Contribution to a Sustainable Society: Biosorption of Precious Metals Using the Microalga Galdieria
Abstract
1. Introduction
2. Results
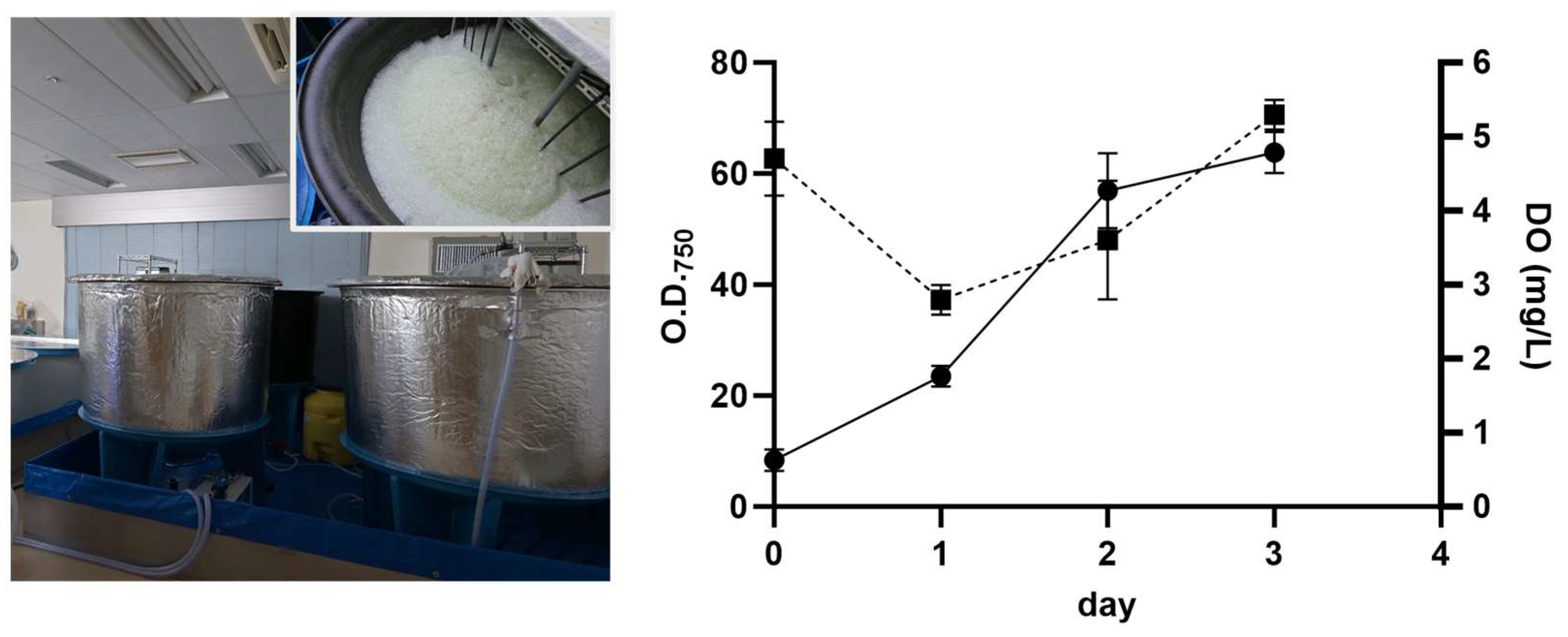
2.1. Large-Scale Production of Galdieria
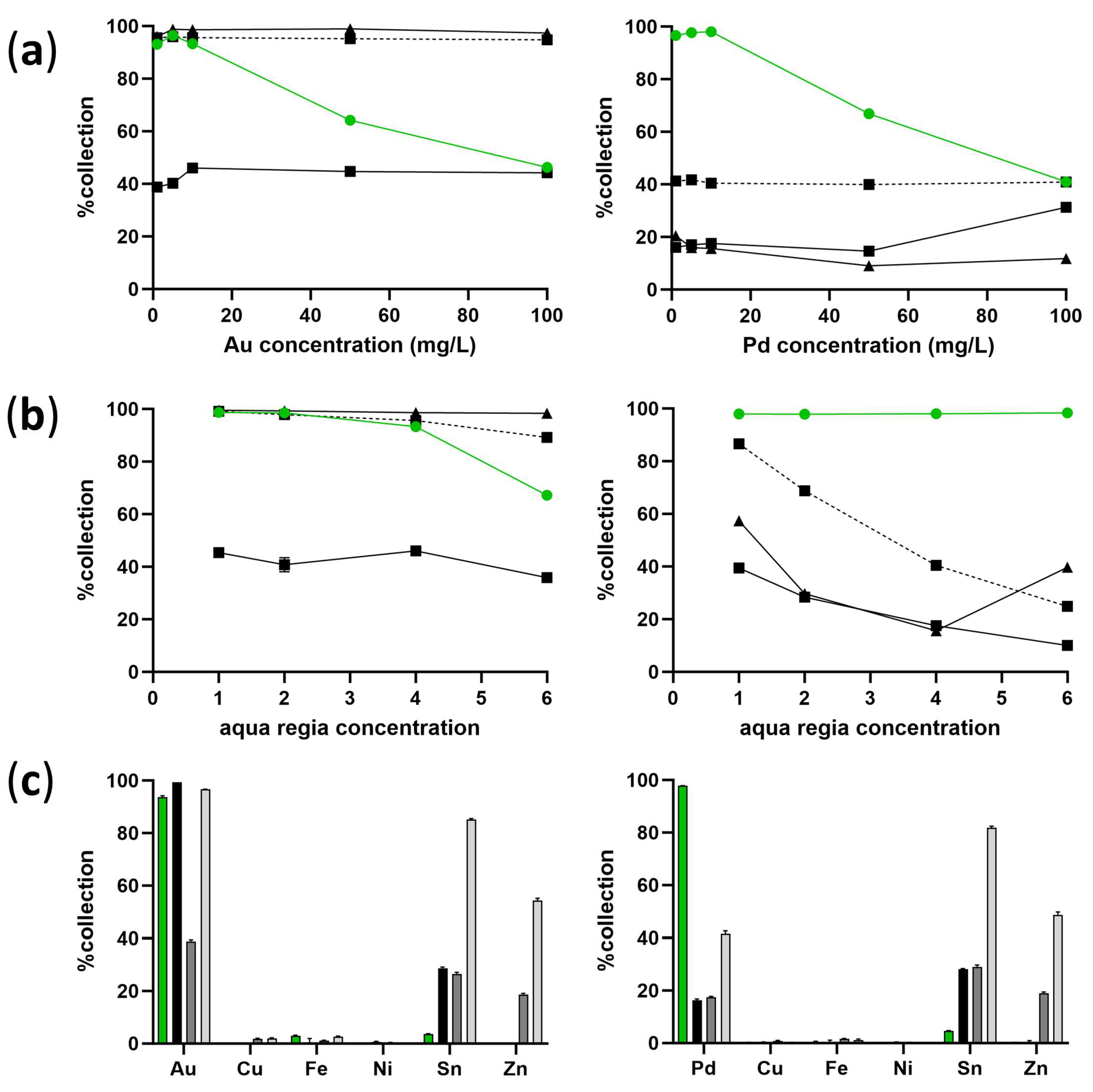
2.2. Precious Metal Collection Capacity of Galdieria in Urban Mines
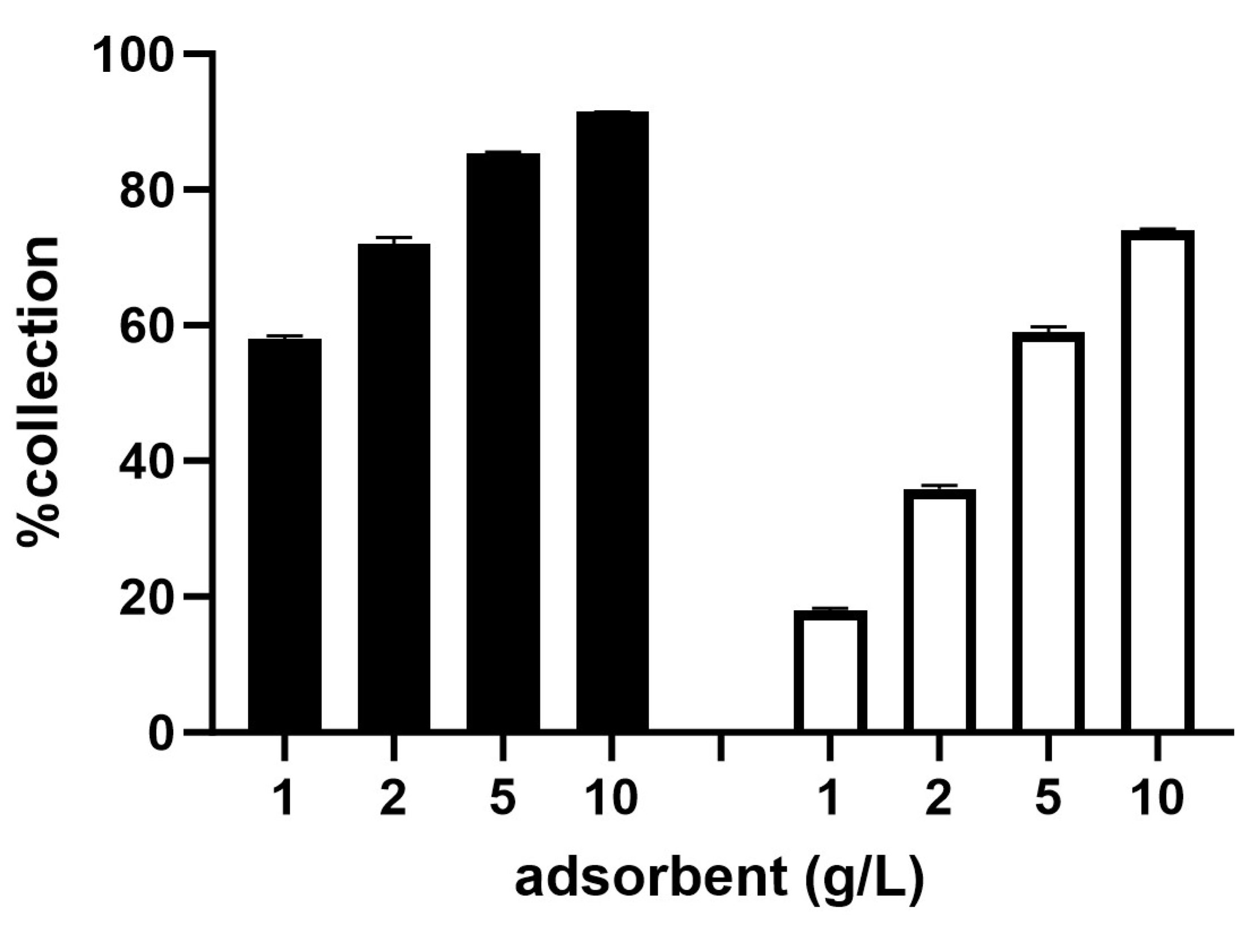
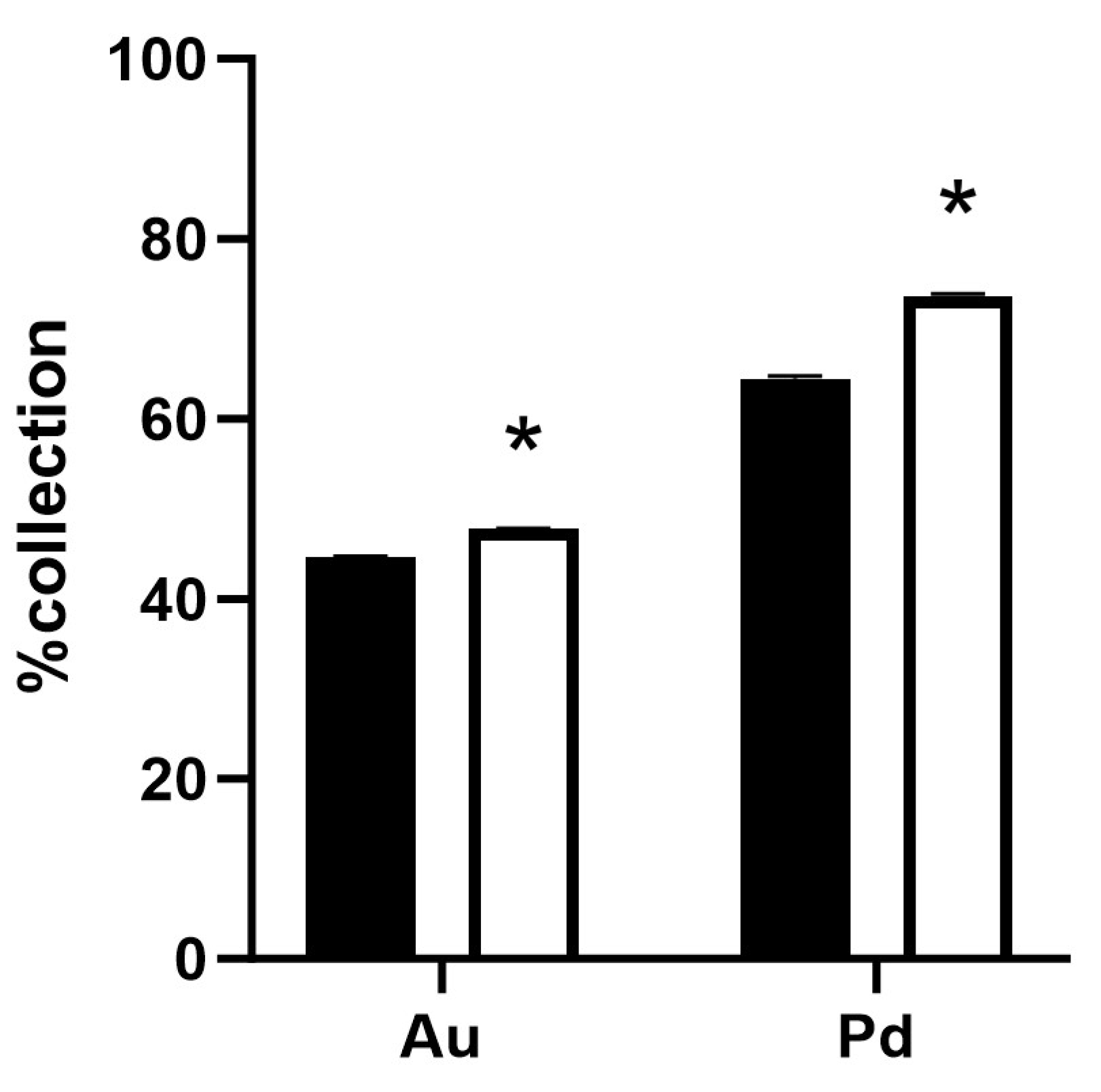
2.3. Ability of Gold Recovery in ASGM
2.4. Content Analysis of Galdieria Cells Grown in Heterotrophy, Mixotrophy and Autotrophy
3. Discussion
4. Materials and Methods
4.1. Galdieria Cultivation and Adsorbent Preparation
4.2. Metal Recovery and Quantification
4.3. Content Analysis
4.4. qRT-PCR Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of Microplastics from the Environment. A Review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Adams, E.; Miyazaki, T.; Hayaishi-Satoh, A.; Han, M.; Kusano, M.; Khandelia, H.; Saito, K.; Shin, R. A Novel Role for Methyl cysteinate, a Cysteine Derivative, in Cesium Accumulation in Arabidopsis thaliana. Sci. Rep. 2017, 7, 43170. [Google Scholar] [CrossRef]
- Hewitt, A.; Keel, T.; Tauber, M.; Le-Fiedler, T. The Ups and Downs of Gold Recycling; World Gold Council: London, UK, 2015. [Google Scholar]
- Karamushka, V.I.; Gadd, G.M. Interaction of Saccharomyces cerevisiae with Gold: Toxicity and Accumulation. Biometals 1999, 12, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Igarashi, K.; Miyashita, S.; Mitsuhashi, H.; Inagaki, K.; Fujii, S.; Sawada, H.; Kuwabara, T.; Minoda, A. Effective and Selective Recovery of Gold and Palladium Ions from Metal Wastewater Using a Sulfothermophilic Red Alga, Galdieria sulphuraria. Bioresour. Technol. 2016, 211, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Chini Zittelli, G.; Lauceri, R.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Valuable Pigments from Microalgae: Phycobiliproteins, Primary Carotenoids, and Fucoxanthin. Photochem. Photobiol. Sci. 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Rigano, C.; Fuggi, A.; Rigano, V.D.M.; Aliotta, G. Studies on Utilization of 2-Ketoglutarate, Glutamate and Other Amino Acids by the Unicellular Alga Cyanidium caldarium. Arch. Microbiol. 1976, 107, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gross, W.D.; Schnarrenberger, C. Heterotrophic Growth of Two Strains of the Acido-Thermophilic Red Alga Galdieria sulphuraria. Plant Cell Physiol. 1995, 36, 633–638. [Google Scholar] [CrossRef]
- Gross, W.; Schnarrenberger, C. Purification and Characterization of a Galactose-1-Phosphate: UDP-Glucose Uridyltransferase from the Red Alga Galdieria sulphuraria. Eur. J. Biochem. 1995, 234, 258–263. [Google Scholar] [CrossRef]
- Oesterhelt, C.; Schnarrenberger, C.; Gross, W. Characterization of a Sugar/Polyol Uptake System in the Red Alga Galdieria sulphuraria. Eur. J. Phycol. 1999, 34, 271–277. [Google Scholar] [CrossRef]
- Adams, E.; Maeda, K.; Kato, T.; Tokoro, C. Mechanism of Gold and Palladium Adsorption on Thermoacidophilic Red Alga Galdieria sulphuraria. Algal Res. 2021, 60, 102549. [Google Scholar] [CrossRef]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as Human Food: Chemical and Nutritional Characteristics of the Thermo-Acidophilic Microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bottone, C.; Camerlingo, R.; Miceli, R.; Salbitani, G.; Sessa, G.; Pirozzi, G.; Carfagna, S. Antioxidant and Anti-Proliferative Properties of Extracts from Heterotrophic Cultures of Galdieria sulphuraria. Nat. Prod. Res. 2019, 33, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.; Hantke, A.; Eriksen, N.T. Purification of the Photosynthetic Pigment C-Phycocyanin from Heterotrophic Galdieria sulphuraria. J. Sci. Food Agric. 2013, 93, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Ahmad, I.; Ahmad, W.; Ishaq, M.; Gul, K.; Khan, R.; Khan, H. Study on Adsorptive Capability of Acid Activated Charcoal for Desulphurization of Model and Commercial Fuel oil Samples. J. Environ. Chem. Eng. 2018, 6, 4037–4043. [Google Scholar] [CrossRef]
- Saleh, T.A.; Danmaliki, G.I. Influence of Acidic and Basic Treatments of Activated Carbon Derived from Waste Rubber Tires on Adsorptive Desulfurization of Thiophenes. J. Taiwan Inst. Chem. Eng. 2016, 60, 460–468. [Google Scholar] [CrossRef]
- UN Environment Programme. Global Mercury Assessment 2018; UN Environment Programme: Gigiri Nairobi, Kenya, 2018. [Google Scholar]
- Saha, S.K.; McHugh, E.; Murray, P.; Walsh, D.J. Microalgae as a Source of Nutraceuticals. In Phycotoxins: Chemistry and Biochemistry, 2nd ed.; Botana, L.M., Alfonso, A., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2015; pp. 255–291. [Google Scholar]
- Barbier, G.; Oesterhelt, C.; Larson, M.D.; Halgren, R.G.; Wilkerson, C.; Garavito, R.M.; Benning, C.; Weber, A.P.M. Comparative Genomics of Two Closely Related Unicellular Thermo-Acidophilic Red Algae, Galdieria sulphuraria and Cyanidioschyzon merolae, Reveals the Molecular Basis of the Metabolic Flexibility of Galdieria sulphuraria and Significant Differences in Carbohydrate Metabolism of Both Algae. Plant Physiol. 2005, 137, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Bumbak, F.; Cook, S.; Zachleder, V.; Hauser, S.; Kovar, K. Best Practices in Heterotrophic High-Cell-Density Microalgal Processes: Achievements, Potential and Possible Limitations. Appl. Microbiol. Biotechnol. 2011, 91, 31–46. [Google Scholar] [CrossRef]
- Hirooka, S.; Tomita, R.; Fujiwara, T.; Ohnuma, M.; Kuroiwa, H.; Kuroiwa, T.; Miyagishima, S.Y. Efficient Open Cultivation of Cyanidialean Red Algae in Acidified Seawater. Sci. Rep. 2020, 10, 13794. [Google Scholar] [CrossRef]
- Schmidt, R.A.; Wiebe, M.G.; Eriksen, N.T. Heterotrophic High Cell-Density Fed-Batch Cultures of the Phycocyanin-Producing Red Alga Galdieria sulphuraria. Biotechnol. Bioeng. 2005, 90, 77–84. [Google Scholar] [CrossRef]
- Wan, M.; Wang, Z.; Zhang, Z.; Wang, J.; Li, S.; Yu, A.; Li, Y. A Novel Paradigm for the High-Efficient Production of Phycocyanin from Galdieria sulphuraria. Bioresour. Technol. 2016, 218, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef] [PubMed]
- Abiusi, F.; Moñino Fernández, P.; Canziani, S.; Janssen, M.; Wijffels, R.H.; Barbosa, M. Mixotrophic Cultivation of Galdieria sulphuraria for C-Phycocyanin and Protein Production. Algal Res. 2022, 61, 102603. [Google Scholar] [CrossRef]
- Canelli, G.; Abiusi, F.; Vidal Garcia, A.; Canziani, S.; Mathys, A. Amino Acid Profile and Protein Bioaccessibility of Two Galdieria sulphuraria Strains Cultivated Autotrophically and Mixotrophically in Pilot-Scale Photobioreactors. Innov. Food Sci. Emerg. 2023, 84, 103287. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, D.; Pohnert, G. The thermoacidophilic red alga Galdieria sulphuraria is a Highly Efficient Cell Factory for Ammonium Recovery from Ultrahigh-NH4+ Industrial Effluent with Co-Production of High-Protein Biomass By photo-fermentation. Chem. Eng. J. 2022, 438, 135598. [Google Scholar] [CrossRef]
- Anwar, S.; Ali, M.A.; Abbas, A.; Wieczorek, K. Arabidopsis Argininosuccinate Lyase and Argininosuccinate Synthase Are Important for Resistance against Pseudomonas syringae. Adv. Life Sci. 2019, 7, 20–26. [Google Scholar]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A Diet-Derived Antioxidant with Therapeutic Potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakamura, K.; Shimizu, Y.; Yokoi, Y.; Ohira, S.; Hagiwara, M.; Wang, Y.; Song, Y.; Aizawa, T.; Ayabe, T. Decrease of A-Defensin Impairs Intestinal Metabolite Homeostasis via Dysbiosis in Mouse Chronic Social Defeat Stress Model. Sci. Rep. 2021, 11, 9915. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Bostanciklioglu, M. The Role of Gut Microbiota in Pathogenesis of Alzheimer’s Disease. J. Appl. Microbiol. 2019, 127, 954–967. [Google Scholar] [CrossRef]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hanninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S.; et al. Faecalibacterium prausnitzii Treatment Improves Hepatic Health and Reduces Adipose Tissue Inflammation in High-Fat Fed Mice. ISME J. 2017, 11, 1667–1679. [Google Scholar] [CrossRef]
- Namba, K.; Hatano, M.; Yaeshima, T.; Takase, M.; Suzuki, K. Effects of Bifidobacterium longum BB536 Administration on Influenza Infection, Influenza Vaccine Antibody Titer, and Cell-Mediated Immunity in the Elderly. Biosci. Biotechnol. Biochem. 2010, 74, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Mishra, S.K.; Kim, C.W.; Suh, W.I.; Park, M.S.; Yang, J.-W. Isolation and Characterization of Thermostable Phycocyanin from Galdieria sulphuraria. Korean J. Chem. Eng. 2014, 31, 490–495. [Google Scholar] [CrossRef]
- Barone, R.; De Napoli, L.; Mayol, L.; Paolucci, M.; Volpe, M.G.; D’Elia, L.; Pollio, A.; Guida, M.; Gambino, E.; Carraturo, F.; et al. Autotrophic and Heterotrophic Growth Conditions Modify Biomolecole Production in the Microalga Galdieria sulphuraria (Cyanidiophyceae, Rhodophyta). Mar. Drugs 2020, 18, 169. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, K.; Seger, M.; Dungan, B.; Hanson, D.T.; Lammers, P.J.; Holguin, F.O. Alterations in Photosynthesis and Energy Reserves in Galdieria sulphuraria during Corn Stover Hydrolysate Supplementation. Bioresour. Technol. Rep. 2019, 7, 100269. [Google Scholar] [CrossRef]
- Liu, L.; Sanchez-Arcos, C.; Pohnert, G.; Wei, D. Untargeted Metabolomics Unveil Changes in Autotrophic and Mixotrophic Galdieria sulphuraria Exposed to High-Light Intensity. Int. J. Mol. Sci. 2021, 22, 1247. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic Cultivation of Galdieria sp. Strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Carbone, D.A.; Olivieri, G.; Pollio, A.; Melkonian, M. Biomass and Phycobiliprotein Production of Galdieria sulphuraria, Immobilized on a Twin-Layer Porous Substrate Photobioreactor. Appl. Microbiol. Biotechnol. 2020, 104, 3109–3119. [Google Scholar] [CrossRef]
- Portillo, F.V.-L.; Sierra-Ibarra, E.; Vera-Estrella, R.; Revah, S.; Ramírez, O.T.; Caspeta, L.; Martinez, A. Growth and Phycocyanin Production with Galdieria sulphuraria UTEX 2919 Using Xylose, Glucose, and Corn Stover Hydrolysates Under Heterotrophy and Mixotrophy. Algal Res. 2022, 65, 102752. [Google Scholar] [CrossRef]
- Jong, L.W.; Fujiwara, T.; Hirooka, S.; Miyagishima, S.-y. Cell Size for Commitment to Cell Division and Number of Successive Cell Divisions in Cyanidialean Red Algae. Protoplasma 2021, 258, 1103–1118. [Google Scholar] [CrossRef]



| Heterotrophy | Mixotrophy | Autotrophy | Unit | |
|---|---|---|---|---|
| Moisture | 4.2 | 2.3 | 1.5 | g |
| Protein | 37.9 | 32.0 | 64.2 | g |
| Fat | 4.9 | 4.2 | 8.3 | g |
| Carbohydrate | 50.6 | 59.8 | 22.8 | g |
| - Fibre | 39.2 | 42.1 | 22.8 | g |
| - Sugar | 11.4 | 17.7 | 0.0 | g |
| Ash | 2.4 | 1.7 | 3.2 | g |
| Sodium | 0.028 | 0.012 | 15 | mg |
| - SCE 1 | 0.071 | 0.030 | 0.038 | g |
| Energy | 320 | 321 | 377 | kcal |
| Heterotrophy | Mixotrophy | Autotrophy | |
|---|---|---|---|
| Asp + Asn | 2.16 | 2.36 | 5.49 |
| Ala | 1.41 | 1.56 | 3.99 |
| Arg | 1.37 | 1.66 | 3.75 |
| Ile | 1.24 | 1.37 | 3.15 |
| Gly | 1.17 | 1.22 | 2.60 |
| Glu + Gln | 4.34 | 4.64 | 8.71 |
| Cys | 0.74 | 0.74 | 1.28 |
| Thr | 1.90 | 2.01 | 3.56 |
| Ser | 2.17 | 2.21 | 4.16 |
| Tyr | 1.85 | 2.03 | 3.92 |
| Trp | 0.40 | 0.41 | 0.73 |
| Val | 1.79 | 1.87 | 3.66 |
| His | 0.51 | 0.53 | 0.77 |
| Phe | 1.15 | 1.22 | 2.47 |
| Pro | 1.49 | 1.56 | 2.61 |
| Met | 0.52 | 0.58 | 1.45 |
| Lys | 1.91 | 2.04 | 3.54 |
| Leu | 1.85 | 2.04 | 4.65 |
| Sum | 27.97 | 30.05 | 60.49 |
| Heterotrophy | Mixotrophy | Autotrophy |
|---|---|---|
| 36 | 35 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adams, E.; Maeda, K.; Kamemoto, Y.; Hirai, K.; Apdila, E.T. Contribution to a Sustainable Society: Biosorption of Precious Metals Using the Microalga Galdieria. Int. J. Mol. Sci. 2024, 25, 704. https://doi.org/10.3390/ijms25020704
Adams E, Maeda K, Kamemoto Y, Hirai K, Apdila ET. Contribution to a Sustainable Society: Biosorption of Precious Metals Using the Microalga Galdieria. International Journal of Molecular Sciences. 2024; 25(2):704. https://doi.org/10.3390/ijms25020704
Chicago/Turabian StyleAdams, Eri, Kazuki Maeda, Yuki Kamemoto, Kazuho Hirai, and Egi Tritya Apdila. 2024. "Contribution to a Sustainable Society: Biosorption of Precious Metals Using the Microalga Galdieria" International Journal of Molecular Sciences 25, no. 2: 704. https://doi.org/10.3390/ijms25020704
APA StyleAdams, E., Maeda, K., Kamemoto, Y., Hirai, K., & Apdila, E. T. (2024). Contribution to a Sustainable Society: Biosorption of Precious Metals Using the Microalga Galdieria. International Journal of Molecular Sciences, 25(2), 704. https://doi.org/10.3390/ijms25020704





