Nerve Wrap for Local Delivery of FK506/Tacrolimus Accelerates Nerve Regeneration
Abstract
1. Introduction
2. Results
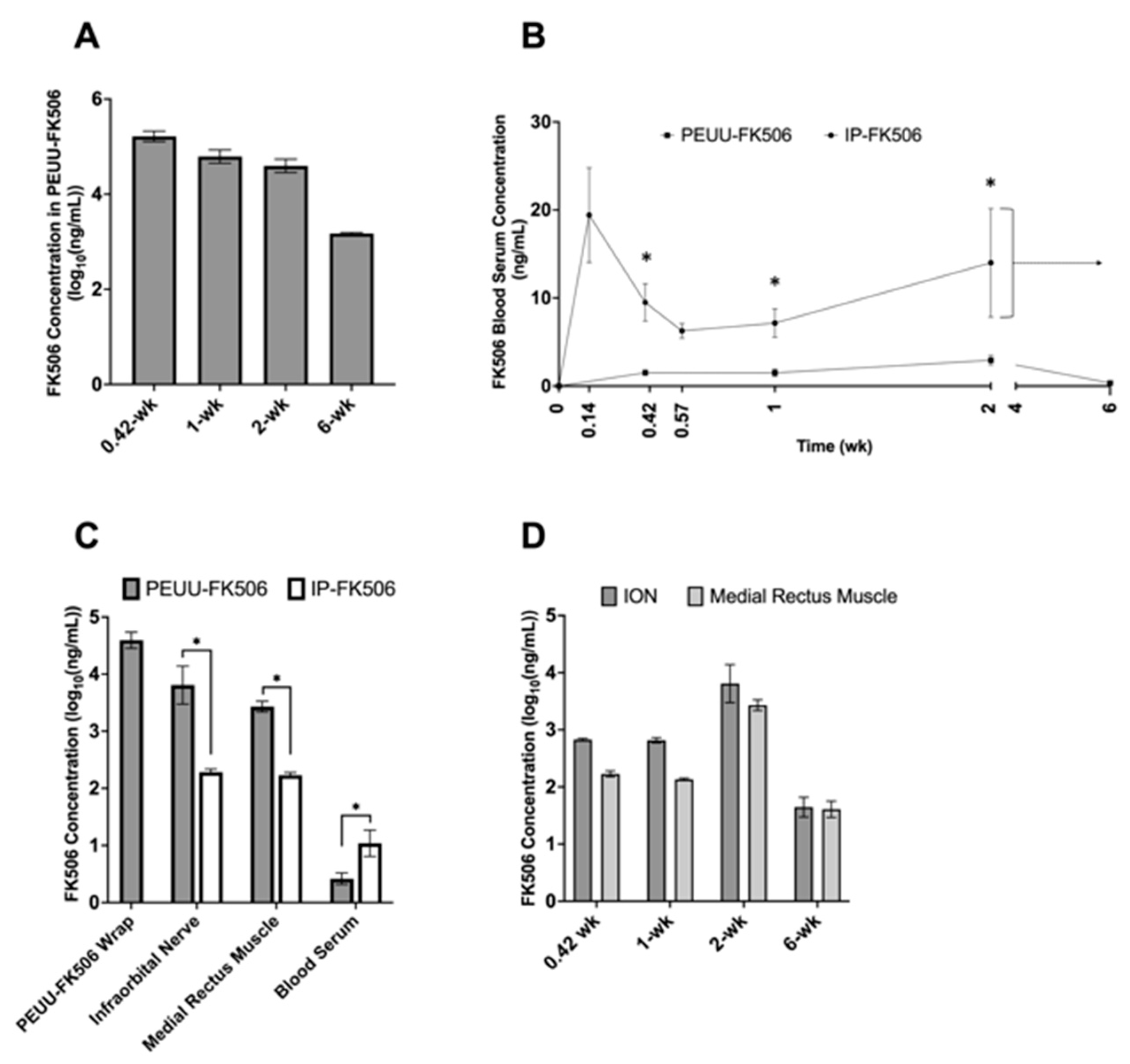
2.1. Concentration of FK506 in PEUU-FK506 Wrap, Serum, and Tissue Samples
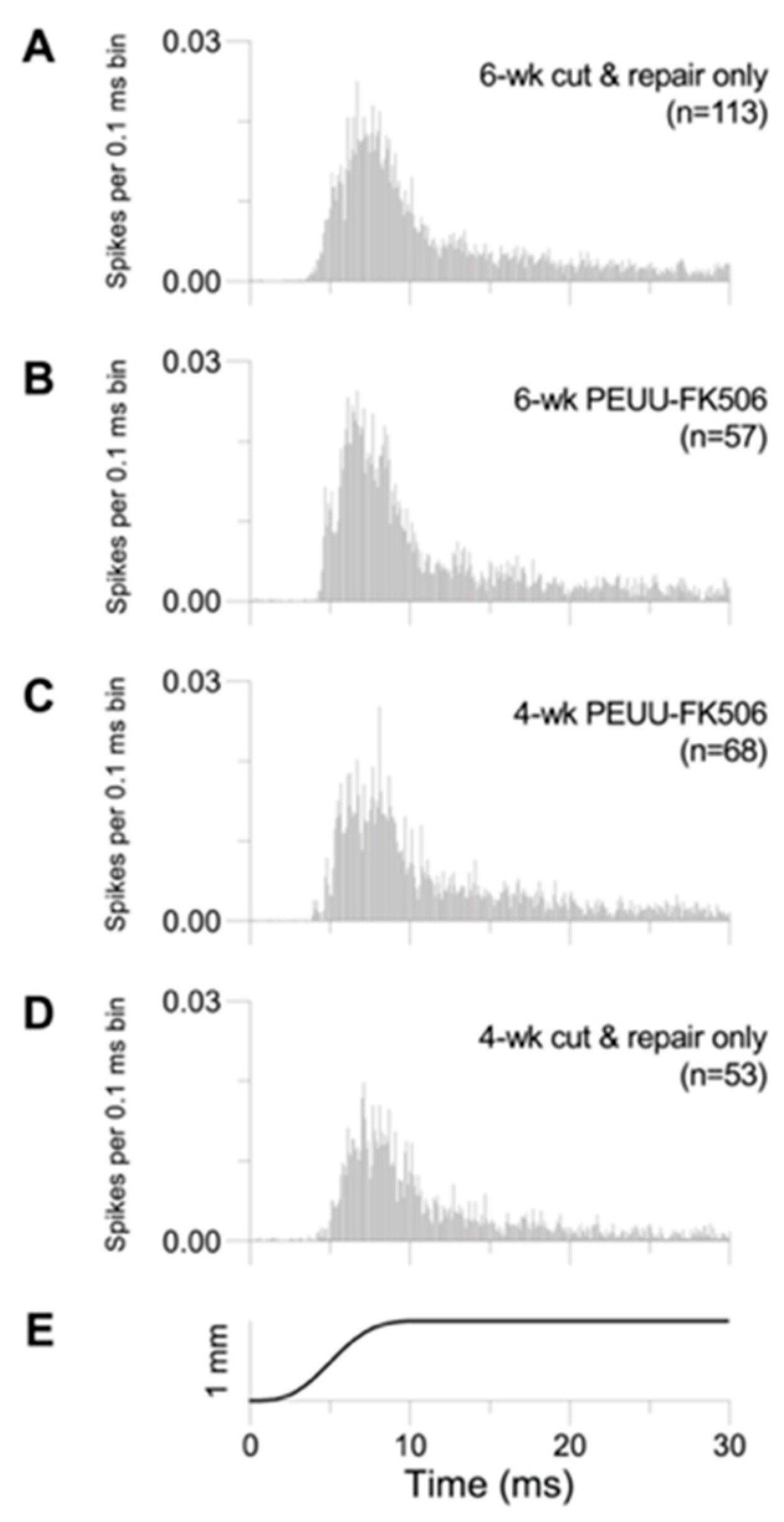
2.2. Trigeminal Ganglion Cell Recordings
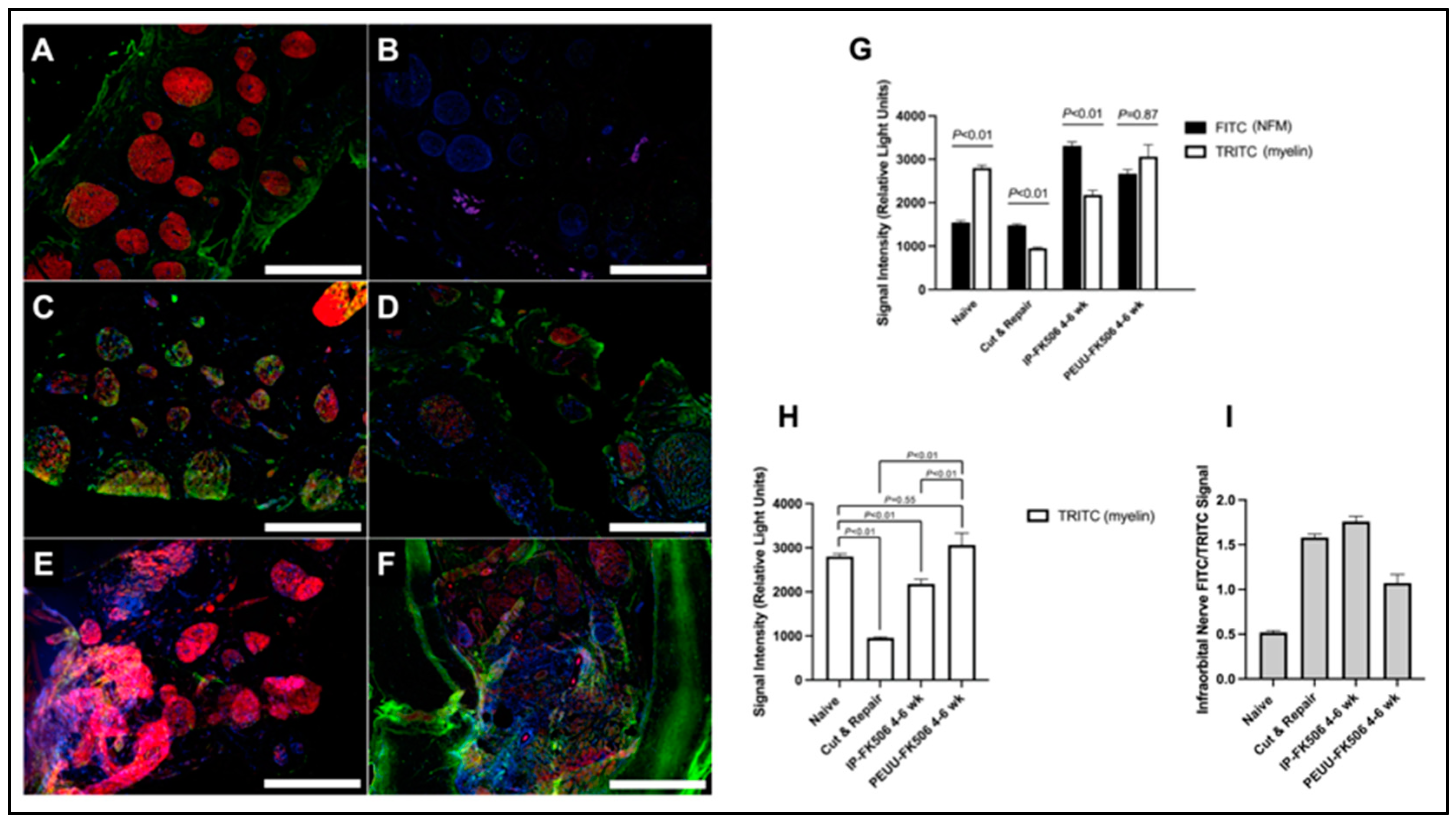
2.3. Infraorbital Nerve Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animals for FK506 Concentration Measurements
4.3. Animals for Trigeminal Ganglion Cell Recordings
4.4. Infraorbital Nerve Transection and Repair
4.5. PEUU-FK506 Sheet Fabrication
4.6. Structural and Mechanical Properties of PEUU-FK506 Sheet
4.7. Extraction of FK506 from Tissue and Wrap
4.8. Quantification of FK506 by LC-Tandem Mass Spectrometry (LC-MS/MS)
4.9. Trigeminal Ganglion Cell Recordings
4.10. Experimental Design and Data Analysis for Trigeminal Ganglion Cell Response
4.11. Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, C.A.; Braza, D.; Rice, J.B.; Dillingham, T. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil. 2008, 87, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Mondelli, M.; Logullo, F.; Grimaldi, S.; Battiston, B.; Sard, A.; Scarinzi, C.; Migliaretti, G.; Faccani, G.; Cocito, D.; et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010, 15, 120–127. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural tissue engineering: Strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Shin, A.Y. Management and complications of traumatic peripheral nerve injuries. Hand Clin. 2015, 31, 151–163. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef]
- Lundborg, G.; Richard, P. Bunge memorial lecture. Nerve injury and repair—A challenge to the plastic brain. J. Peripher. Nerv. Syst. 2003, 8, 209–226. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef]
- Glenn, T.D.; Talbot, W.S. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr. Opin. Neurobiol. 2013, 23, 1041–1048. [Google Scholar] [CrossRef]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Hoke, A. Schwann cell pheno-type is regulated by axon modality and central-peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef]
- Gordon, T.; Tetzlaff, W. Regeneration-associated genes decline in chronically injured rat sciatic motoneurons. Eur. J. Neurosci. 2015, 42, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; You, S.; Cassar, S.L.; Tetzlaff, W. Reduced expression of regeneration associated genes in chronically axotomized facial motoneurons. Exp. Neurol. 2015, 264, 26–32. [Google Scholar] [CrossRef]
- You, S.; Petrov, T.; Chung, P.H.; Gordon, T. The expression of the low affinity nerve growth factor receptor in long-term dener-vated Schwann cells. Glia 1997, 20, 87–100. [Google Scholar] [CrossRef]
- Schnider, J.T.; Weinstock, M.; Plock, J.A.; Solari, M.G.; Venkataramanan, R.; Zheng, X.X.; Gorantla, V.S. Site-specific immuno-suppression in vascularized composite allotransplantation: Prospects and potential. Clin. Dev. Immunol. 2013, 2013, 495212. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Todo, S.; Fung, J.; Demetris, A.J.; Venkataramman, R.; Jain, A. FK 506 for liver, kidney, and pancreas transplanta-tion. Lancet 1989, 2, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Schwaninger, M.; Blume, R.; Oetjen, E.; Knepel, W. The immunosuppressive drugs cyclosporin A and FK506 inhibit calcineurin phosphatase activity and gene transcription mediated through the cAMP-responsive element in a nonimmune cell line. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1993, 348, 541–545. [Google Scholar] [CrossRef]
- Saffari, T.M.; Bedar, M.; Zuidam, J.M.; Shin, A.Y.; Baan, C.C.; Hesselink, D.A.; Hundepool, C.A. Exploring the neuroregenerative potential of tacrolimus. Expert. Rev. Clin. Pharmacol. 2019, 12, 1047–1057. [Google Scholar] [CrossRef]
- Butcher, S.P.; Henshall, D.C.; Teramura, Y.; Iwasaki, K.; Sharkey, J. Neuroprotective actions of FK506 in experimental stroke: In vivo evidence against an antiexcitotoxic mechanism. J. Neurosci. 1997, 17, 6939–6946. [Google Scholar] [CrossRef]
- Furuichi, Y.; Maeda, M.; Moriguchi, A.; Sawamoto, T.; Kawamura, A.; Matsuoka, N.; Mutoh, S.; Yanagihara, T. Tacrolimus, a potential neuroprotective agent, ameliorates ischemic brain damage and neurologic deficits after focal cerebral ischemia in nonhuman primates. J. Cereb. Blood Flow. Metab. 2003, 23, 1183–1194. [Google Scholar] [CrossRef]
- Quinta, H.R.; Galigniana, M.D. The neuroregenerative mechanism mediated by the Hsp90-binding immunophilin FKBP52 resembles the early steps of neuronal differentiation. Br. J. Pharmacol. 2012, 166, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.G.; Zhong, Y.P. FK506 requires stimulation of the extracellular signal-regulated kinase 1/2 and the steroid receptor chaperone protein p23 for neurite elongation. Neurosignals 2004, 13, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G.; Altmann, S.; Plogmeier, K.; Wolf, G.; Schneider, W. The effect of the immunosuppressant FK 506 on pe-ripheral nerve regeneration following nerve grafting. J. Hand Surg. Br. 1999, 24, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Keilhoff, G.; Horn, T.; Altmann, S.; Wolf, G.; Schneider, W. Stimulation of Schwann cell proliferation and axonal regeneration by FK 506. Restor. Neurol. Neurosci. 2000, 16, 77–86. [Google Scholar] [PubMed]
- Que, J.; Cao, Q.; Sui, T.; Du, S.; Zhang, A.; Kong, D.; Cao, X. Tacrolimus reduces scar formation and promotes sciatic nerve re-generation. Neural Regen. Res. 2012, 7, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, B.L.; Perez-Abadia, G.; Onifer, S.M.; Magnuson, D.S.; Burke, D.A.; Grossi, F.V.; Francois, C.G.; Barker, J.H.; Maldonado, C. Neuroregeneration in composite tissue allografts: Effect of low-dose FK506 and mycophenolate mofetil immunotherapy. Plast. Reconstr. Surg. 2006, 118, 615–623; discussion 615–624. [Google Scholar] [CrossRef] [PubMed]
- Konofaos, P.; Terzis, J.K. FK506 and nerve regeneration: Past, present, and future. J. Reconstr. Microsurg. 2013, 29, 141–148. [Google Scholar] [CrossRef]
- Kim, J.S.; Baek, G.H.; Chung, M.S.; Park, H.Y.; Sun, J.H. Effects of FK-506 and CTLA4--Ig on nerve allografts in mice. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2014, 67, e49–e53. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.J.; Li, J.; Kan, Q.C. Effect of FK506 nanospheres on regeneration of allogeneic nerve after transplant. Asian Pac. J. Trop. Med. 2014, 7, 478–482. [Google Scholar] [CrossRef]
- Shahraki, M.; Mohammadi, R.; Najafpour, A. Influence of Tacrolimus (FK506) on Nerve Regeneration Using Allografts: A Rat Sciatic Nerve Model. J. Oral. Maxil Surg. 2015, 73, 1438.E1–1438.E9. [Google Scholar] [CrossRef]
- Yang, L.M.; Wu, Y.X.; Zhang, X.P.; Li, X.H. Experimental research on end-to-side anastomosis of peripheral nerves and effect of FK506 on end-to-side anastomosis. Bratisl. Lek. Listy 2014, 115, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Tricot, L.; Lebbe, C.; Pillebout, E.; Martinez, F.; Legendre, C.; Thervet, E. Tacrolimus-induced alopecia in female kidney-pancreas transplant recipients. Transplantation 2005, 80, 1546–1549. [Google Scholar] [CrossRef]
- Oto, T.; Okazaki, M.; Takata, K.; Egi, M.; Yamane, M.; Toyooka, S.; Sano, Y.; Snell, G.I.; Goto, K.; Miyoshi, S. Calcineurin inhib-itor-related cholestasis complicating lung transplantation. Ann. Thorac. Surg. 2010, 89, 1664–1665. [Google Scholar] [CrossRef] [PubMed]
- Leroy, S.; Isapof, A.; Fargue, S.; Fakhoury, M.; Bensman, A.; Deschenes, G.; Jacqz-Aigrain, E.; Ulinski, T. Tacrolimus nephrotox-icity: Beware of the association of diarrhea, drug interaction and pharmacogenetics. Pediatr. Nephrol. 2010, 25, 965–969. [Google Scholar] [CrossRef]
- Gnatta, D.; Keitel, E.; Heineck, I.; Cardoso, B.; Rodrigues, A.; Michel, K.; Garcia, V. Use of tacrolimus and the development of posttransplant diabetes mellitus: A Brazilian single-center, observational study. In Transplantation Proceedings; Elsevier Sci-ence Publishers: Amsterdam, The Netherlands, 2010; pp. 475–478. [Google Scholar]
- Dehghani, S.M.; Haghighat, M.; Imanieh, M.H.; Zahmatkeshan, M.; Borzooei, M.; Amoozegar, H.; Zamirian, M.; Gholami, S.; Bahador, A.; Nikeghbalian, S.; et al. Tacrolimus related hypertrophic cardiomyopathy in liver transplant recipients. Arch. Iran. Med. 2010, 13, 116–119. [Google Scholar]
- Davaus Gasparetto, T.; Marchiori, E.; Menezes, P.; Zanetti, G. Pulmonary toxicity associated with sirolimus following kidney transplantation: Computed tomography findings. Nefrologia 2010, 30, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.C.; Cendales, L.C. Vascularized composite allotransplantation in the United States: A retrospective analysis of the Organ Procurement and Transplantation Network data after 5 years of the Final Rule. Am. J. Transplant. 2021, 21, 291–296. [Google Scholar] [CrossRef]
- Lee, W.P.; Yaremchuk, M.J.; Pan, Y.C.; Randolph, M.A.; Tan, C.M.; Weiland, A.J. Relative antigenicity of components of a vascu-larized limb allograft. Plast. Reconstr. Surg. 1991, 87, 401–411. [Google Scholar] [CrossRef]
- Davis, B.; Hilgart, D.; Erickson, S.; Labroo, P.; Burton, J.; Sant, H.; Shea, J.; Gale, B.; Agarwal, J. Local FK506 delivery at the di-rect nerve repair site improves nerve regeneration. Muscle Nerve 2019, 60, 613–620. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Schramm, P.; Isenmann, S.; Brecht, S.; Eickmeier, C.; Burger, E.; Herdegen, T.; Sievers, J.; Lucius, R. Differential effects of immunophilin-ligands (FK506 and V-10,367) on survival and regeneration of rat retinal ganglion cells in vitro and after optic nerve crush in vivo. J. Neurotrauma 2003, 20, 297–307. [Google Scholar] [CrossRef]
- Tajdaran, K.; Chan, K.; Zhang, J.; Gordon, T.; Borschel, G.H. Local FK506 dose-dependent study using a novel three-dimensional organotypic assay. Biotechnol. Bioeng. 2019, 116, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Tajdaran, K.; Shoichet, M.S.; Gordon, T.; Borschel, G.H. A novel polymeric drug delivery system for localized and sustained release of tacrolimus (FK506). Biotechnol. Bioeng. 2015, 112, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, J.; Shao, X.; Zhang, L. Application of FK506 slow-releasing film in peripheral nerve allotransplantation: An experimental study in mice. Chin. J. Hand Surg. 2007, 23, 8–10. [Google Scholar]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Design of pH-sensitive microspheres for the colonic delivery of the immunosuppressive drug tacrolimus. Eur. J. Pharm. Biopharm. 2004, 58, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Taddeo, A.; Tsai, C.; Vogelin, E.; Rieben, R. Novel targeted drug delivery systems to minimize systemic immunosuppression in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Dellon, A.L. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast. Reconstr. Surg. 1990, 85, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.A.; Breidenbach, W.C.; Brown, R.E.; Jabaley, M.E.; Mass, D.P. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast. Reconstr. Surg. 2000, 106, 1036–1045; discussion 1038–1046. [Google Scholar] [CrossRef]
- Meek, M.F.; Coert, J.H. Recovery of two-point discrimination function after digital nerve repair in the hand using resorbable FDA- and CE-approved nerve conduits. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2013, 66, 1307–1315. [Google Scholar] [CrossRef]
- van der Merwe, Y.; Faust, A.E.; Conner, I.; Gu, X.; Feturi, F.; Zhao, W.; Leonard, B.; Roy, S.; Gorantla, V.S.; Venkataramanan, R.; et al. An Elastomeric Polymer Matrix, PEUU-Tac, Delivers Bioactive Tacrolimus Transdurally to the CNS in Rat. EBioMedicine 2017, 26, 47–59. [Google Scholar] [CrossRef]
- Woolsey, T.A.; Van der Loos, H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970, 17, 205–242. [Google Scholar] [CrossRef]
- Simons, D.J. Response properties of vibrissa units in rat SI somatosensory neocortex. J. Neurophysiol. 1978, 41, 798–820. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zanoun, R.R.; Carvell, G.E.; Simons, D.J.; Washington, K.M. Response properties of whisker-associated primary afferent neurons following infraorbital nerve transection with microsurgical repair in adult rats. J. Neurophysiol. 2016, 115, 1458–1467. [Google Scholar] [CrossRef]
- Fraser, G.; Hartings, J.A.; Simons, D.J. Adaptation of trigeminal ganglion cells to periodic whisker deflections. Somatosens. Mot. Res. 2006, 23, 111–118. [Google Scholar] [CrossRef]
- Zuo, K.J.; Saffari, T.M.; Chan, K.; Shin, A.Y.; Borschel, G.H. Systemic and Local FK506 (Tacrolimus) and its Application in Pe-ripheral Nerve Surgery. J. Hand Surg. Am. 2020, 45, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Gad, S.F.; Chobisa, D.; Li, Y.; Yeo, Y. Local drug delivery systems for inflammatory diseases: Status quo, challenges, and opportunities. J. Control. Release 2021, 330, 438–460. [Google Scholar] [CrossRef]
- Yang, R.K.; Lowe, J.B., 3rd; Sobol, J.B.; Sen, S.K.; Hunter, D.A.; Mackinnon, S.E. Dose-dependent effects of FK506 on neuroregen-eration in a rat model. Plast. Reconstr. Surg. 2003, 112, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.S.; Zeleny-Pooley, M.; Gold, B.G. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of ax-onal regeneration in the rat sciatic nerve. J. Pharmacol. Exp. Ther. 1997, 282, 1084–1093. [Google Scholar]
- Udina, E.; Ceballos, D.; Verdu, E.; Gold, B.G.; Navarro, X. Bimodal dose-dependence of FK506 on the rate of axonal regeneration in mouse peripheral nerve. Muscle Nerve 2002, 26, 348–355. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef]
- Steiner, J.P.; Connolly, M.A.; Valentine, H.L.; Hamilton, G.S.; Dawson, T.M.; Hester, L.; Snyder, S.H. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat. Med. 1997, 3, 421–428. [Google Scholar] [CrossRef]
- Gold, B.G.; Zeleny-Pooley, M.; Wang, M.S.; Chaturvedi, P.; Armistead, D.M. A nonimmunosuppressant FKBP-12 ligand in-creases nerve regeneration. Exp. Neurol. 1997, 147, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Harty, B.L.; Coelho, F.; Pease-Raissi, S.E.; Mogha, A.; Ackerman, S.D.; Herbert, A.L.; Gereau, R.W.; Golden, J.P.; Lyons, D.A.; Chan, J.R.; et al. Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7. Nat. Com-mun. 2019, 10, 2976. [Google Scholar] [CrossRef] [PubMed]
- Benga, A.; Zor, F.; Korkmaz, A.; Marinescu, B.; Gorantla, V. The neurochemistry of peripheral nerve regeneration. Indian. J. Plast. Surg. 2017, 50, 5–15. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Pan, Y.; Chen, L.; Zhang, Z.; Lu, L. Peripheral nerve injuries treatment: A systematic review. Cell Biochem. Biophys. 2014, 68, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. The anatomy and physiology of nerve injury. Muscle Nerve 1990, 13, 771–784. [Google Scholar] [CrossRef]
- Dominguez, C.A.; Kouya, P.F.; Wu, W.P.; Hao, J.X.; Xu, X.J.; Wiesenfeld-Hallin, Z. Sex differences in the development of local-ized and spread mechanical hypersensitivity in rats after injury to the infraorbital or sciatic nerves to create a model for neu-ropathic pain. Gend. Med. 2009, 6 (Suppl. S2), 225–234. [Google Scholar] [CrossRef]
- Carty, M.J.; Bueno, E.; Lehmann, L.S.; Pomahac, B. A position paper in support of hand transplantation in the blind. Plast. Re-constr. Surg. 2011, 128, 510e–515e. [Google Scholar] [CrossRef]
- Kramer, M.R.; Amital, A.; Fuks, L.; Shitrit, D. Voriconazole and itraconazole in lung transplant recipients receiving tacroli-mus (FK 506): Efficacy and drug interaction. Clin. Transplant. 2011, 25, E163–E167. [Google Scholar] [CrossRef]
- Stankus, J.J.; Guan, J.; Wagner, W.R. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J. Bi-omed. Mater. Res. Part A 2004, 70, 603–614. [Google Scholar] [CrossRef]
- Guan, J.; Sacks, M.S.; Beckman, E.J.; Wagner, W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biode-gradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J. Biomed. Mater. Res. 2002, 61, 493–503. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Chapin, J.K.; Sorensen, S.M.; Woodward, D.J. Acute ethanol effects on sensory responses of single units in the somatosensory cortex of rats during different behavioral states. Pharmacol. Biochem. Behav. 1986, 25, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.J. Multi-whisker stimulation and its effects on vibrissa units in rat SmI barrel cortex. Brain Res. 1983, 276, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Faust, A.; van der Merwe, Y.; Xiao, B.; Johnson, S.; Kandakatla, A.; Gorantla, V.S.; Badylak, S.F.; Washington, K.M.; Ste-ketee, M.B. Fetal extracellular matrix nerve wraps locally improve peripheral nerve remodeling after complete transection and direct repair in rat. Sci. Rep. 2018, 8, 4474. [Google Scholar] [CrossRef]
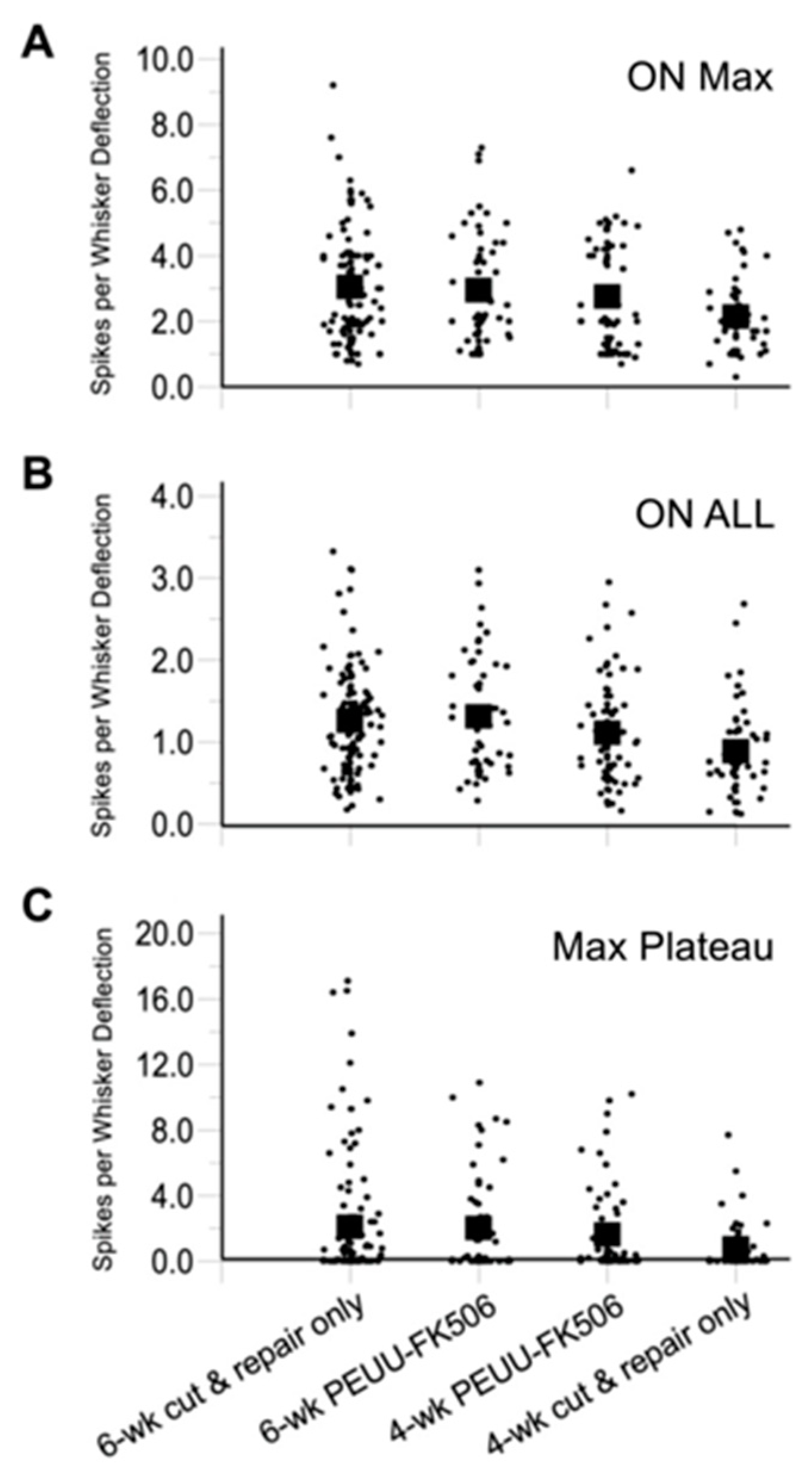

| Group | ON Max (20 ms) | ON ALL (20 ms) | Max Plateau (100 ms) |
|---|---|---|---|
| 6-wk cut & repair only (n = 113) | 3.05 ± 1.61 | 1.26 ± 0.65 | 2.10 ± 3.80 |
| 6-wk PEEU-FK506 (n = 57) | 2.95 ± 1.67; p = 0.97 | 1.30 ± 0.68; p = 0.96 | 1.99 ± 3.07; p = 0.99 |
| 4-wk PEEU-FK506 (n = 68) | 2.76 ± 1.51; p = 0.49 | 1.15 ± 0.64; p = 0.34 | 1.62 ± 2.56; p = 0.65 |
| 4-wk cut & repair only (n = 53) | 2.15 ± 1.08; p = 0.001 | 0.89 ± 0.55; p = 0.002 | 0.78 ± 1.50; p = 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, B.; Feturi, F.; Su, A.-J.A.; Van der Merwe, Y.; Barnett, J.M.; Jabbari, K.; Khatter, N.J.; Li, B.; Katzel, E.B.; Venkataramanan, R.; et al. Nerve Wrap for Local Delivery of FK506/Tacrolimus Accelerates Nerve Regeneration. Int. J. Mol. Sci. 2024, 25, 847. https://doi.org/10.3390/ijms25020847
Xiao B, Feturi F, Su A-JA, Van der Merwe Y, Barnett JM, Jabbari K, Khatter NJ, Li B, Katzel EB, Venkataramanan R, et al. Nerve Wrap for Local Delivery of FK506/Tacrolimus Accelerates Nerve Regeneration. International Journal of Molecular Sciences. 2024; 25(2):847. https://doi.org/10.3390/ijms25020847
Chicago/Turabian StyleXiao, Bo, Firuz Feturi, An-Jey A. Su, Yolandi Van der Merwe, Joshua M. Barnett, Kayvon Jabbari, Neil J. Khatter, Bing Li, Evan B. Katzel, Raman Venkataramanan, and et al. 2024. "Nerve Wrap for Local Delivery of FK506/Tacrolimus Accelerates Nerve Regeneration" International Journal of Molecular Sciences 25, no. 2: 847. https://doi.org/10.3390/ijms25020847
APA StyleXiao, B., Feturi, F., Su, A.-J. A., Van der Merwe, Y., Barnett, J. M., Jabbari, K., Khatter, N. J., Li, B., Katzel, E. B., Venkataramanan, R., Solari, M. G., Wagner, W. R., Steketee, M. B., Simons, D. J., & Washington, K. M. (2024). Nerve Wrap for Local Delivery of FK506/Tacrolimus Accelerates Nerve Regeneration. International Journal of Molecular Sciences, 25(2), 847. https://doi.org/10.3390/ijms25020847







