Force-Induced Alignment of Nanofibrillated Bacterial Cellulose for the Enhancement of Cellulose Composite Macrofibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Properties of the HPBC Spinning Dope
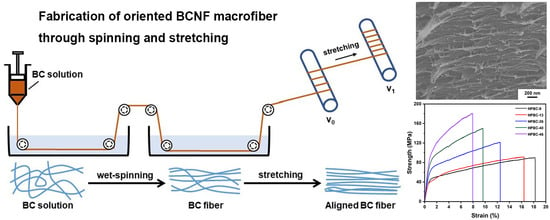
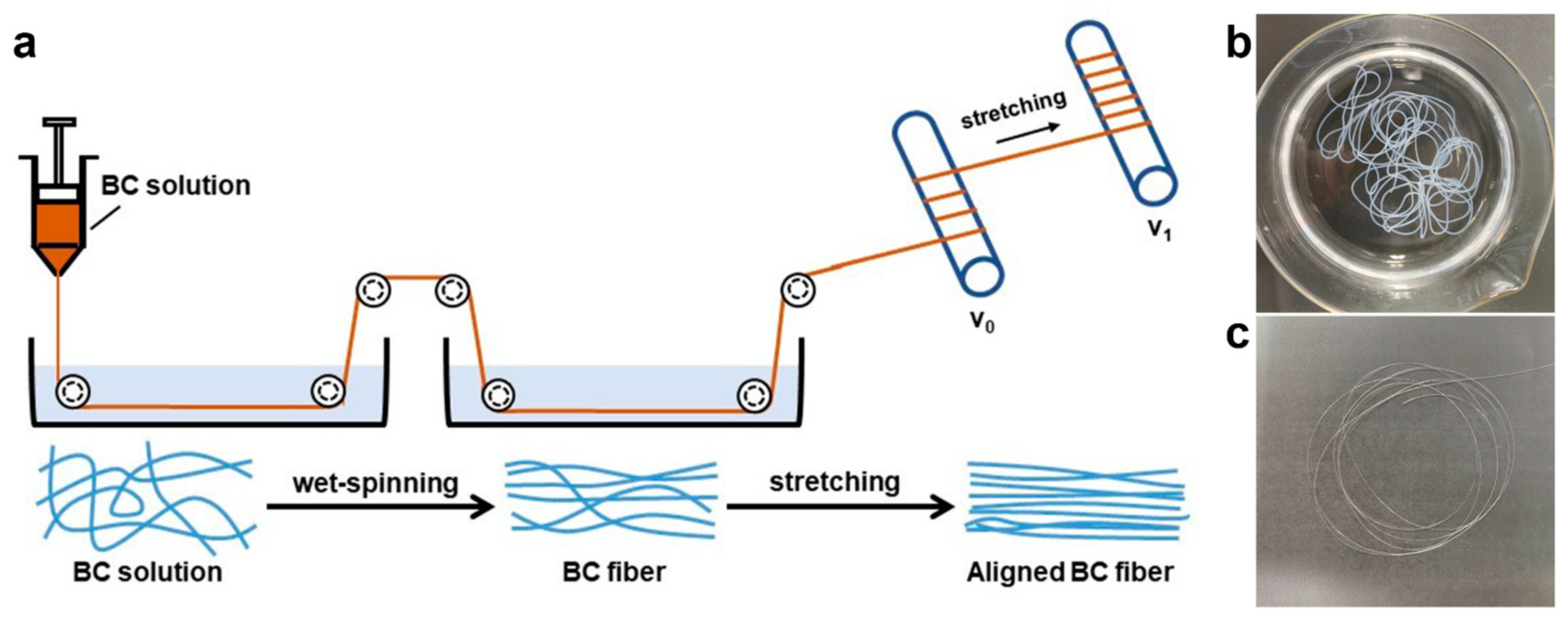
2.2. Dry Jet-Wet Spinning of HPBC
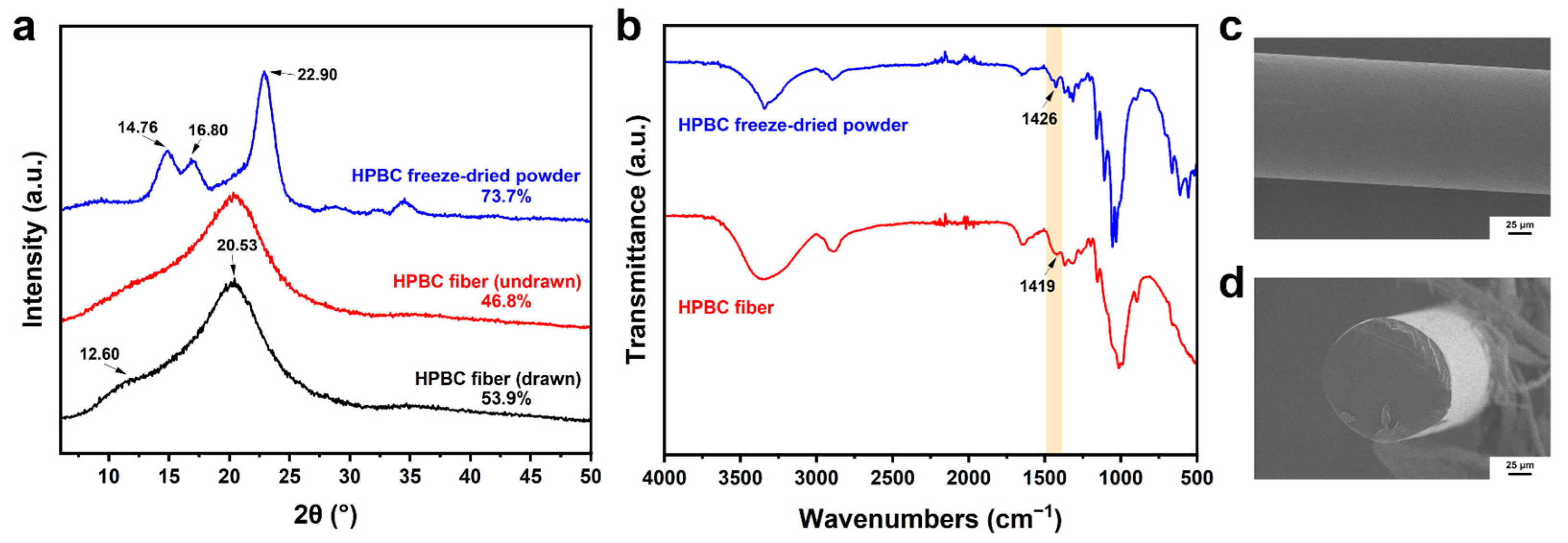
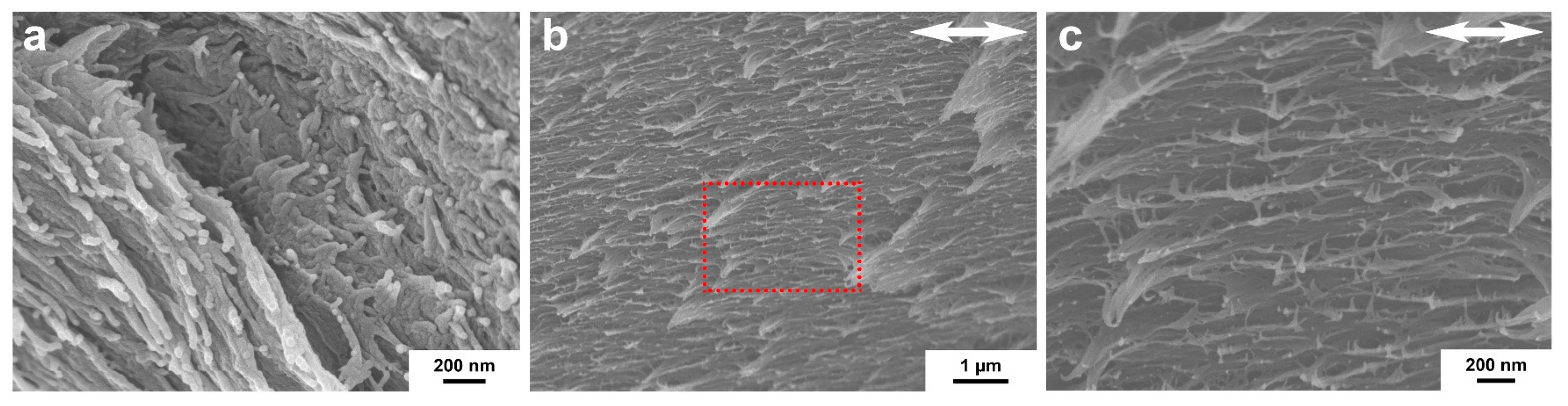
2.3. The Aligned Interior Microstructure of the HPBC Fiber
2.4. Mechanical Properties of the HPBC Fiber
2.5. Co-Spinning of CMBC and Chitosan
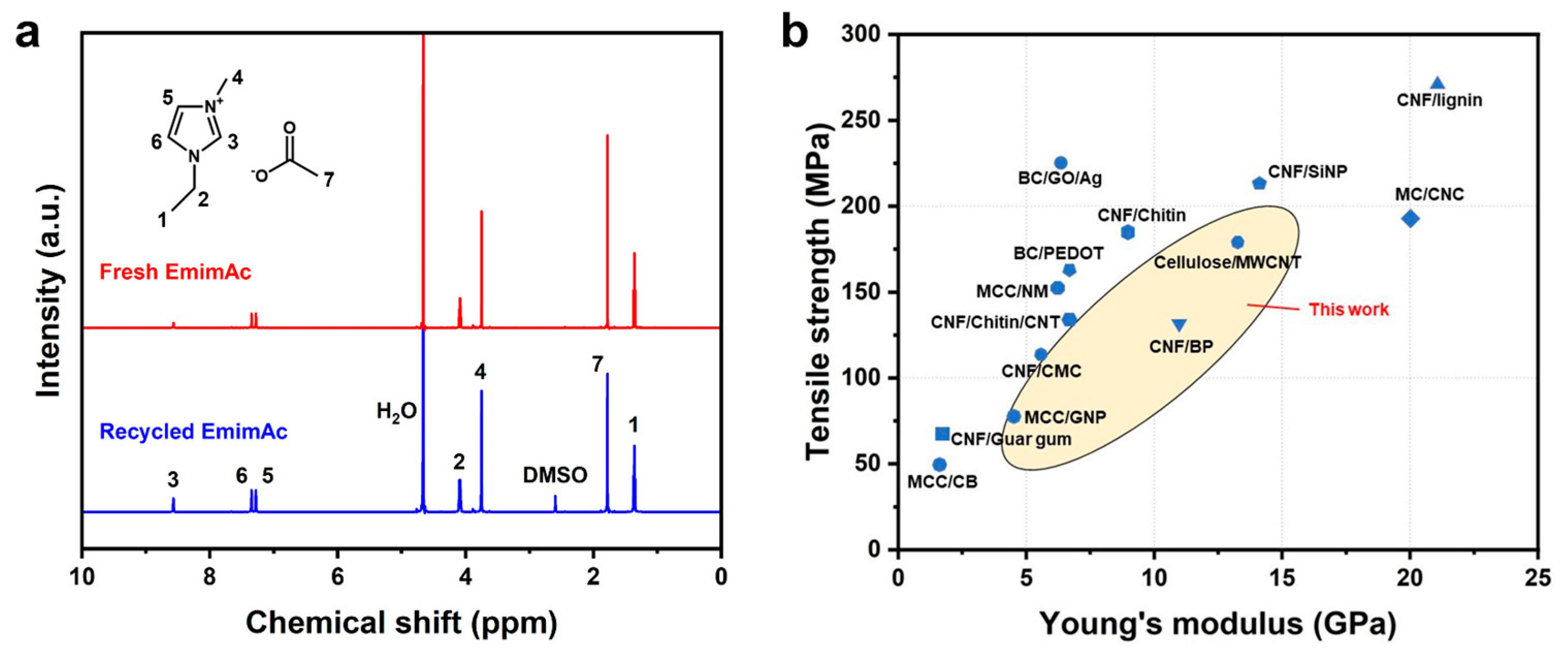
2.6. Recycling of the IL
3. Materials and Methods
3.1. Materials
3.2. Dry Jet-Wet Spinning of HPBC
3.3. Wet Stretching of Macrofibers
3.4. Dry Jet-Wet Spinning of CMBC and HEBC
3.5. Dry Jet-Wet Spinning of CMBC and Chitosan (CMCS Fiber)
3.6. Recycling of IL
3.7. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | bacterial cellulose |
| BP | benzophenone |
| CB | carbon black |
| CMC | carboxymethyl cellulose |
| CNC | cellulose nanocrystal |
| CNF | cellulose nanofiber |
| CNT | carbon nanotube |
| GNP | graphene nanoplatelets |
| GO | graphene oxide |
| MC | methyl cellulose |
| MCC | microcrystalline cellulose |
| MWCNT | multiwall carbon nanotube |
| NM | nanomagnetite particles |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| SiNP | silica nanoparticles |
References
- Shin, S.-K.; Um, N.; Kim, Y.-J.; Cho, N.-H.; Jeon, T.-W. New Policy Framework with Plastic Waste Control Plan for Effective Plastic Waste Management. Sustainability 2020, 12, 6049. [Google Scholar] [CrossRef]
- Su, L.; Xiong, X.; Zhang, Y.; Wu, C.; Xu, X.; Sun, C.; Shi, H. Global transportation of plastics and microplastics: A critical review of pathways and influences. Sci. Total Environ. 2022, 831, 154884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current technologies for plastic waste treatment: A review. J. Clean. Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Narancic, T.; O’Connor, K.E. Plastic waste as a global challenge: Are biodegradable plastics the answer to the plastic waste problem? Microbiology 2019, 165, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263 Pt A, 114469. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Ji, Q.; Zhou, C.; Li, Z.; Boateng, I.D.; Liu, X. Is nanocellulose a good substitute for non-renewable raw materials? A comprehensive review of the state of the art, preparations, and industrial applications. Ind. Crops Prod. 2023, 202, 117093. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X.; Ma, K.; Xia, G.; Wu, M.; Cheung, Y.H.; Yu, H.; Zou, B.; Zhang, X.; Farha, O.K.; et al. Functional Textiles with Smart Properties: Their Fabrications and Sustainable Applications. Adv. Funct. Mater. 2023, 33, 2301607. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the cellulose-based textiles: Raw materials and technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef]
- Kramar, A.; Gonzalez-Benito, F.J. Cellulose-Based Nanofibers Processing Techniques and Methods Based on Bottom-Up Approach-A Review. Polymers 2022, 14, 286. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; Michud, A.; Sixta, H. Dry jet-wet spinning of strong cellulose filaments from ionic liquid solution. Cellulose 2014, 21, 4471–4481. [Google Scholar] [CrossRef]
- Zhu, C.; Richardson, R.M.; Potter, K.D.; Koutsomitopoulou, A.F.; van Duijneveldt, J.S.; Vincent, S.R.; Wanasekara, N.D.; Eichhorn, S.J.; Rahatekar, S.S. High Modulus Regenerated Cellulose Fibers Spun from a Low Molecular Weight Microcrystalline Cellulose Solution. ACS Sustain. Chem. Eng. 2016, 4, 4545–4553. [Google Scholar] [CrossRef]
- Guizani, C.; Larkiala, S.; Moriam, K.; Sawada, D.; Elsayed, S.; Rantasalo, S.; Hummel, M.; Sixta, H. Air gap spinning of a cellulose solution in [DBNH][OAc] ionic liquid with a novel vertically arranged spinning bath to simulate a closed loop operation in the Ioncell® process. J. Appl. Polym. Sci. 2020, 138, 49787. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Bochek, A. Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Kim, T.; Kim, D.; Park, Y. Recent progress in regenerated fibers for “green” textile products. J. Clean. Prod. 2022, 376, 134226. [Google Scholar] [CrossRef]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent advances in the application of cellulose derivatives for removal of contaminants from aquatic environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, P.K.; Pattnaik, R.; Kumar, S.; Ojha, S.K.; Srichandan, H.; Parhi, P.K.; Jyothi, R.K.; Sarangi, P.K. Biochemistry, Synthesis, and Applications of Bacterial Cellulose: A Review. Front. Bioeng. Biotechnol. 2022, 10, 780409. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Huang, L.H.; Zhao, X.Q.; Li, W.C.; Wang, Y.Y.; Jia, S.R.; Zhong, C. Bacterial cellulose and its potential for biomedical applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef]
- Provin, A.P.; Cubas, A.L.V.; Dutra, A.R.d.A.; Schulte, N.K. Textile industry and environment: Can the use of bacterial cellulose in the manufacture of biotextiles contribute to the sector? Clean. Technol. Environ. Policy 2021, 23, 2813–2825. [Google Scholar] [CrossRef]
- Chen, C.; Ding, W.; Zhang, H.; Zhang, L.; Huang, Y.; Fan, M.; Yang, J.; Sun, D. Bacterial cellulose-based biomaterials: From fabrication to application. Carbohydr. Polym. 2022, 278, 118995. [Google Scholar] [CrossRef] [PubMed]
- Asanarong, O.; Minh Quan, V.; Boonrungsiman, S.; Sukyai, P. Bioactive wound dressing using bacterial cellulose loaded with papain composite: Morphology, loading/release and antibacterial properties. Eur. Polym. J. 2021, 143, 110224. [Google Scholar] [CrossRef]
- Yang, H.B.; Liu, Z.X.; Yin, C.H.; Han, Z.M.; Guan, Q.F.; Zhao, Y.X.; Ling, Z.C.; Liu, H.C.; Yang, K.P.; Sun, W.B.; et al. Edible, Ultrastrong, and Microplastic-Free Bacterial Cellulose-Based Straws by Biosynthesis. Adv. Funct. Mater. 2021, 32, 2111713. [Google Scholar] [CrossRef]
- Fernandes, I.A.A.; Pedro, A.C.; Ribeiro, V.R.; Bortolini, D.G.; Ozaki, M.S.C.; Maciel, G.M.; Haminiuk, C.W.I. Bacterial cellulose: From production optimization to new applications. Int. J. Biol. Macromol. 2020, 164, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R. Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, S.; Chen, Z.; Hu, Y.; Shi, G.; Zhuo, H.; Zhang, H.; Zhong, L. Assembling nanocelluloses into fibrous materials and their emerging applications. Carbohydr. Polym. 2023, 299, 120008. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Netravali, A.N. Aligned Bacterial Cellulose Arrays as “Green” Nanofibers for Composite Materials. ACS Macro Lett. 2016, 5, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Panicker, P.S.; Kim, H.C.; Agumba, D.O.; Muthoka, R.M.; Kim, J. Electric field-assisted wet spinning to fabricate strong, tough, and continuous nanocellulose long fibers. Cellulose 2022, 29, 3499–3511. [Google Scholar] [CrossRef]
- Ye, D.; Cheng, Q.; Zhang, Q.; Wang, Y.; Chang, C.; Li, L.; Peng, H.; Zhang, L. Deformation Drives Alignment of Nanofibers in Framework for Inducing Anisotropic Cellulose Hydrogels with High Toughness. ACS Appl. Mater. Interfaces 2017, 9, 43154–43162. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-Strong, Super-Stiff Macrofibers with Aligned, Long Bacterial Cellulose Nanofibers. Adv. Mater. 2017, 29, 1702498. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; Wu, R.; Sheng, N.; Zhang, M.; Ji, P.; Wang, H. Top-down peeling bacterial cellulose to high strength ultrathin films and multifunctional fibers. Chem. Eng. J. 2020, 391, 123527. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Wu, Z.; Sheng, N.; Zhang, M.; Liang, Q.; Han, Z.; Wang, H. Toward continuous high-performance bacterial cellulose macrofibers by implementing grading-stretching in spinning. Carbohydr. Polym. 2022, 282, 119133. [Google Scholar] [CrossRef]
- Saleh, A.K.; Tolba, E.; Salama, A. In situ development of bacterial cellulose/hydroxyapatite nanocomposite membrane based on two different fermentation strategies: Characterization and cytotoxicity evaluation. Biomass Convers. Biorefinery 2023, 1–11. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Bi, J.C.; Liu, S.X.; Li, C.F.; Li, J.; Liu, L.X.; Deng, J.; Yang, Y.C. Morphology and structure characterization of bacterial celluloses produced by different strains in agitated culture. J. Appl. Microbiol. 2014, 117, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, S.S.; Maciel Filho, R. Are ionic liquids eco-friendly? Renew. Sustain. Energy Rev. 2022, 157, 112039. [Google Scholar] [CrossRef]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids--promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Kusumoto, R.; Kose, R.; Kono, H.; Matsushima, T.; Isono, T.; Yamamoto, T.; Satoh, T. One-Step Production of Amphiphilic Nanofibrillated Cellulose Using a Cellulose-Producing Bacterium. Biomacromolecules 2017, 18, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Fujie, T.; Sasaki, R.; Matsushima, T.; Takahashi, K. Direct synthesis of a robust cellulosic composite from cellulose acetate and a nanofibrillated bacterial cellulose sol. Polym. J. 2022, 54, 735–740. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Zhang, L.; Chen, L. Rapid dissolution of spruce cellulose in H2SO4 aqueous solution at low temperature. Cellulose 2016, 23, 3463–3473. [Google Scholar] [CrossRef]
- Kafy, A.; Kim, H.C.; Zhai, L.; Kim, J.W.; Hai, L.V.; Kang, T.J.; Kim, J. Cellulose long fibers fabricated from cellulose nanofibers and its strong and tough characteristics. Sci. Rep. 2017, 7, 17683. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Zhang, H.; Zhang, Q.; Wang, L.; Li, C.; Dai, B.; Yang, J.; Liu, J.; Sun, D. Electrically-responsive core-shell hybrid microfibers for controlled drug release and cell culture. Acta Biomater. 2017, 55, 434–442. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T.; Dai, B.; Zhang, H.; Chen, X.; Yang, J.; Liu, J.; Sun, D. Rapid Fabrication of Composite Hydrogel Microfibers for Weavable and Sustainable Antibacterial Applications. ACS Sustain. Chem. Eng. 2016, 4, 6534–6542. [Google Scholar] [CrossRef]
- Härdelin, L.; Hagström, B. Wet spun fibers from solutions of cellulose in an ionic liquid with suspended carbon nanoparticles. J. Appl. Polym. Sci. 2014, 132, 41417. [Google Scholar] [CrossRef]
- Hynninen, V.; Mohammadi, P.; Wagermaier, W.; Hietala, S.; Linder, M.B.; Ikkala, O. Nonappa, Methyl cellulose/cellulose nanocrystal nanocomposite fibers with high ductility. Eur. Polym. J. 2019, 112, 334–345. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Cunha, A.G.; Rojo, E.; Papageorgiou, A.C.; Rautkari, L.; Arboleda, J.C.; Rojas, O.J. Strength and Water Interactions of Cellulose I Filaments Wet-Spun from Cellulose Nanofibril Hydrogels. Sci. Rep. 2016, 6, 30695. [Google Scholar] [CrossRef] [PubMed]
- Maxim, M.L.; Sun, N.; Wang, H.; Sterner, J.R.; Haque, A.; Rogers, R.D. Reinforced magnetic cellulose fiber from ionic liquid solution. Nanomater. Energy 2012, 1, 225–236. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Bordes, R.; Kohnke, T. Wet Spinning of Flame-Retardant Cellulosic Fibers Supported by Interfacial Complexation of Cellulose Nanofibrils with Silica Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 39069–39077. [Google Scholar] [CrossRef] [PubMed]
- Rahatekar, S.S.; Rasheed, A.; Jain, R.; Zammarano, M.; Koziol, K.K.; Windle, A.H.; Gilman, J.W.; Kumar, S. Solution spinning of cellulose carbon nanotube composites using room temperature ionic liquids. Polymer 2009, 50, 4577–4583. [Google Scholar] [CrossRef]
- Vuoriluoto, M.; Orelma, H.; Lundahl, M.; Borghei, M.; Rojas, O.J. Filaments with Affinity Binding and Wet Strength Can Be Achieved by Spinning Bifunctional Cellulose Nanofibrils. Biomacromolecules 2017, 18, 1803–1813. [Google Scholar] [CrossRef]
- Zhang, K.; Ketterle, L.; Järvinen, T.; Hong, S.; Liimatainen, H. Conductive hybrid filaments of carbon nanotubes, chitin nanocrystals and cellulose nanofibers formed by interfacial nanoparticle complexation. Mater. Des. 2020, 191, 108594. [Google Scholar] [CrossRef]
- Zhang, K.; Liimatainen, H. Hierarchical Assembly of Nanocellulose-Based Filaments by Interfacial Complexation. Small 2018, 14, e1801937. [Google Scholar] [CrossRef]




| Entry | Extrusion Rate (m min−1) | Stretching Ratio (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|
| 1 | 15 | 0 | 67.2 ± 11.4 | 5.3 ± 0.3 |
| 2 | 15 | 26 | 96.9 ± 22.0 | 8.1 ± 1.1 |
| 3 | 15 | 46 | 110.5 ± 18.3 | 8.4 ± 0.9 |
| 4 | 20 | 0 | 85.0 ± 12.9 | 7.7 ± 0.8 |
| 5 | 20 | 26 | 107.3 ± 10.1 | 8.9 ± 0.5 |
| 6 | 20 | 46 | 124.7 ± 18.1 | 9.7 ± 0.8 |
| 7 | 25 | 0 | 96.7 ± 6.8 | 7.3 ± 0.7 |
| 8 | 25 | 26 | 108.8 ± 7.3 | 8.3 ± 0.7 |
| 9 | 25 | 46 | 149.2 ± 26.6 | 11.1 ± 0.6 |
| 10 | 30 | 0 | 91.5 ± 17.1 | 7.7 ± 0.6 |
| 11 | 30 | 26 | 119.0 ± 19.2 | 9.3 ± 0.9 |
| 12 | 30 | 46 | 173.8 ± 8.3 | 13.7 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Fujie, T.; Itaya, H.; Wada, N.; Takahashi, K. Force-Induced Alignment of Nanofibrillated Bacterial Cellulose for the Enhancement of Cellulose Composite Macrofibers. Int. J. Mol. Sci. 2024, 25, 69. https://doi.org/10.3390/ijms25010069
Wang R, Fujie T, Itaya H, Wada N, Takahashi K. Force-Induced Alignment of Nanofibrillated Bacterial Cellulose for the Enhancement of Cellulose Composite Macrofibers. International Journal of Molecular Sciences. 2024; 25(1):69. https://doi.org/10.3390/ijms25010069
Chicago/Turabian StyleWang, Ruochun, Tetsuo Fujie, Hiroyuki Itaya, Naoki Wada, and Kenji Takahashi. 2024. "Force-Induced Alignment of Nanofibrillated Bacterial Cellulose for the Enhancement of Cellulose Composite Macrofibers" International Journal of Molecular Sciences 25, no. 1: 69. https://doi.org/10.3390/ijms25010069
APA StyleWang, R., Fujie, T., Itaya, H., Wada, N., & Takahashi, K. (2024). Force-Induced Alignment of Nanofibrillated Bacterial Cellulose for the Enhancement of Cellulose Composite Macrofibers. International Journal of Molecular Sciences, 25(1), 69. https://doi.org/10.3390/ijms25010069