Studying the Stability of Anthocyanin Pigments Isolated from Juices of Colored-Fleshed Potatoes
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Potato Juices
2.2. Characterization of Potato Pigments
2.3. Model Study
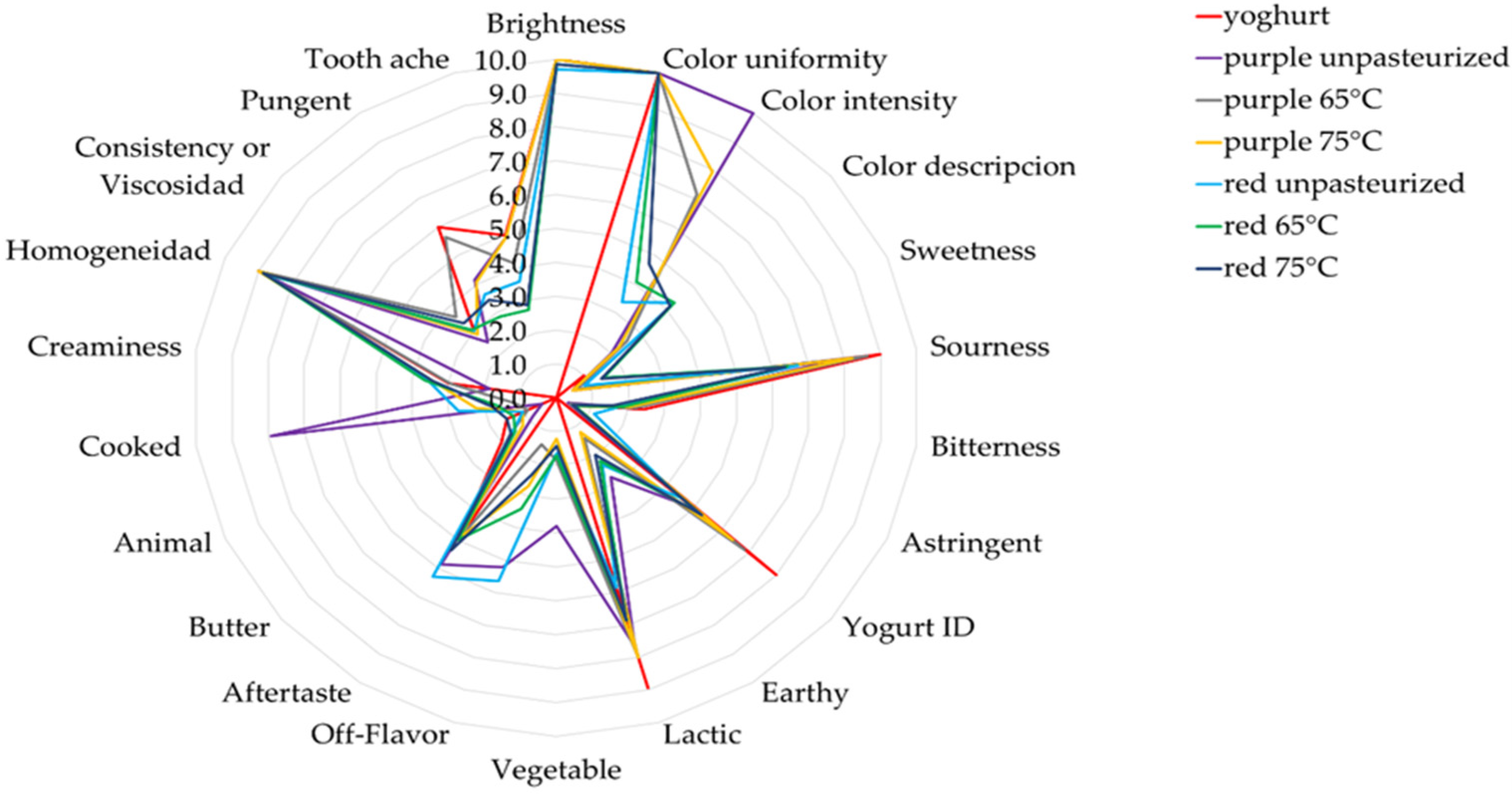
2.4. Analysis of Natural Yogurt with Added Anthocyanin Pigments
3. Materials and Methods
3.1. Colored-Fleshed Potato Juices
3.1.1. Preparation of Potato Juices
Potato Juices with the Addition of Fruit and Vegetable Juice Concentrates
Pasteurized Juices
3.1.2. Preparation of Potato Pigments
3.1.3. Lyophilization
3.2. Model Study of Anthocyanin Pigment Extracts
3.2.1. Determination of the Influence of Temperature on Anthocyanin Stability
3.2.2. Determination of the Effect of pH on Anthocyanin Stability
3.3. Preparation of Yoghurts
3.4. Sensory Assessment
3.5. Analytical Methods
3.5.1. Potato Juices
3.5.2. Anthocyanin Pigments
3.5.3. Yoghurts
3.5.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrés-Bello, A.; Barreto-Palacios, V.I.V.I.A.N.; García-Segovia, P.; Mir-Bel, J.; Martínez-Monzó, J. Effect of pH on color and texture of food products. J. Food Eng. 2013, 5, 158–170. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. CRFSFS 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Tkaczyńska, A.; Rytel, E.; Kucharska, A.Z.; Kolniak-Ostek, J.; Sokół-Łętwska, A. Stability of color and biologically active compounds of pasteurized juices from potatoes with colored flesh. Food Sci. Nutr. 2024, 12, 4637–4655. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Patras, A. Stability and colour evaluation of red cabbage waste hydroethanolic extract in presence of different food additives or ingredients. Food Chem. 2019, 275, 539–548. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sun, D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin pigments: Beyond aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Aadil, R.M. Natural pigments: Anthocyanins, carotenoids, chlorophylls, and betalains as colorants in food products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Aura, A.M.; Mattila, I.; Hyotylainen, T.; Gopalacharyulu, P.; Cheynier, V.; Souquet, J.M.; Bes, M.; Bourvellec, C.L.; Guyot, S.; Oresic, M. Characterization of microbial metabolism of Syrah grape products in an in vitro colon model using targeted and non-targeted analytical approaches. Eur. J. Nutr. 2013, 52, 833–846. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An insight into anti-inflammatory activities and inflammation relate dieases of anthocyanins: A review of both in vivo and in vitro investigations. Int. J. Mol. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohara, N.; Tsukoi, A. Stability of anthocyanins in various vegetable and fruits. Food Sci. Technol. Int. 1996, 2, 30–33. [Google Scholar] [CrossRef][Green Version]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Strugala, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 3126–3147. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic Effect of Diacylated Anthocyanin Derived from Ipomoea batatas Cultivar Ayamurasaki can be Achieved through the r-Glucosidase Inhibitory Action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef]
- Tkaczyńska, A.; Rytel, E. Influence of potato varieties with red and purple flesh on enzymatic darkening of tubers and antioxidant properties. ŻNTJ 2022, 4, 85–99. [Google Scholar]
- Fang, S.; Lin, F.; Qu, D.; Liang, X.; Wang, L. Characterization of Purified Red Cabbage Anthocyanins: Improvement in HPLC Separation and Protective Effect against H2O2-Induced Oxidative Stress in HepG2 Cells. Molecules 2019, 24, 124–133. [Google Scholar] [CrossRef]
- Askin, B.; Türkyılmaz, M.; Özkan, M.; Küçüköner, E. Changes in anthocyanins and colour of black mulberry (Morus nigra) juice during clarification and pasteurization. J. Food Meas. Charact. 2022, 16, 784–792. [Google Scholar] [CrossRef]
- Jackman, R.; Yada, R.; Tung, M.; Speers, R. Anthocyanins as food colorants—A review. J. Food BioChem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.C.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 10–157. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzel, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Effect of vacuum cooking treatment on physicochemical and structural characteristics of purple-flesh potato. IJFST 2014, 49, 943–951. [Google Scholar] [CrossRef]
- Tkaczyńska, A.; Rytel, E.; Kucharska, A.Z.; Kolniak-Ostek, J.; Sokół-Łętowska, A. The Influence of the Addition of Fruit and Vegetable Concentrates on the Stability of Anthocyanins in Juices from Colored Potatoes. Int. J. Mol. Sci. 2024, 25, 7584–7598. [Google Scholar] [CrossRef]
- Rios-Romero, E.A.; Ochoa-Martínez, L.A.; Bello-Pérez, L.A.; Morales-Castro, J.; Quintero-Ramos, A.; Gallegos-Infante, J.A. Effect of ultrasound and steam treatments on bioaccessibility of β-carotene and physicochemical parameters in orange-fleshed sweet potato juice. Heliyon 2021, 7, 6632–6638. [Google Scholar] [CrossRef]
- Li, J.; Li, X.D.; Zhang, Y.; Zheng, Z.D.; Qu, Z.Y.; Liu, M.; Qu, L. Identification and thermal stability of purple-fleshed potato 588 anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem. 2013, 136, 1429–1434. [Google Scholar] [CrossRef]
- Kouniaki, S.; Kajda, P.; Zabetakis, I. The effect if high hydrostatic pressure on anthocyanins and ascorbic acid in blackcurrants (Ribes nigrum). Flavour Fragr J. 2004, 19, 281–286. [Google Scholar] [CrossRef]
- Kita, A.; Bakowska-Barczak, A.; Hamouz, K.; Kulakowska, K.; Lisinska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). Food Chem. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Francis, F.J. Food colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.J.; Ruan, S.Y.; Xie, Z.H.; Dong, Y.; Yu, L.L. Anthocyanin and glucosinolate occurrences in the roots of Chinese red radish (Raphanus sativus L.), and their stability to heat and pH. Food Chem. 2012, 133, 1569–1576. [Google Scholar]
- Dobson, G.; McDougall, G.J.; Stewart, D.; Cubero, M.Á.; Karjalainen, R.O. Effects of juice matrix and pasteurization on stability of black currant anthocyanins during storage. J. Food Sci. 2017, 82, 44–52. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Engin. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar]
- Sampaio, S.L.; Lonchamp, J.; Dias, M.I.; Liddle, C.; Petropoulos, S.A.; Glamočlija, J.; Barros, L. Anthocyanin-rich extracts from purple and red potatoes as natural colourants: Bioactive properties, application in a soft drink formulation and sensory analysis. Food Chem. 2021, 342, 128526–128535. [Google Scholar]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; He, J. Research advances of purple sweet potato anthocyanins: Extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef]
- Suda, I.; Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. JARQ 2003, 37, 167–173. [Google Scholar] [CrossRef]
- Castaneda-Ovando, A.; De Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins. Food Chem. 2009, 113, 859–871. [Google Scholar]
- Reyes, L.F.; Cisneros-Zevallos, L. Degradation kinetics and color of anthocyanins in aqueous extracts of purple and red-flesh potatoes (Solanum tuberosum L.). Food Chem. 2007, 100, 885–894. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Czapski, J. Color changes of a preparation from red cabbage during storage in a model system. Food Chem. 2007, 104, 709–714. [Google Scholar] [CrossRef]
- Fan, G.; Han, Y.; Gu, Z.; Gu, F. Composition and color stability of anthocyanins extracted from fermented purple sweet potato culture. LWT-FS&T 2008, 41, 1412–1416. [Google Scholar]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Sendra, E.; Fayos, P.; Lario, Y.; Fernández-López, J.; Sayas-Barberá, E.; Angel Pérez-Alvarez, J. Incorporation of citrus fibers in fermented milk containing probiotic bacteria. Food Microbiol. 2008, 25, 13–21. [Google Scholar] [CrossRef]
- Szołtysik, M.; Kucharska, A.Z.; Dąbrowska, A.; Zięba, T.; Bobak, Ł.; Chrzanowska, J. Effect of two combined functional additives on yoghurt properties. Foods 2021, 10, 1159. [Google Scholar] [CrossRef]
- Szołtysik, M.; Kucharska, A.Z.; Sokół-Łętowska, A.; Dąbrowska, A.; Bobak, Ł.; Chrzanowska, J. The effect of Rosa spinosissima fruits extract on lactic acid bacteria growth and other yoghurt parameters. Foods 2020, 9, 1167. [Google Scholar] [CrossRef]
- Trigueros, L.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Nadal, E.S. Low fat set yoghurt rich in pomegranate juice: A new antioxidant dairy product. Milk Sci. Int. 2012, 67, 177–180. [Google Scholar]
- Wrolstad, R.; Durst, R.; Lee, J. Tracking color and pigment changes in anthocyanins product. Trends Food Sci. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Nemś, A.; Pęksa, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kita, A.; Drożdż, W.; Hamouz, K. Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef]
- Pȩksa, A.; Gołubowska, G.; Rytel, E.; Lisińska, G.; Aniołowski, K. Influence of harvest date on glycoalkaloid contents of three potato varieties. Food Chem. 2002, 78, 313–317. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokoł-Łeztowska, A.; Oszmianski, J.; Piorecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.; Chen, H. Antioxidant activity of various tea extracts in relations to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Trigueros, L.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Sendra, E. Anthocyanins decay in pomegranate enriched fermented milks as a function of bacterial strain and processing conditions. LWT 2017, 80, 193–199. [Google Scholar] [CrossRef]
- Amanpour, A.; Soltani, M.; Lipan, L.; Garcia-Garví, J.M.; Hernández-García, F.; Carbonell-Barrachina, Á.A.; Nadal, E.S. Comparative study on nutraceutical and sensorial characteristics of saffron (Crocus sativus L.) cultivated in Iran, Spain, and Türkiye. J. Sci. Food. Agri. 2024, 104, 7580–7591. [Google Scholar] [CrossRef]

| Flesh Color | Variety | Variant | 0 h | 1 h | 4 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |||
| purple | Provita | CS | 0.61 aB | 3.32 aC | 1.25 aC | 0.34 aA | 1.73 aAB | 0.44 aB | 0.30 aA | 1.61 aA | 0.33 aA |
| 65 °C | 4.36 bA | 5.38 cA | 11.6 bA | 20.1 cC | 34.4 dC | 32.1 bC | 16.9 cB | 31.7 cB | 27.8 bB | ||
| 75 °C | 5.63 bA | 4.31 cA | 12.0 bA | 21.4 cC | 35.5 dC | 33.3 bC | 19.5 dB | 33.6 cB | 3.8 cB | ||
| Le | 2.34 aB | 2.93 aA | 1.54 aA | 1.78 abA | 6.51 bC | 1.95 aB | 1.75 bA | 6.20 bB | 2.33 aC | ||
| Li | 2.34 aB | 9.80 bB | 2.38 aA | 3.19 bC | 11.8 cC | 4.12 aC | 1.80 bA | 7.05 bA | 2.49 aB | ||
| Rh | 0.12 aA | 0.93 aA | 0.57 aA | 1.22 abB | 5.44 abB | 1.37 aB | 1.70 bC | 6.37 bC | 2.30 aC | ||
| Double Fun | CS | 0.19 aB | 0.23 aA | −0.05 aA | 0.16 aA | 0.28 aAB | 0.05 aB | 0.15 aA | 0,36 aB | 0.03 aB | |
| 65 °C | 1.94 dB | 8.14 dB | 1.38 cA | 1.88 dA | 7.32 dA | 1.53 dB | 1.89 cA | 8.46 deC | 1.92 dC | ||
| 75 °C | 2.25 eB | 9.80 eC | 1.62 dA | 1.94 dA | 7.90 dA | 1.67 dA | 2.09 cAB | 9.42 eB | 2.17 dB | ||
| Le | 0.72 cA | 3.01 cA | 0.39 bA | 0.85 cAB | 4.28 cB | 0.77 cAB | 1.22 bB | 6.29 cdC | 1.34 cB | ||
| Li | 0.45 bA | 1.80 bA | 0.26 bA | 0.60 bcAB | 2.90 bB | 0.48 bAB | 0.90 bB | 4.55 bcC | 0.87 bcB | ||
| Rh | 0.46 bA | 1.28 bA | −0.01 aA | 0.54 bAB | 1.80 bB | 0.33 bB | 0.81 bB | 3.48 bC | 0.74 bC | ||
| red | Lily Rose | CS | 0.56 aB | 3.06 aC | 0.92 aC | 0.44 aAB | 2.58 aB | 0.73 aB | 0.32 aA | 1.70 aA | 0.45 aA |
| 65 °C | 28.3 cB | 36.3 eC | 36.1 dB | 25.5 dAB | 34.8 deB | 38.7 dC | 18.5 dA | 29.9 cdA | 30.0 dA | ||
| 75 °C | 32.1 cC | 38.3 eC | 36.5 dAB | 29.2 dB | 36.6 eB | 40.7 eB | 22.0 eA | 32.2 cdA | 35.0 dA | ||
| Le | 7.67 bB | 23.4 bB | 9.81 bC | 5.19 bA | 21.0 bA | 6.11 bA | 5.75 bAB | 23.2 bB | 7.04 abB | ||
| Li | 8.12 bB | 27.2 cBC | 10.8 bB | 6.56 bA | 24.5 cA | 8.44 bA | 10.3 bcC | 28.6 cC | 14.3 bcC | ||
| Rh | 12.2 bA | 32.0 dA | 17.5 cA | 13.2 cB | 32.8 dB | 19.2 cB | 12.7 cAB | 32.8 dB | 18.5 cAB | ||
| Flesh Color | Variety | Variant | Juice | Pigment | ||
|---|---|---|---|---|---|---|
| TP | TA | TP | TA | |||
| purple | Provita | CS | 7.26 ± 0.15 | 27.8 ± 0.13 | 138.2 ± 0.10 | 1098.1 ± 0.10 |
| 65 °C | 3.95 ± 0.13 | 15.8 ± 0.15 | 160.4 ± 0.10 | 1321.5 ± 0.13 | ||
| 75 °C | 4.54 ± 0.12 | 12.2 ± 0.12 | 164.3 ± 0.10 | 1343.9 ± 0.13 | ||
| Le | 10.7 ± 0.14 | 54.7 ± 0.14 | 152.6 ± 0.01 | 4279.9 ± 0.10 | ||
| Li | 14.6 ± 0.16 | 109 ± 0.11 | 121.5 ± 0.01 | 3707.8 ± 0.30 | ||
| Rh | 11.7 ± 0.12 | 118 ± 0.12 | 156.3 ± 0.01 | 4499.1 ± 0.14 | ||
| Double Fun | CS | 9.45 ± 0.13 | 70.6 ± 0.07 | 147.2 ± 0.01 | 5487.8 ± 0.15 | |
| 65 °C | 13.2 ± 0.16 | 37.4 ± 0.17 | 163.8 ± 0.01 | 1084.8 ± 0.10 | ||
| 75 °C | 9.83 ± 0.10 | 7.50 ± 0.05 | 166.6 ± 0.01 | 1095.6 ± 0.09 | ||
| Le | 17.3 ± 0.17 | 117.8 ± 0.17 | 155.4 ± 0.10 | 5878.5 ± 0.15 | ||
| Li | 16.3 ± 0.15 | 146.6 ± 0.14 | 125.5 ± 0.01 | 5460.6 ± 0.50 | ||
| Rh | 13.8 ± 0.19 | 130.9 ± 0.13 | 158.8 ± 0.01 | 6368.3 ± 0.50 | ||
| red | Lily Rose | CS | 7.32 ± 0.18 | 13.9 ± 0.12 | 115.5 ± 0.01 | 1157.2 ± 0.12 |
| 65 °C | 2.69 ± 0.19 | 14.4 ± 0.14 | 97.7 ± 0.51 | 1474.6 ± 0.14 | ||
| 75 °C | 3.65 ± 0.16 | 9.40 ± 0.09 | 114.9 ± 0.01 | 1722.7 ± 0.17 | ||
| Le | 20.5 ± 0.11 | 152.0 ± 0.15 | 59.4 ± 0.01 | 5934.3 ± 0.52 | ||
| Li | 23.0 ± 0.18 | 145.6 ± 0.14 | 92.2 ± 0.01 | 5562.6 ± 0.53 | ||
| Rh | 15.2 ± 0.17 | 89.8 ± 0.09 | 129.4 ± 0.01 | 6225.1 ± 0.51 | ||
| LSD | 5.18 | 9.00 | 21.0 | 100 | ||
| Compound | Provita | Double Fun | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 65 °C | 75 °C | Le | Li | Rh | CS | 65 °C | 75 °C | Le | Li | Rh | |
| Petunidin 3-rutinoside 5-glucoside | 0.72 | 0.51 | 0.53 | 1.94 | 3.96 | 3.54 | 0.64 | - | - | - | - | - |
| Peonidin 3-rutinoside 5-glucoside isomer 1 | 0.39 | 0.28 | 0.27 | - | - | - | - | - | - | 2.96 | 3.32 | 2.69 |
| Peonidin 3-rutinoside 5-glucoside isomer 2 | - | - | - | - | - | - | - | - | - | 2.09 | 2.34 | 2.21 |
| Petunidin 3-caffeoylrutinoside 5-glucoside isomer 1 | - | - | - | - | - | - | - | - | - | 0.72 | 0.83 | 0.81 |
| Petunidin 3-caffeoylrutinoside 5-glucoside isomer 2 | - | - | - | - | - | - | - | - | - | 1.03 | 1.30 | 1.33 |
| Delphinidin 3-coumaroylrutinoside 5-glucoside | 0.83 | 0.40 | 0.44 | 0.95 | 1.09 | 1.48 | - | - | - | - | - | - |
| Malvidin derivative | - | - | - | - | - | - | 0.84 | 0.58 | - | 0.81 | 0.84 | 0.87 |
| Petunidin 3-coumaroylrutinoside 5-glucoside | 8.61 | 3.39 | 2.38 | 12.8 | 21.9 | 33.7 | 12.8 | 4.61 | - | 3.97 | 4.86 | 7.16 |
| Malvidin 3-coumarylorutinoside 5-glucoside isomer 1 | - | - | - | - | - | - | 60.9 | 33.0 | 7.50 | 102.7 | 129.2 | 112.8 |
| Malvidin 3-feruloylrutinoside 5-glucoside | - | - | - | - | - | - | 4.56 | 2.81 | - | 3.37 | 3.77 | 3.72 |
| Malvidin 3-coumarylorutinoside 5-glucoside isomer 2 | - | - | - | - | - | - | 0.55 | 0.77 | - | 0.27 | 0.24 | 0.25 |
| Petunidin 3-feruloylrutinoside 5-glucoside | 0.64 | 0.45 | 0.49 | 0.61 | 1.13 | 2.01 | - | - | - | - | - | - |
| Peonidin 3-coumaroylrutinoside 5-glucoside | 15.7 | 10.0 | 7.25 | 36.8 | 77.1 | 73.5 | - | - | - | - | - | - |
| Peonidin 3-feruloylrutinoside 5-glucoside | 0.89 | 0.70 | 0.50 | 1.53 | 3.82 | 3.84 | - | - | - | - | - | - |
| Peonidin derivative | - | - | 0.30 | - | - | - | - | - | - | - | - | - |
| Compound | Lily Rose | |||||
|---|---|---|---|---|---|---|
| CS | 65 °C | 75 °C | Le | Li | Rh | |
| Pelargonidin 3-rutinoside 5-glucoside | 0.80 | 0.64 | 0.47 | 12.6 | 12.8 | 7.61 |
| Pelargonidin derivative 1 | 0.59 | 0.41 | 0.33 | 1.44 | 1.24 | 0.96 |
| Pelargonidin derivative 2 | 0.40 | 0.29 | - | 1.08 | 1.06 | 0.75 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 1 | 0.41 | 0.68 | 0.37 | - | - | - |
| Pelargonidin 3-caffeoylrutinoside 5-glucoside isomer 1 | 0.64 | 0.75 | 0.53 | 4.19 | 3.68 | 2.69 |
| Pelargonidin 3-caffeoylrutinoside 5-glucoside isomer 2 | 0.38 | - | - | - | - | - |
| Unidentified | 0.29 | - | - | 4.63 | 4.65 | 3.07 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 2 | 1.19 | 0.72 | 0.48 | 3.87 | 3.49 | 2.35 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 3 | 7.73 | 9.40 | 5.71 | 116.8 | 112.0 | 67.9 |
| Pelargonidin 3-feruloylrutinoside 5-glucoside | 0.64 | 0.75 | 0.50 | 7.36 | 6.80 | 4.42 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 4 | 0.93 | 0.36 | 0.41 | - | - | - |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 5 | - | 0.32 | 0.49 | - | - | - |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 6 | - | 0.75 | 0.74 | - | - | - |
| Flesh Color | Variety | Variant | 0 h | 1 h | 4 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |||
| purple | Provita | Cs | 36.3 eA | 2.70 eA | −3.57 aA | 36.2 eA | 2.45 dA | −3.51 aA | 36.6 fA | 2.50 dA | −3.60 aA |
| 65 °C | 32.7 aA | 0.12 bA | −1.00 cA | 32.8 aA | 0.06 aA | −0.88 dA | 32.6 aA | 0.13 aA | −0.95 cA | ||
| 75 °C | 33.3 bA | 0.05 aA | −1.02 cA | 33.3 bA | 0.08 aA | −1.00 cA | 33.3 cA | 0.14 aB | −1.00 cA | ||
| Le | 34.5 dA | 0.40 cA | −0.86 dA | 34.6 dA | 0.35 bA | −0.85 dA | 34.5 eA | 0.37 bA | −0.95 cA | ||
| Li | 34.1 cA | 0.45 cA | −0.76 eA | 34.3 cA | 0.39 bA | −0.71 eA | 34.3 dA | 0.42 bA | −0.69 dA | ||
| Rh | 32.8 aA | 0.96 dA | −1.31 bA | 32.8 aA | 0.98 cA | −1.30 bA | 32.7 bA | 0.99 cA | −1.32 bA | ||
| Double Fun | CS | 33.0 aA | 0.10 bA | −1.28 bA | 33.1 aA | 0.07 bA | −1.23 bA | 33.2 bA | 0.17 bA | −1.32 bA | |
| 65 °C | 34.1 eA | −0.01 aA | −1.26 bA | 34.1 cA | −0.07 aA | −1.15 cA | 34.0 dA | −0.05 aA | −1.21 cA | ||
| 75 °C | 33.8 dA | 0.47 cA | −1.43 aA | 33.8 bA | 0.47 cA | −1.40 aA | 33.5 cA | 0.47 cA | −1.47 aA | ||
| Le | 34.3 fA | 0.86 dA | −0.07 eA | 34.1 cA | 0.83 dA | −0.18 eA | 34.5 eA | 0.87 dA | −0.15 eA | ||
| Li | 33.5 cA | 1.03 eA | −0.92 dA | 33.1 aA | 0.85 dA | −0.97 dA | 33.0 bA | 0.98 eA | −0.97 dA | ||
| Rh | 33.3 bA | 1.08 eA | −1.17 cA | 33.0 aA | 1.06 eA | −1.14 cA | 32.8 aA | 0.93 deA | −1.14 cA | ||
| red | Lily Rose | CS | 36.6 dA | 4.18 dA | −0.31 bA | 36.6 dA | 4.34 dA | −0.11 bA | 36.8 dA | 4.54 cA | −0.12 cA |
| 65 °C | 35.3 cB | 1.84 bA | −0.74 aA | 35.3 cB | 1.81 aA | −0.68 aA | 34.9 bA | 2.02 aA | −0.54 aA | ||
| 75 °C | 35.1 bB | 1.54 aA | −0.82 aA | 34.9 bA | 1.92 bA | −0.64 aA | 34.9 bA | 2.04 aA | −0.57 aA | ||
| Le | 33.7 aA | 2.78 cA | −0.38 bA | 33.9 aA | 2.83 cA | −0.17 bA | 33.8 aA | 2.86 bA | −0.27 bA | ||
| Li | 36.6 dA | 12.1 eA | 1.62 cA | 36.7 dA | 12.3 eA | 1.75 cA | 36.7 cA | 12.5 dA | 1.72 dA | ||
| Rh | 41.1 eA | 22.7 fA | 3.50 dA | 41.3 eA | 23.1 fA | 3.58 dA | 41.3 eA | 23.0 eA | 3.57 eA | ||
| Compound | Provita | Double Fun | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 65 °C | 75 °C | Le | Li | Rh | CS | 65 °C | 75 °C | Le | Li | Rh | |
| Petunidin3-rutinoside 5-glucoside | 80.6 | 87.2 | 85.5 | 393.9 | 362.0 | 373.2 | 191.1 | - | - | - | - | - |
| Peonidin3-rutinoside 5-glucoside isomer 1 | 33.0 | 57.1 | 61.3 | 263.9 | 265.6 | 264.8 | 190.1 | - | - | 342.5 | 336.6 | 337.7 |
| Peonidin3-rutinoside 5-glucoside isomer 2 | - | - | - | - | - | - | - | - | - | 320.7 | 312.6 | 327.3 |
| Petunidin 3-caffeoylrutinoside 5-glucoside isomer 2 | - | - | - | - | - | - | 101.0 | - | - | 281.0 | 278.7 | 282.7 |
| Petunidin 3-caffeoylrutinoside 5-glucoside isomer 1 | - | - | - | - | - | - | 141.2 | 96.1 | 103.8 | 333.3 | 327.3 | 348.4 |
| Delphinidin 3-coumaroylrutinoside 5-glucoside | 81.1 | 31.1 | 35.3 | 266.3 | 262.4 | 275.0 | - | - | - | - | - | - |
| Malvidin derivative | - | - | - | - | - | - | 115.6 | 92.5 | 93.2 | 299.3 | 298.7 | 309.3 |
| Petunidin 3-coumaroylrutinoside 5-glucoside | 338.1 | 243.8 | 260.6 | 703.9 | 544.5 | 839.2 | 344.7 | 110.9 | 118.1 | 379.4 | 347.7 | 402.3 |
| Malvidin 3-coumarylorutinoside 5-glucoside isomer 1 | - | - | - | - | - | - | 4118.4 | 785.3 | 780.5 | 3596.7 | 3244.9 | 4014.9 |
| Malvidin 3-feruloylrutinoside 5-glucoside | - | - | - | - | - | - | 285.7 | - | - | 325.6 | 314.1 | 345.7 |
| Petunidin 3-feruloylrutinoside 5-glucoside | 58.2 | 58.4 | 59.7 | 266.3 | 262.9 | 271.9 | - | - | - | - | - | - |
| Peonidin 3-coumaroylrutinoside 5-glucoside | 503.7 | 835.8 | 834.9 | 2065.5 | 1707.4 | 2149.3 | - | - | - | - | - | - |
| Peonidin 3-feruloylrutinoside 5-glucoside | 39.8 | 47.2 | 48.6 | 320.0 | 303.0 | 325.6 | - | - | - | - | - | - |
| Compound | Lily Rose | |||||
|---|---|---|---|---|---|---|
| CS | 65 °C | 75 °C | Le | Li | Rh | |
| Pelargonidin3-rutinoside 5-glucoside | 121.6 | 137.8 | 159.57 | 632.35 | 611.57 | 646.27 |
| Unidentified | - | - | - | 97.20 | 106.17 | - |
| Pelargonidin derivative 1 | - | 95.4 | 101.27 | 286.52 | 281.07 | 284.98 |
| Pelargonidin derivative 2 | - | 99.4 | 107.61 | 277.33 | 270.20 | 273.43 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 1 | - | 114.8 | 140.78 | - | - | - |
| Pelargonidin3-caffeoylrutinoside 5-glucoside isomer 1 | 98.3 | 118.9 | 132.56 | 334.32 | 312.48 | 345.73 |
| Pelargonidin3-caffeoylrutinoside 5-glucoside isomer 2 | 100.5 | 104.7 | 111.71 | - | - | - |
| unidentified | 102.4 | - | - | 366.33 | 368.06 | 366.48 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 2 | 108.1 | - | - | 335.15 | 318.81 | 346.21 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 3 | 517.4 | 684.9 | 842.32 | 3034.61 | 2744.79 | 3321.29 |
| Pelargonidin3-feruloylrutinoside 5-glucoside | 108.9 | 118.7 | 126.86 | 398.7605 | 381.93 | 418.17 |
| Pelargonidin 3-coumaroylrutinoside 5-glucoside isomer 4 | - | - | - | 440.772 | 441.24 | 445.22 |
| Temperature | Color Parameters | Purple | Red | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 65 °C | 75 °C | Le | Li | Rh | CS | 65 °C | 75 °C | Le | Li | Rh | ||
| Non-pasteurized | L* | 5.49 bA | 21.0 eA | 1.79 aA | 10.8 cA | 14.3 dA | 5.30 bA | 70.2 hC | 68.4 hD | 65.6 gE | 69.6 iE | 77.7 jD | 63.1 fA |
| a* | 17.9 gA | 23.8 hB | 6.43 aA | 31.0 kF | 30.1 jF | 25.9 iE | 8.11 bB | 10.7 cA | 8.79 bA | 14.6 eB | 12.9 dB | 16.3 fA | |
| b* | 4.18 eA | 26.8 hA | 0.42 dA | −20.3 bA | −18.5 cA | −25.7 aA | 29.4 jA | 39.1 lA | 37.0 kA | 15.5 gA | 13.9 fA | 28.3 iA | |
| 60 °C | L* | 24.9 bB | 34.2 dB | 12.1 aB | 38.6 eB | 38.2 eB | 25.2 cB | 64.5 gB | 64.9 gC | 61.7 fD | 66.3 hC | 73.8 iA | 64.8 gB |
| a* | 19.9 jB | 20.7 kA | 19.7 jC | 6.8 cE | 5.53 bE | −3.71 aD | 8.47 dB | 11.4 fB | 9.34 eB | 14.6 hB | 12.0 gB | 18.3 iB | |
| b* | 35.1 hB | 49.7 kB | 16.1 dB | 8.96 bB | 10.0 cB | −6.46 aB | 32.3 gB | 40.9 jB | 39.3 iB | 21.1 fC | 19.8 eB | 32.3 gB | |
| 70 °C | L* | 32.9 cC | 36.1 dC | 25.9 aC | 40.4 eC | 40.1 fC | 31.3 bC | 63.9 gA | 64.4 gC | 59.6 hC | 71.9 jF | 75.7 kC | 68.7 iD |
| a* | 19.6 hB | 20.9 iA | 18.9 gB | 3.11 cD | 1.94 bD | −9.56 aC | 7.96 dA | 11.8 fB | 9.36 eB | 11.6 fA | 11.6 fA | 18.1 gB | |
| b* | 48.1 jC | 54.6 kC | 39.0 hC | 11.8 bC | 12.2 cC | 2.03 aC | 34.0 gC | 41.7 iC | 40.9 iC | 16.3 dB | 19.9 eB | 32.5 fB | |
| 80 °C | L* | 34.4 cD | 36.9 dC | 29.3 aD | 40.4 eC | 41.2 fD | 31.9 bC | 63.2 iA | 63.1 iB | 58.7 gB | 61.8 hA | 74.6 kB | 68.3 jD |
| a* | 19.8 iB | 20.8 jA | 19.3 iC | 1.90 cC | 0.92 bC | −12.3 aA | 8.40 dB | 12.3 fC | 9.81 eB | 17.1 hD | 13.5 gC | 19.0 iC | |
| b* | 51.3 iD | 56.0 jD | 44.2 hD | 12.9 bD | 14.4 cD | 5.65 aD | 34.9 fC | 42.7 gD | 42.1 gD | 26.2 eD | 21.1 dC | 34.8 fC | |
| 90 °C | L* | 38.5 cE | 38.8 cD | 33.5 aE | 41.8 dD | 43.7 eE | 37.1 bD | 63.3 hA | 62.6 gA | 57.5 fA | 62.5 gB | 74.3 jB | 67.9 iC |
| a* | 21.2 jC | 23.5 kB | 20.4 iD | 1.62 cB | −1.5 bB | −10.9 aB | 8.97 dB | 12.5 fC | 10.8 eC | 16.6 hC | 13.5 gC | 19.8 iD | |
| b* | 57.4 hE | 58.9 iE | 50.9 gE | 16.6 aE | 16.5 aE | 15.9 aE | 37.2 eD | 43.1 fE | 43.3 fE | 27.2 cE | 21.8 bC | 35.8 dD | |
| 100 °C | L* | 38.3 cE | 38.2 cD | 34.0 aF | 42.7 dE | 43.4 eE | 37.3 bD | 63.5 gA | 63.2 gB | 58.6 fB | 67.3 hD | 74.7 jB | 68.3 iD |
| a* | 21.1 jC | 23.6 kB | 19.8 iC | −2.12 bA | −2.09 cA | −12.9 aA | 9.47 dC | 12.5 fC | 11.6 eD | 14.9 hB | 13.7 gC | 20.3 iE | |
| b* | 57.4 iE | 58.3 jE | 50.9 hE | 17.8 bF | 19.2 cF | 15.7 aE | 38.3 fE | 43.2 gE | 42.9 gD | 26.4 eD | 23.7 dD | 37.1 fE | |
| Color Flesh | Variant | Non-Pasteurized | 60 °C | 70 °C | 80 °C | 90 °C | 100 °C |
|---|---|---|---|---|---|---|---|
| purple | CS | 150.1 ± 0.01 | 145.7 ± 0.01 | 161.0 ± 0.01 | 157.3 ± 0.15 | 147.8 ± 0.15 | 157.3 ± 0.15 |
| 65 °C | 158.0 ± 0.01 | 154.9 ± 0.01 | 144.9 ± 0.15 | 139.8 ± 0.15 | 146.6 ± 0.15 | 151.2 ± 0.15 | |
| 75 °C | 167.5 ± 0.01 | 166.1 ± 0.01 | 161.7 ± 0.01 | 161.5 ± 0.15 | 158.3 ± 0.15 | 157.2 ± 0.15 | |
| Le | 154.4 ± 0.01 | 143.8 ± 0.05 | 143.1 ± 0.05 | 143.5 ± 0.05 | 142.9 ± 0.01 | 136.4 ± 0.01 | |
| Li | 130.3 ± 0.01 | 121.1 ± 0.05 | 111.1 ± 0.05 | 132.7 ± 0.05 | 135.4 ± 0.01 | 136.1 ± 0.01 | |
| Rh | 152.1 ± 0.01 | 141.0 ± 0.01 | 111.4 ± 0.05 | 146.8 ± 0.05 | 141.9 ± 0.05 | 147.7 ± 0.01 | |
| red | CS | 108.4 ± 0.01 | 100.2 ± 0.01 | 101.5 ± 0.01 | 78.2 ± 0.01 | 100.8 ± 0.01 | 102.3 ± 0.01 |
| 65 °C | 97.1 ± 0.01 | 108.3 ± 0.01 | 99.5 ± 0.01 | 97.5 ± 0.01 | 97.6 ± 0.01 | 99.1 ± 0.01 | |
| 75 °C | 115.7 ± 0.01 | 109.4 ± 0.01 | 109.8 ± 0.01 | 116.7 ± 0.01 | 109.5 ± 0.01 | 109.6 ± 0.1 | |
| Le | 56.9 ± 0.15 | 140.8 ± 0.15 | 141.2 ± 0.05 | 93.2 ± 0.01 | 145.0 ± 0.01 | 105.3 ± 0.01 | |
| Li | 110.8 ± 0.15 | 118.3 ± 0.15 | 132.5 ± 0.15 | 114.2 ± 0.01 | 130.4 ± 0.01 | 112.8 ± 0.01 | |
| Rh | 145.9 ± 0.01 | 145.1 ± 0.05 | 121.9 ± 0.05 | 132.8 ± 0.01 | 106.9 ± 0.01 | 146.4 ± 0.01 |
| pH | Color Parameters | Purple | Red | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS | 65 °C | 75 °C | Le | Li | Rh | CS | 65 °C | 75 °C | Le | Li | Rh | ||
| 1 | L* | 28.6 bC | 33.2 dD | 25.5 aE | 33.9 dB | 34.1 eD | 32.4 cC | 62.9 hC | 62.0 hC | 62.0 hC | 52.5 fC | 57.5 gB | 57.7 gD |
| a* | 60.5 dF | 63.0 fF | 57.9 cF | 66.2 hF | 66.3 hF | 65.9 gF | 56.3 bE | 51.8 aF | 51.9 aF | 61.6 eE | 60.7 dE | 63.7 fF | |
| b* | 40.2 dE | 38.2 cE | 39.9 cdF | 25.8 aD | 25.6 aD | 29.1 bD | 55.4 gD | 53.8 fD | 50.4 eD | 80.8 iF | 79.4 hE | 82.1 jF | |
| 3 | L* | 29.8 bD | 38.9 fE | 22.9 aD | 34.6 dC | 32.9 cC | 36.4 eD | 67.8 jD | 65.9 iD | 68.6 kE | 52.0 gC | 58.7 hC | 57.9 hD |
| a* | 52.4 fE | 50.9 dE | 51.2 eE | 55.8 gE | 60.5 iE | 60.2 iE | 41.9 cD | 23.9 aE | 27.6 bE | 57.9 hD | 52.9 fD | 60.2 iE | |
| b* | 12.6 dD | 22.0 fC | 20.6 eD | −0.46 bB | 6.80 cB | −9.24 aB | 31.5 gB | 33.3 iA | 32.5 hA | 64.9 kD | 51.9 jC | 64.3 kD | |
| 5 | L* | 32.7 dF | 45.6 fF | 25.1 cE | 36.3 eD | 22.1 bA | 11.7 aA | 70.9 kF | 71.7 kF | 68.9 iE | 66.1 gE | 67.6 hE | 69.6 jE |
| a* | 47.6 iD | 32.9 gD | 50.4 jD | 53.4 kD | −25.2 aB | −14.8 bC | 8.44 cC | 12.9 eD | 22.8 fD | 12.9 eC | 9.39 dB | 39.7 hD | |
| b* | 9.89 dC | 25.8 hD | 10.9 eC | −3.23 bA | −1.61 cA | −16.0 aA | 29.0 iA | 33.2 kA | 32.1 jA | 18.9 gA | 19.5 gA | 15.9 fA | |
| 7 | L* | 16.9 cB | 18.6 dB | 16.5 aB | 20.7 eA | 23.1 fB | 12.4 bB | 70.3 kE | 69.3 jE | 67.7 iD | 61.9 hD | 74.4 lF | 56.0 gC |
| a* | 35.3 jC | 21.7 iC | 6.49 dB | −40.5 aA | −33.8 cA | −34.9 bA | 8.04 eC | 10.5 gC | 10.0 gC | 10.3 gA | 9.92 fB | 11.9 hA | |
| b* | 3.94 bB | 20.1 eB | −0.56 aA | 6.30 dC | 24.7 fC | 4.48 cC | 29.0 gA | 38.9 iB | 35.8 hB | 21.2 eB | 20.9 eB | 40.4 jB | |
| 9 | L* | 2.36 bA | 7.34 cA | 1.29 aA | 39.4 fE | 38.0 eE | 36.3 dD | 44.1 hA | 46.9 iA | 42.6 gA | 46.1 iA | 53.9 jA | 44.2 hA |
| a* | 0.49 fA | 2.69 hA | 0.87 gA | −15.9 cB | −18.5 bC | −32.9 aB | −2.34 dA | 5.81 iA | −1.30 eA | 11.6 kB | 10.8 jC | 20.3 lC | |
| b* | 2.30 bA | 9.89 cA | 0.74 aB | 52.2 jE | 50.2 iE | 42.7 fE | 32.1 dC | 43.9 gC | 37.6 eC | 45.9 hC | 58.2 kD | 58.9 lC | |
| 11 | L* | 30.3 bE | 32.6 cC | 17.6 aC | 60.4 iF | 59.8 hF | 57.3 fE | 60.9 jB | 58.8 gB | 57.3 fB | 48.3 dB | 65.7 kD | 54.1 eB |
| a* | 14.6 hB | 19.2 jB | 7.94 dC | 5.26 cC | 3.96 bD | −7.36 aD | 4.61 bB | 9.53 fB | 7.82 dB | 12.2 gC | 8.69 eA | 17.4 iB | |
| b* | 50.2 bF | 54.6 cF | 28.9 aE | 85.3 jF | 83.6 iF | 77.2 gF | 66.9 dE | 69.6 fE | 67.7 eE | 67.2 deE | 89.4 kF | 80.9 hE | |
| Color Flesh | Variant | 1 | 3 | 5 | 7 | 9 | 11 |
|---|---|---|---|---|---|---|---|
| purple | CS | 214.2 ± 0.01 | 205.4 ± 0.01 | 216.7 ± 0.01 | 203.6 ± 0.01 | 192.3 ± 0.01 | 169.2 ± 0.01 |
| 65 °C | 201.7 ± 0.01 | 201.1 ± 0.01 | 191.1 ± 0.01 | 197.3 ± 0.01 | 164.8 ± 0.01 | 171.7 ± 0.01 | |
| 75 °C | 203.6 ± 0.01 | 213.6 ± 0.01 | 208.6 ± 0.01 | 209.8 ± 0.01 | 183.6 ± 0.01 | 164.8 ± 0.01 | |
| Le | 163.6 ± 0.01 | 177.3 ± 0.01 | 180.4 ± 0.01 | 146.7 ± 0.01 | 136.1 ± 0.01 | 111.1 ± 0.01 | |
| Li | 166.1 ± 0.01 | 166.7 ± 0.01 | 163.6 ± 0.01 | 146.7 ± 0.01 | 131.7 ± 0.01 | 111.7 ± 0.01 | |
| Rh | 187.9 ± 0.01 | 188.6 ± 0.01 | 187.9 ± 0.01 | 154.2 ± 0.01 | 147.3 ± 0.01 | 108.6 ± 0.01 | |
| red | CS | 135.9 ± 0.01 | 147.1 ± 0.01 | 209.3 ± 0.01 | 164.3 ± 0.01 | 128.2 ± 0.01 | 113.7 ± 0.01 |
| 65 °C | 127.6 ± 0.01 | 134.8 ± 0.01 | 129.8 ± 0.01 | 131.5 ± 0.01 | 123.7 ± 0.01 | 105.4 ± 0.01 | |
| 75 °C | 159.3 ± 0.01 | 173.2 ± 0.01 | 174.3 ± 0.01 | 161.5 ± 0.01 | 134.3 ± 0.01 | 138.7 ± 0.01 | |
| Le | 203.2 ± 0.01 | 197.1 ± 0.01 | 198.2 ± 0.01 | 184.3 ± 0.01 | 122.1 ± 0.01 | 101.5 ± 0.01 | |
| Li | 207.6 ± 0.01 | 215.4 ± 0.01 | 165.4 ± 0.01 | 163.2 ± 0.01 | 99.3 ± 0.01 | 72.1 ± 0.01 | |
| Rh | 172.6 ± 0.01 | 182.1 ± 0.01 | 174.3 ± 0.01 | 169.8 ± 0.01 | 123.2 ± 0.01 | 88.7 ± 0.01 |
| Flesh Color | Variety | Variant | 0 Day | 2 Day | 7 Day | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |||
| yoghurt | 81.6 f A | −2.12 aA | 5.47 jA | 81.3 gA | −2.17 aA | 5.41 hA | 82.1 gA | −2.38 Aa | 5.44 gA | ||
| purple | Double Fun | CS | 68.0 aA | 8.53 hA | −3.32 cA | 68.6 aA | 8.12 gA | −2.71 cB | 69.5 aB | 8.13 fA | −2.54 bB |
| 65 °C | 68.7 aA | 6.81 fA | −1.21 eA | 69.7 bB | 6.81 eA | −1.04 dA | 70.6 bC | 6.72 dA | −0.81 cA | ||
| 75 °C | 69.1 bAB | 6.62 fA | −0.68 fA | 69.5 bB | 6.44 eA | −0.41 eA | 68.9 aA | 6.15 Da | −0.05 cA | ||
| Le | 69.5 aB | 9.51 iB | −4.60 bA | 68.5 aA | 9.53 hB | −4.90 aA | 70.2 bC | 8.32 Fa | −3.28 aB | ||
| Li | 68.2 aA | 9.90 iB | −5.11 aA | 69.4 bB | 8.64 gA | −3.93 bB | 69.6 aB | 8.85 fA | −3.88 aB | ||
| Rh | 69.9 bA | 8.08 hA | −2.81 dA | 69.8 bA | 7.99 fA | −2.74 cA | 70.2 bA | 7.77 Ea | −2.18 bA | ||
| Provita | CS | 73.8 bA | 2.55 bA | 5.31 jA | 73.9 cA | 2.55 bA | 5.77 hAB | 74.8 dB | 2.48 Ba | 6.01 hB | |
| 65 °C | 74.5 cB | 2.78 bA | 6.32 kA | 73.9 cA | 2.77 bA | 6.26 iA | 73.7 cA | 2.72 bA | 6.15 hA | ||
| 75 °C | 74.5 cA | 2.62 bA | 6.09 kA | 74.5 dA | 2.78 bA | 6.30 iA | 73.9 cA | 2.65 bA | 6.06 hA | ||
| Le | 76.8 eA | 2.83 bA | 4.94 iA | 76.5 fA | 2.80 bA | 5.23 hA | 76.5 fA | 2.94 Ba | 5.18 gA | ||
| Li | 76.3 eA | 2.74 bA | 5.85 jA | 75.8 eA | 2.70 bA | 6.06 iA | 75.9 eA | 2.88 Ba | 5.90 gA | ||
| Rh | 76.4 eB | 2.74 bA | 5.92 jA | 74.2 dA | 3.09 cB | 5.64 hA | 74.6 dA | 3.19 cB | 5.57 gA | ||
| red | Lily Rose | CS | 74.7 cA | 2.72 bA | 6.87 kA | 74.5 dA | 2.59 bA | 6.91 iA | 74.2 dA | 2.42 bA | 6.79 hA |
| 65 °C | 74.6 cA | 3.45 cA | 5.82 jA | 74.8 dA | 3.26 cA | 5.94 hA | 75.9 eB | 3.18 Ca | 6.15 hA | ||
| 75 °C | 74.2 cA | 4.02 dA | 5.37 jA | 74.6 dA | 3.95 cA | 5.48 hA | 73.8 cA | 3.78 Ca | 5.33 gA | ||
| Le | 75.5 dB | 7.65 gB | 1.93 gA | 74.3 dA | 7.80 fB | 2.27 Fb | 75.1 eB | 6.39 dA | 3.19 eC | ||
| Li | 75.9 eB | 6.73 fB | 2.42 hA | 75.2 eB | 5.55 dA | 3.99 gB | 74.2 dA | 8.10 Fc | 2.01 dA | ||
| Rh | 75.0 dB | 5.71 eA | 4.76 iA | 73.7 cA | 5.69 dA | 5.07 hA | 74.4 dAB | 6.09 Da | 4.76 fA | ||
| Flesh Colour | Variant | 2 Days | 7 Days | 0 Days | 2 Days | 7 Day | ||
|---|---|---|---|---|---|---|---|---|
| Lab-MRS-5 | Lac-M17-7 | Lab-MRS-5 | Lac-M17-7 | pH | ||||
| yoghurt | 7.67 | 7.93 | 7.56 | 8.32 | 4.10 | 4.30 | 4.15 | |
| purple | CS | 7.54 | 8.04 | 7.65 | 8.79 | 4.12 | 4.29 | 4.20 |
| 65 °C | 7.75 | 8.45 | 7.71 | 8.67 | 3.98 | 4.15 | 4.07 | |
| 75 °C | 7.50 | 7.71 | 7.59 | 8.72 | 3.92 | 4.07 | 4.16 | |
| Le | 7.39 | 8.79 | 7.72 | 9.38 | 4.35 | 4.17 | 4.38 | |
| Li | 8.05 | 9.82 | 7.95 | 9.19 | 4.38 | 4.33 | 4.34 | |
| Rh | 7.98 | 9.49 | 7.95 | 9.49 | 4.38 | 4.36 | 4.34 | |
| red | CS | 7.62 | 9.85 | 7.71 | 8.57 | 4.01 | 4.11 | 4.14 |
| 65 °C | 7.50 | 9.49 | 7.73 | 9.72 | 4.10 | 4.28 | 4.19 | |
| 75 °C | 7.56 | 9.74 | 7.57 | 9.58 | 4.02 | 4.12 | 4.23 | |
| Le | 7.84 | 9.25 | 7.55 | 8.91 | 4.53 | 4.39 | 4.34 | |
| Li | 7.79 | 8.36 | 7.73 | 8.70 | 4.52 | 4.37 | 4.34 | |
| Rh | 7.85 | 9.56 | 7.60 | 9.32 | 4.53 | 4.37 | 4.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkaczyńska, A.; Sendra, E.; Jiménez-Redondo, N.; Rytel, E. Studying the Stability of Anthocyanin Pigments Isolated from Juices of Colored-Fleshed Potatoes. Int. J. Mol. Sci. 2024, 25, 11116. https://doi.org/10.3390/ijms252011116
Tkaczyńska A, Sendra E, Jiménez-Redondo N, Rytel E. Studying the Stability of Anthocyanin Pigments Isolated from Juices of Colored-Fleshed Potatoes. International Journal of Molecular Sciences. 2024; 25(20):11116. https://doi.org/10.3390/ijms252011116
Chicago/Turabian StyleTkaczyńska, Agnieszka, Esther Sendra, Nuria Jiménez-Redondo, and Elżbieta Rytel. 2024. "Studying the Stability of Anthocyanin Pigments Isolated from Juices of Colored-Fleshed Potatoes" International Journal of Molecular Sciences 25, no. 20: 11116. https://doi.org/10.3390/ijms252011116
APA StyleTkaczyńska, A., Sendra, E., Jiménez-Redondo, N., & Rytel, E. (2024). Studying the Stability of Anthocyanin Pigments Isolated from Juices of Colored-Fleshed Potatoes. International Journal of Molecular Sciences, 25(20), 11116. https://doi.org/10.3390/ijms252011116








