; Jones, D.P.; Roman, J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L972–L981. [Google Scholar] [CrossRef] [PubMed]
- Ritzenthaler, J.D.; Roser-Page, S.; Guidot, D.M.; Roman, J. Nicotinic acetylcholine receptors are sensors for ethanol in lung fibroblasts. Alcohol. Clin. Exp. Res. 2013, 37, 914–923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watson, W.H.; Ritzenthaler, J.D.; Torres-Gonzalez, E.; Arteel, G.E.; Roman, J. Mice lacking alpha4 nicotinic acetylcholine receptors are protected against alcohol-associated liver injury. Alcohol. Clin. Exp. Res. 2022, 46, 1371–1383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindstrom, J.; Anand, R.; Gerzanich, V.; Peng, X.; Wang, F.; Wells, G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog. Brain Res. 1996, 109, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Ndoye, A.; Nguyen, V.T.; Grando, S.A. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res. Commun. Mol. Pathol. Pharmacol. 1997, 97, 243–262. [Google Scholar] [PubMed]
- Maus, A.D.; Pereira, E.F.; Karachunski, P.I.; Horton, R.M.; Navaneetham, D.; Macklin, K.; Cortes, W.S.; Albuquerque, E.X.; Conti-Fine, B.M. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol. Pharmacol. 1998, 54, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Borkar, N.A.; Thompson, M.A.; Bartman, C.M.; Khalfaoui, L.; Sine, S.; Sathish, V.; Prakash, Y.S.; Pabelick, C.M. Nicotinic receptors in airway disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 326, L149–L163. [Google Scholar] [CrossRef] [PubMed]
- Banzato, R.; Pinheiro-Menegasso, N.M.; Roncon Santana Novelli, F.P.; Olivo, C.R.; Taguchi, L.; de Oliviera Santos, S.; Fukuzaki, S.; Paganelli Rosolia Teodoro, W.; Lopez, F.D.T.Q.S.; Tiberio, I.F.L.C.; et al. Alpha-7 nicotinic receptor agonist protects mice against pulmonary emphysema induced by elastase. Inflammation 2024, 47, 958–974. [Google Scholar] [CrossRef]
- Hollenhorst, M.I.; Krasteva-Christ, G. Nicotinic acetylcholine receptors in the respiratory tract. Molecules 2021, 26, 6097. [Google Scholar] [CrossRef] [PubMed]
- Bele, T.; Turk, T.; Kirzaj, I. Nicotinic acetylcholine receptors in cancer: Limitations and prospects. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166875. [Google Scholar] [CrossRef]
- Fatemika, H.; Dehghanian, A.; Ziaian, B.; Farokhipous, M.; Ketabchi, F. Roles of alpha-7 nicotinic acetylcholine receptors and spleen in lung injury induced by a repeated saline lavage in rat. BMC Pulm Med. 2022, 22, 367. [Google Scholar] [CrossRef]
- Mizuno, Y.; Dosch, H.M.; Gelfand, E.W. Carbamycholine modulation of E-rosette formation: Identification of nicotinic acetylcholine receptors on a subpopulation of human T lymphocytes. J. Clin. Immunol. 1982, 2, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.D.; Hoss, W.; Lin, J.P.; Lionetti, F. Evidence for a noncholinergic nicotine receptor on human phagocytic leukocytes. Mol. Cell Biochem. 1982, 44, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Q.; Yu, K.; Hu, J.; Kuo, Y.P.; Segerberg, M.; St John, P.A.; Lukas, R.J. Roles of nicotinic acetylcholine receptor beta subunits in function of human alpha4-containing nicotinic receptors. J. Physiol. 2006, 576, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Woods, C.R.; Mora, A.L.; Zu, J.; Brigham, K.L. Endotoxin-induced lung injury in mice: Structural, functional, and biochemical responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L333–L334. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-x.; Yakel, J.L. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol. Sin. 2009, 30, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Li, L.; Zhao, C.; Pan, M.; Qian, Z.; Su, X. Deficiency of α7 nicotinic acetylcholine receptor attenuates bleomycin-induced lung fibrosis in mice. Mol. Med. 2017, 23, 34–39. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and function of the cholinergic system in immune cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Zheng, Y.; Ritzenthaler, J.D.; Burke, T.J.; Otero, J.; Roman, J.; Watson, W.H. Age-dependent oxidation of extracellular cysteine/cystine redox state (Eh(Cys/CySS)) in mouse lung fibroblasts is mediated by a decline in Slc7a11 expression. Free Radic. Biol. Med. 2018, 118, 13–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, H.-K.; Liin, A.-H.; Perng, D.-W.; Lee, T.-S.; Kou, Y.R. Lung epithelial TRPA1 mediates lipopolysaccharide-induced lung inflammation in bronchial epithelial cells and mice. Front. Physiol. 2020, 11, 596314. [Google Scholar] [CrossRef]
- Moss, M.; Parsons, P.E.; Steinberg, K.P.; Hudson, L.D.; Guidot, D.M.; Burnham, E.L.; Eaton, S.; Cotsonis, G.A. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit. Care Med. 2003, 31, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Massey, V.L.; Poole, L.G.; Siow, D.L.; Torres, E.; Warner, N.L.; Schmidt, R.H.; Ritzenthaler, J.D.; Roman, J.; Arteel, G.E. Chronic Alcohol Exposure Enhances Lipopolysaccharide-Induced Lung Injury in Mice: Potential Role of Systemic Tumor Necrosis Factor-Alpha. Alcohol Clin. Exp. Res. 2015, 39, 1978–1988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wells, G.B. Structural answers and persistent questions about how nicotinic receptors work. Front. Biosci. 2008, 13, 5479–5510. [Google Scholar] [CrossRef]
- Lai, J.-L.; Liu, Y.-H.; Liu, C.; Qi, M.-P.; Liu, R.-N.; Zhu, X.-F.; Zhou, Q.-G.; Chen, Y.-Y.; Guo, A.-Z.; Hu, C.-M. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced lung injury by regulating the TLR4/NF-KB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Ren, W.; Zhu, L. Plasminogen activator inhibitor type-1 deficiency exaggerates LPS-induced acute lung injury through enhancing Toll-like receptor 4 signaling pathway. Blood Coagul. Fibrinolysis 2011, 2, 480–486. [Google Scholar] [CrossRef]
- Ueda, J.; Starr, M.E.; Takahashi, H.; Du, J.; Chang, L.Y.; Crapo, J.D.; Evers, B.M.; Saito, H. Decreased pulmonary extracellular superoxide dismutase during systemic inflammation. Free Radic. Biol. Med. 2008, 45, 897–904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adachi, Y.; Mio, T.; Striz, I.; Carnevali, S.; Romberger, D.J.; Spurzem, J.R.; Heire, P.; Illig, M.G.; Ertl, R.F.; Rennard, S.I. Lipopolysaccharide increases fibronectin production and release from cultured lung fibroblasts partially through proteolytic activity. J. Lab. Clin. Med. 1996, 127, 448–455. [Google Scholar] [CrossRef]
- Vandewalle, J.; Steeland, S.; Van Ryckeghem, S.; Eggermont, M.; Van Wonterghem, E.; Vandenbroucke, R.E.; Libert, C. A Study of Cecal Ligation and Puncture-Induced Sepsis in Tissue-Specific Tumor Necrosis Factor Receptor 1-Deficient Mice. Front. Immunol. 2019, 10, 2574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, H.; Shiiya, A.; Kimata, M.; Maebara, K.; Tamba, M.; Sakakura, Y.; Makino, N.; Sugiyama, F.; Yagami, K.-i.; Moriguchi, T.; et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005, 280, 37423–37429. [Google Scholar] [CrossRef]
- Watson, W.H.; Burke, T.J.; Zelko, I.N.; Torres-Gonzalez, E.; Ritzenthaler, J.D.; Roman, J. Differential Regulation of the Extracellular Cysteine/Cystine Redox State (EhCySS) by Lung Fibroblasts from Young and Old Mice. Oxid. Med. Cell. Longev. 2016, 2016, 1561305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritzenthaler, J.D.; Torres-Gonzalez, E.; Zheng, Y.; Zelko, I.N.; van Berkel, V.; Nunley, D.R.; Kindane, B.; Halayko, A.J.; Summer, R.; Watson, W.H.; et al. The profibrotic and senescence phenotype of old lung fibroblasts is reversed or ameliorated by genetic and pharmacological manipulation of Slc7a11 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L449–L461. [Google Scholar] [CrossRef] [PubMed Central]
- Giebelen, I.A.J.; van Westerloo, D.J.; LaRosa, G.J.; de Vos, A.F.; van der Poll, T. Local stimulation of α7 cholinergic receptors inhibits LPS-induced the TNF-α release in the mouse lung. Shock 2007, 28, 700–703. [Google Scholar] [CrossRef]
- Wang, Q.; Sundar, I.K.; Li, D.; Lucas, J.H.; Muthumalage, T.; McDonough, S.R.; Rahman, I. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR a7 receptor: Role of nAChR a7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir. Res. 2020, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Vicary, G.W.; Ritzenthaler, J.D.; Panchabhai, T.S.; Torres-Gonzalez, E.; Roman, J. Nicotine stimulates collagen type I expression in lung via α7 nicotinic acetylcholine receptors. Respir. Res. 2017, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Saika, F.; Kobayashi, Y.; Ko, M.-C. TC-2559, an a4b2 nicotinic acetylcholine receptor agonist, suppresses the expression of CCL3 and IL-1b through STAT3 inhibition in cultured murine macrophages. J. Pharmacol. Sci. 2015, 128, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.; Verplaetse, T.L.; Moore, K.; Oberleitner, L.; Picciotto, M.R.; McKee, S.A. Effects of varenicline on alcohol self-administration and craving in drinkers with depressive symptoms. J. Psychopharmacol. 2017, 31, 906–914. [Google Scholar] [CrossRef]
- Jones, D.P.; Park, Y.; Gletsu-Miller, N.; Liang, Y.; Yu, T.; Accardi, C.J.; Ziegler, T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition 2011, 27, 199–205. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritzenthaler, J.D.; Watson, W.H.; Roman, J. α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation. Int. J. Mol. Sci. 2024, 25, 11305. https://doi.org/10.3390/ijms252011305
Ritzenthaler JD, Watson WH, Roman J. α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation. International Journal of Molecular Sciences. 2024; 25(20):11305. https://doi.org/10.3390/ijms252011305
Chicago/Turabian StyleRitzenthaler, Jeffrey D., Walter H. Watson, and Jesse Roman. 2024. "α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation" International Journal of Molecular Sciences 25, no. 20: 11305. https://doi.org/10.3390/ijms252011305
APA StyleRitzenthaler, J. D., Watson, W. H., & Roman, J. (2024). α4 Nicotinic Acetylcholine Receptors in Lipopolysaccharide-Related Lung Inflammation. International Journal of Molecular Sciences, 25(20), 11305. https://doi.org/10.3390/ijms252011305




