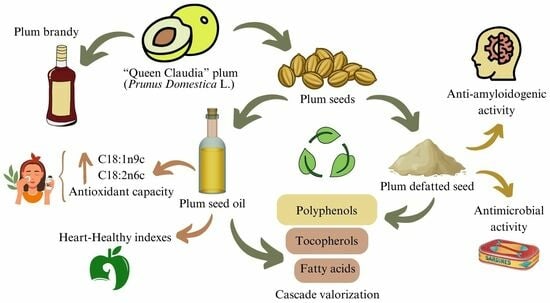
The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Soxhlet Extraction Yields of Plum Seeds
2.2. Evaluation of the Lipid Profile of Plum Seed Oils
2.2.1. Fatty Acid Composition
2.2.2. Heart-Healthy Lipid Quality Indexes
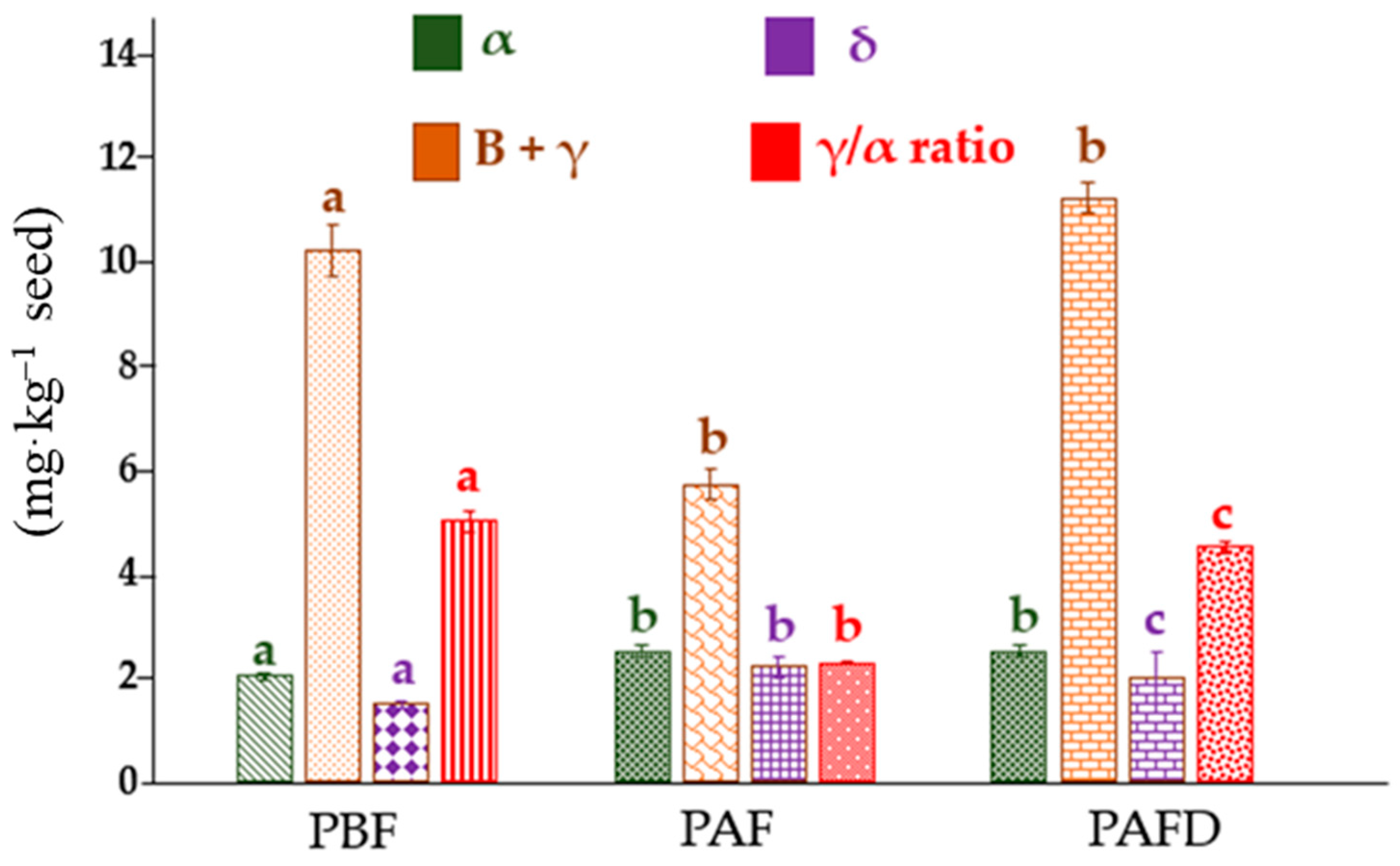
2.3. Determination of Tocopherols Content of Plum Seed Oils
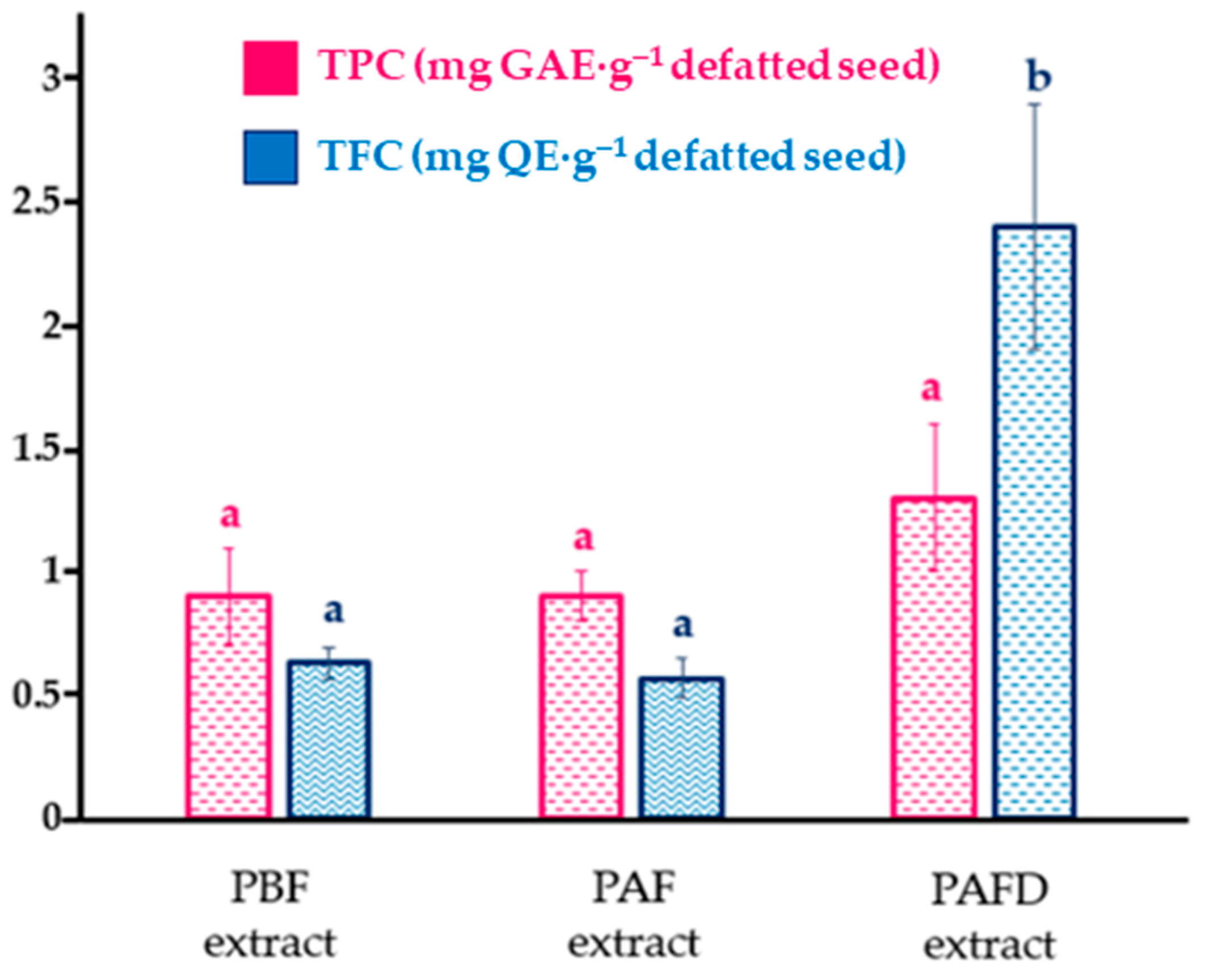
2.4. Determination of Total Phenolic Content and Individual Polyphenols of Defatted Plum Seeds
2.5. Evaluation of Bioactive Properties of Plum Seed Oils and Defatted Plum Seeds
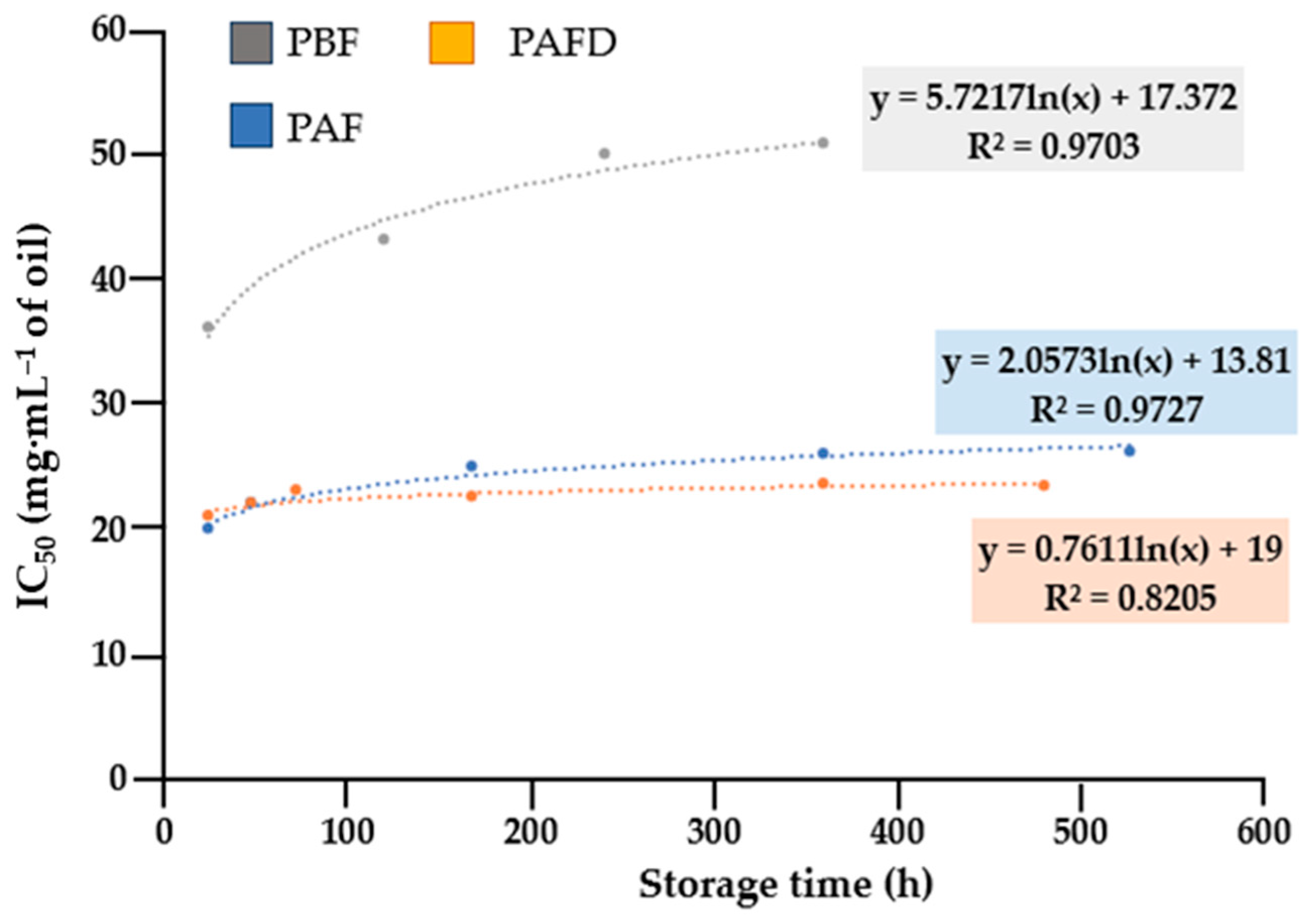
2.5.1. Antioxidant Activity
- Plum seed oils
- Defatted seed phenolic extracts:
2.5.2. Neuroprotective Activity
2.5.3. Antimicrobial Activity
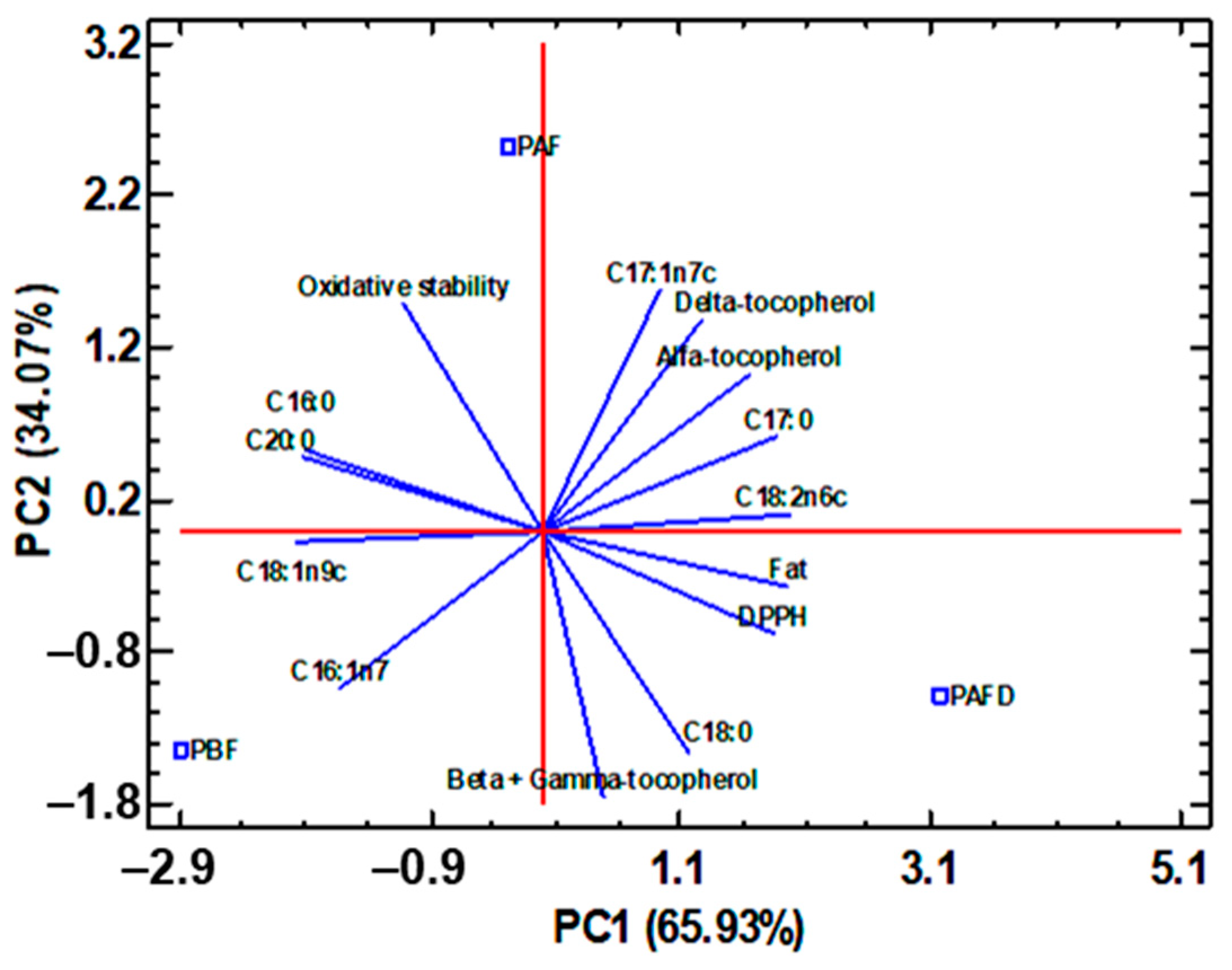
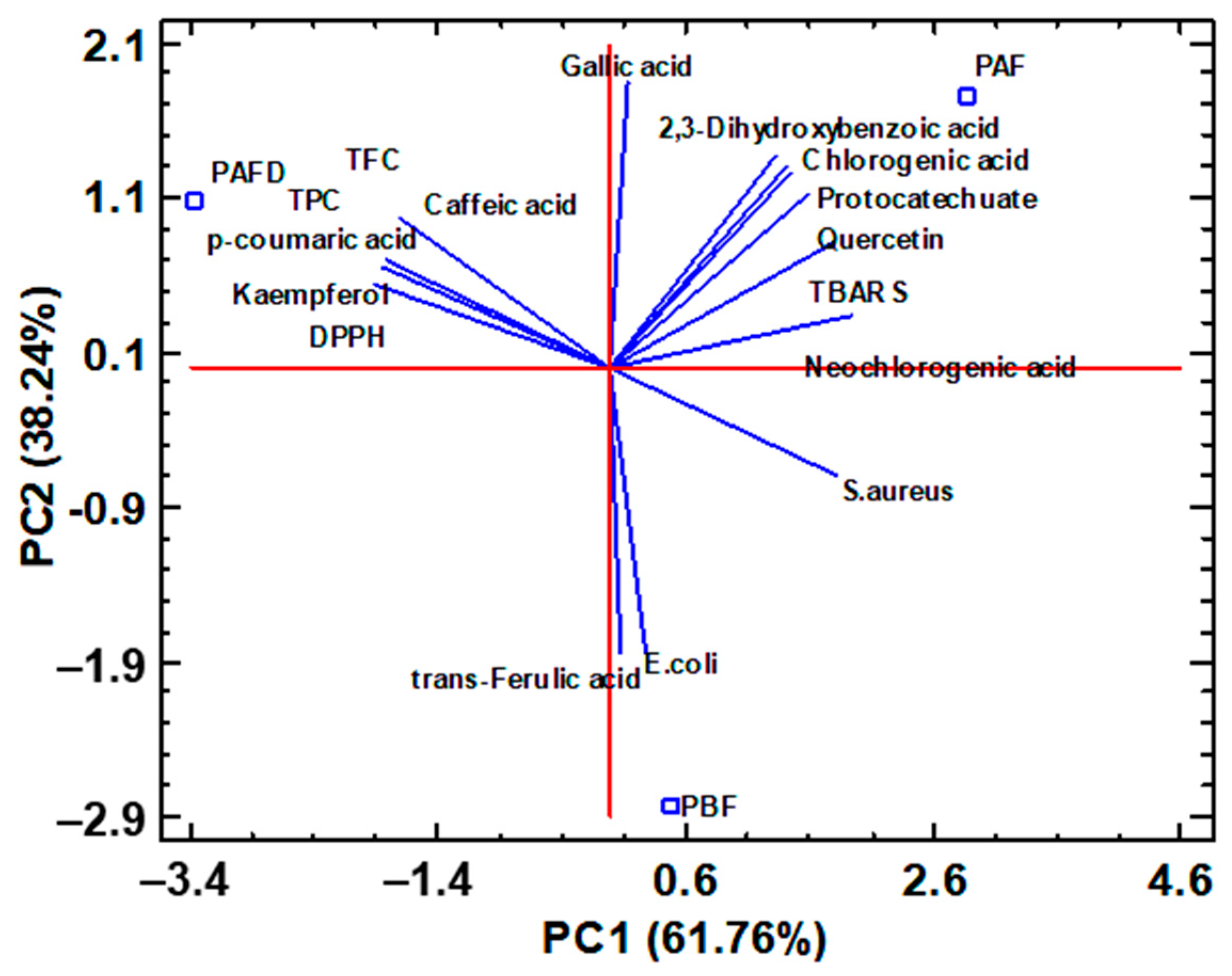
2.6. Multivariate Statistical Analysis
3. Materials and Methods
3.1. Reagents, Standards, Bacterial Strains, and Solvents
3.2. Raw Materials
3.3. Moisture Content of Seed Samples
3.4. Soxhlet Extraction of Plum Seeds
3.5. Phenolic Extraction of Defatted Plum Seeds
3.6. Characterization of the Lipid Profile and Evaluation of the Health Lipid Indexes of Plum Seed Oils
3.7. Characterization of Tocopherols in Plum Seed Oils
3.8. Determination of Phenolic Compounds from Defatted Plum Seed Extracts
3.8.1. Spectrophotometric Methods
3.8.2. Chromatographic Method
3.9. Evaluation of Bioactive Properties of Plum Seed Oils and Defatted Plum Seed Extracts
3.9.1. Antioxidant Activity
3.9.2. Anti-Amyloidogenic Activity
3.9.3. Antibacterial Activity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonilla, J.; Vargas, F.C.; de Oliveira, T.G.; da Aparecida Makishi, G.L.; do Amaral Sobral, P.J. Recent Patents on the Application of Bioactive Compounds in Food: A Short Review. Curr. Opin. Food Sci. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Bahaa Eldin, A.; Khalifa, I. Valorization and Extraction Optimization of Prunus Seeds for Food and Functional Food Applications: A Review with Further Perspectives. Food Chem. 2022, 388, 132955. [Google Scholar] [CrossRef] [PubMed]
- Kumar Joshi, V.; Sharma, R.; Shyam Abrol, G. 5 Stone Fruit: Wine and Brandy. In Handbook of Plant-Based Fermented Food and Beverage Technology; CRC: Boca Raton, FL, USA, 2011. [Google Scholar]
- Rabrenović, B.B.; Demin, M.A.; Basić, M.G.; Pezo, L.L.; Paunović, D.M.; Sovtić, F.S. Impact of Plum Processing on the Quality and Oxidative Stability of Cold-Pressed Kernel Oil. Grasas Aceites 2021, 72, e395. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; García-Sánchez, B.; Miranda, R. Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules 2023, 28, 7045. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Rudzińska, M.; Soliven, A. Industrial By-Products of Plum Prunus domestica L. and Prunus cerasifera Ehrh. as Potential Biodiesel Feedstock: Impact of Variety. Ind. Crops Prod. 2017, 100, 77–84. [Google Scholar] [CrossRef]
- Vladić, J.; Gavarić, A.; Jokić, S.; Pavlović, N.; Moslavac, T.; Popović, L.; Matias, A.; Agostinho, A.; Banožić, M.; Vidović, S. Alternative to Conventional Edible Oil Sources: Cold Pressing and Supercritical CO2 Extraction of Plum (Prunus domestica L.) Kernel Seed. Acta Chim. Slov. 2020, 67, 778–784. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, N.; Manzoor, M.F.; Shabbir, U.; Ahmed, S.; Ismail, T.; Saeed, F.; Nisa, M.; Anjum, F.M.; Hussain, S. Health Lipid Indices and Physicochemical Properties of Dual Fortified Yogurt with Extruded Flaxseed Omega Fatty Acids and Fibers for Hypercholesterolemic Subjects. Food Sci. Nutr. 2020, 8, 273–280. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-Tocopherol) Levels in Plant Oils. Flavour. Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Barouh, N.; Bourlieu-Lacanal, C.; Figueroa-Espinoza, M.C.; Durand, E.; Villeneuve, P. Tocopherols as Antioxidants in Lipid-Based Systems: The Combination of Chemical and Physicochemical Interactions Determines Their Efficiency. Compr. Rev. Food Sci. Food Saf. 2022, 21, 642–688. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Fernández-Ávila, L.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Miranda, R. Valorization of Defatted Cherry Seed Residues from Liquor Processing by Matrix Solid-Phase Dispersion Extraction: A Sustainable Strategy for Production of Phenolic-Rich Extracts with Antioxidant Potential. Antioxidants 2023, 12, 2041. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M. Optimization Study on Extraction of Antioxidants from Plum Seeds (Prunus domestica L.). Optim. Eng. 2021, 22, 141–158. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Saini, C.S.; Sharma, H.K. Synergistic Effect of Microwave Heating and Hydrothermal Treatment on Cyanogenic Glycosides and Bioactive Compounds of Plum (Prunus domestica L.) Kernels: An Analytical Approach. Curr. Res. Food Sci. 2022, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Jahangir Alam, M.; Barua Agriculture, R.; Food Canada, A. In Vitro Regeneration and Antibacterial Activity of Prunus domestica L. J. BioSci. Biotechnol. 2015, 4, 9–15. [Google Scholar]
- Mehta, S.; Soni, N.; Satpathy, G.; Gupta, R.K. Evaluation of Nutritional, Phytochemical, Antioxidant and Antibacterial Activity of Dried Plum (Prunus domestica). J. Pharmacogn. Phytochem. 2014, 3, 166–171. [Google Scholar]
- Shukla, R.K.; Kishan; Shukla, A.; Singh, R. Evaluation of Nutritive Value, Phytochemical Screening, Total Phenolic Content and in-Vitro Antioxidant Activity of the Seed of Prunus domestica L. Plant Sci. Today 2021, 8, 830–835. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Kashyap, P.; Singh Riar, C.; Jindal, N. Detoxification of Meghalayan Cherry (Prunus nepalensis) Kernel and Its Effect on Structural and Thermal Properties of Proteins. Food Res. Int. 2023, 164, 112437. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ünver, A.; Arslan, D. A Research on Evaluation of Some Fruit Kernels and/or Seeds as a Raw Material of Vegetable Oil Industry. Qual. Assur. Saf. Crops Foods 2015, 7, 187–191. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Valli, E.; Gallina Toschi, T. Quality Indexes and Composition of 13 Commercial Hemp Seed Oils. J. Food Compos. Anal. 2023, 117, 105112. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.A.; Hamed, I.M.; Mohammed, S.E. Utilization of Grape and Apricot Fruits By-Products as Cheap Source for Biologically Active Compounds for Health Promotion. Egypt. J. Chem. 2021, 64, 2037–2045. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Method 960.39, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Segliņa, D. Impact of Cultivar on Profile and Concentration of Lipophilic Bioactive Compounds in Kernel Oils Recovered from Sweet Cherry (Prunus avium L.) by-Products. Plant Foods Hum. Nutr. 2016, 71, 158–164. [Google Scholar] [CrossRef]
- Bjelica, M.; Vujasinović, V.; Rabrenović, B.; Dimić, S. Some Chemical Characteristics and Oxidative Stability of Cold Pressed Grape Seed Oils Obtained from Different Winery Waste. Eur. J. Lipid Sci. Technol. 2019, 121, 1800416. [Google Scholar] [CrossRef]
- Fratianni, F.; d’Acierno, A.; Ombra, M.N.; Amato, G.; De Feo, V.; Ayala-Zavala, J.F.; Coppola, R.; Nazzaro, F. Fatty Acid Composition, Antioxidant, and in vitro Anti-Inflammatory Activity of Five Cold-Pressed Prunus Seed Oils, and Their Anti-Biofilm Effect Against Pathogenic Bacteria. Front. Nutr. 2021, 8, 775751. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef]
- Song, J.; Kim, Y.S.; Lee, D.H.; Lee, S.H.; Park, H.J.; Lee, D.; Kim, H. Neuroprotective Effects of Oleic Acid in Rodent Models of Cerebral Ischaemia. Sci. Rep. 2019, 9, 10732. [Google Scholar] [CrossRef] [PubMed]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary Linoleic Acid and Human Health: Focus on Cardiovascular and Cardiometabolic Effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Karsli, B. Comparative Analysis of the Fatty Acid Composition of Commercially Available Fish Oil Supplements in Turkey: Public Health Risks and Benefits. J. Food Compos. Anal. 2021, 103, 104105. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Chemical, Rheological and Nutritional Characteristics of Sesame and Olive Oils Blended with Linseed Oil. Adv. Pharm. Bull. 2018, 8, 107–113. [Google Scholar] [CrossRef]
- Davis, J.P.; Raleigh, A.; Price, K.; Lisa Dean, M.L.; Sweigart Jane Cottonaro Timothy H Sanders, D.S.; Davis, J.P.; Price, K.; Dean, L.; Sweigart, D.; Cottonaro, J.; et al. Peanut Oil Stability and Physical Properties Across a Range of Industrially Relevant Oleic Acid/Linoleic Acid Ratios. Peanut Sci. 2016, 43, 1–11. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłuzewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Zbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef]
- Lamine, M.; Mliki, A.; Mlikia, A. Genetic Diversity and Salt Tolerance in Barley Germplasm: Physiological, Biochemical & Molecular Characterization View Project Citrus Waste Valorization View Project Nutritional Quality Perceptions through Fatty Acid Profiling, Health Lipid Indices and Antioxidant Potentialities. Open J. Nutr. Food Sci. 2021, 3, 1015. [Google Scholar]
- Gliszczyńska-Świgło, A.; Sikorska, E.; Khmelinskii, I.; Sikorski, M. Tocopherol Content in Edible Plant Oils. Pol. J. Food Nutr. Sci. 2007, 57, 157–161. [Google Scholar]
- Bakir, D.S.; Yalcin, G.; Cucu, A.K. Isolation and Determination of Tocopherols and Tocotrienols from the Seed of Capparis Ovata Grown in Turkey by Reversed-Phase High-Performance Liquid Chromatography. Chromatographia 2020, 83, 77–86. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Sikorska, E. Simple Reversed-Phase Liquid Chromatography Method for Determination of Tocopherols in Edible Plant Oils. J. Chromatogr. A 2004, 1048, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Abidi, S.L.; Mounts, T.L. Reversed-Phase High-Performance Liquid Chromatographic Separations of Tocopherols. J. Chromatogr. A 1997, 782, 25–32. [Google Scholar] [CrossRef]
- Popa, V.M.; Bele, C.; Poiana, M.A.; Dumbrava, D.; Raba, D.N.; Jianu, C. Evaluation of Bioactive Compounds and of Antioxidant Properties in Some Oils Obtained from Food Industry By-Products. Rom. Biotechnol. Lett. 2011, 16, 6234–6241. [Google Scholar]
- Górnaś, P.; Mišina, I.; Grāvīte, I.; Lācis, G.; Radenkovs, V.; Olšteine, A.; Segliņa, D.; Kaufmane, E.; Rubauskis, E. Composition of Tocochromanols in the Kernels Recovered from Plum Pits: The Impact of the Varieties and Species on the Potential Utility Value for Industrial Application. Eur. Food Res. Technol. 2015, 241, 513–520. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of Fermentation on the Phytochemical Composition and Antioxidant Properties of Soy Germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Breyer, L. Composition and Oxidative Stability of Crude Oil Extracts of Corn Germ and Distillers Grains. Ind. Crops Prod. 2011, 33, 572–578. [Google Scholar] [CrossRef]
- Bruscatto, M.H.; Pestana-Bauer, V.R.; Otero, D.M.; Zambiazi, R.C. Effects of Heating Temperature on the Tocopherol Contents of Chemically and Physically Refined Rice Bran Oil. Grasas Aceites 2019, 70, 294. [Google Scholar] [CrossRef]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New Perspectives on Vitamin E: γ-Tocopherol and Carboxyethylhydroxychroman Metabolites in Biology and Medicine. Free. Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Saari Csallany, A. The Antioxidant Functions of Tocopherol and Tocotrienol Homologues in Oils, Fats, and Food Systems. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Soares Mateus, A.R.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Sanches Silva, A. By-Products of Dates, Cherries, Plums and Artichokes: A Source of Valuable Bioactive Compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- Badr, J.; Tawfik, M.K.; Badr, J.M. Analytical and Pharmacological Investigation of Amygdalin in Prunus armeniaca L. Kernels. J. Pharm. Res. 2010, 3, 2134–2137. [Google Scholar]
- Blaheta, R.A.; Nelson, K.; Haferkamp, A.; Juengel, E. Amygdalin, Quackery or Cure? Phytomedicine 2016, 23, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18, e06223. [Google Scholar] [CrossRef] [PubMed]
- Makovi, C.M.; Parker, C.H.; Zhang, K. Determination of Amygdalin in Apricot Kernels and Almonds Using LC-MS/MS. J. AOAC Int. 2023, 106, 457–463. [Google Scholar] [CrossRef]
- Song, Z.; Xu, X. Advanced Research on Anti-Tumor Effects of Amygdalin. J. Cancer Res. Ther. 2014, 10, C3–C7. [Google Scholar] [CrossRef]
- Salama, R.H.; Ramadan, A.E.R.G.; Alsanory, T.A.; Herdan, M.O.; Fathallah, O.M.; Alsanory, A.A. Experimental and Therapeutic Trials of Amygdalin. Int. J. Biochem. Pharmacol. 2019, 1, 21–26. [Google Scholar] [CrossRef]
- Jasar, D.; Filipovski, V.; Kubelka -Sabit, K.; Curcic-Trajkovska, B. Potential Benefits and Controversies Related to Use Amygdalin (Vitamin B17). J. Eng. Des. 2008, 23, 57–63. [Google Scholar]
- Tsaur, I.; Thomas, A.; Monecke, M.; Zugelder, M.; Rutz, J.; Grein, T.; Maxeiner, S.; Xie, H.; Chun, F.K.H.; Rothweiler, F.; et al. Amygdalin Exerts Antitumor Activity in Taxane-Resistant Prostate Cancer Cells. Cancers 2022, 14, 3111. [Google Scholar] [CrossRef]
- Gago-López, N.; Lagunas Arnal, C.; Perez, J.J.; Wagner, E.F. Topical Application of an Amygdalin Analogue Reduces Inflammation and Keratinocyte Proliferation in a Psoriasis Mouse Model. Exp. Dermatol. 2021, 30, 1662–1674. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Hung, T.W.; Wang, C.C.; Wu, S.W.; Yang, T.W.; Yang, C.Y.; Tseng, T.H.; Wang, C.J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating Mir-34a in Vitro. Int. J. Mol. Sci. 2021, 22, 13163. [Google Scholar] [CrossRef] [PubMed]
- Karthikesan, K.; Pari, L.; Menon, V.P. Antihyperlipidemic Effect of Chlorogenic Acid and Tetrahydrocurcumin in Rats Subjected to Diabetogenic Agents. Chem. Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Silva, R.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Partitioning and Antioxidative Effect of Protocatechuates in Soybean Oil Emulsions: Relevance of Emulsifier Concentration. Eur. J. Lipid Sci. Technol. 2017, 119, 1600274. [Google Scholar] [CrossRef]
- Littlefield, D.; Zhu, T.; Buckholt, M. Effects of 2,3-Dihydroxybenzoate on Growth and Antibiotic Resistance in E. coli. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2023. [Google Scholar]
- Prasad, N.R.; Jeyanthimala, K.; Ramachandran, S. Caffeic Acid Modulates Ultraviolet Radiation-B Induced Oxidative Damage in Human Blood Lymphocytes. J. Photochem. Photobiol. B 2009, 95, 196–203. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory Mechanism against Oxidative Stress of Caffeic Acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Jericó Santos, T.R.; Santos Vasconcelos, A.G.; Lins de Aquino Santana, L.C.; Gualberto, N.C.; Buarque Feitosa, P.R.; Pires de Siqueira, A.C. Solid-State Fermentation as a Tool to Enhance the Polyphenolic Compound Contents of Acidic Tamarindus Indica by-Products. Biocatal. Agric. Biotechnol. 2020, 30, 101851. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant Phenolics and Their Microbial Production by Submerged and Solid State Fermentation Process: A Review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Rizzi, C.; Mihaylova, D.; Lante, A. Effect of the Distillation Process on Polyphenols Content of Grape Pomace. Eur. Food Res. Technol. 2022, 248, 929–935. [Google Scholar] [CrossRef]
- Marinova, E.M.; Yanishlieva, N. V Antioxidant Activity and Mechanism of Action of Some Phenolic Acids at Ambient and High Temperatures. Food Chem. 2003, 81, 189–197. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of Grape (Vitis vinifera L.) Seed Oil Production as a Valuable Source of Phenolic Antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of Black Mulberry and Grape Seeds: Chemical Characterization and Bioactive Potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, Characterization and Antioxidant Activity of Fenugreek (Trigonella-Foenum Graecum) Seed Oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Chamberlin, K.D.; Melouk, H.A.; Madden, R.; Dillwith, J.W.; Bannore, Y.; El Rassi, Z.; Payton, M. Determining the Oleic/Linoleic Acid Ratio in a Single Peanut Seed: A Comparison of Two Methods. Peanut Sci. 2011, 38, 78–84. [Google Scholar] [CrossRef][Green Version]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Natić, M.; Zagorac, D.D.; Ćirić, I.; Meland, M.; Rabrenović, B.; Akšić, M.F. Cold Pressed Oils from Genus Prunus. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 637–658. [Google Scholar] [CrossRef]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to Increase the Oxidative Stability of Cold Pressed Oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Zaki Rizk, M. Possible Neuromodulating Role of Different Grape (Vitis vinifera L.) Derived Polyphenols against Alzheimer’s Dementia: Treatment and Mechanisms. Bull. Natl. Res. Cent. 2019, 43, 108. [Google Scholar] [CrossRef]
- Vahedi-Mazdabadi, Y.; Karimpour-Razkenari, E.; Akbarzadeh, T.; Lotfian, H.; Toushih, M.; Roshanravan, N.; Saeedi, M.; Ostadrahimi, A. Anti-Cholinesterase and Neuroprotective Activities of Sweet and Bitter Apricot Kernels (Prunus armeniaca L.). Iran. J. Pharm. Res. 2020, 19, 216–224. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Screening the Extraction Process of Phenolic Compounds from Pressed Grape Seed Residue: Towards an Integrated and Sustainable Management of Viticultural Waste. LWT 2022, 169, 113988. [Google Scholar] [CrossRef]
- Dhellot, J.R.; Matouba, E.; Maloumbi, M.G.; Nzikou, J.M.; Ngoma, D.G.S.; Linder, M.; Desobry, S.; Parmentier, M. Extraction, Chemical Composition and Nutrional Characterization of Vegetable Oils: Case of Amaranthus Hybridus (Var 1 and 2) of Congo Brazzaville. Afr. J. Biotechnol. 2006, 5, 1095–1101. [Google Scholar]
- Muir, R.M.; Ibáñez, A.M.; Uratsu, S.L.; Ingham, E.S.; Leslie, C.A.; McGranahan, G.H.; Batra, N.; Goyal, S.; Joseph, J.; Jemmis, E.D.; et al. Mechanism of Gallic Acid Biosynthesis in Bacteria (Escherichia coli) and Walnut (Juglans regia). Plant Mol. Biol. 2011, 75, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus Aureus Clinical Strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. P-Coumaric Acid as an Active Ingredient in Cosmetics: A Review Focusing on Its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Ogbuta, A.A.; Given, O.T.; Ebinistei, P.; Alexis, E.O.; Wellington, E.O.; Teke, E.C.; Manda, A.E. Protective Effects of Trans-Ferulic Acid on Tamoxifen Induced Heptatic and Renal Oxidative Stress in Male Wister Albino Rat. J. Appl. Sci. Environ. Manag. 2023, 27, 1727–1732. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). AOAC Method 925.10 (Air Oven Method) for Moisture in Flour, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- The Comission of the European Communities. Commission Regulation (EC) No 796/2002 of 6 May 2002 Amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Pomace Oil and on the Relevant Methods of Analysis and the Additional Notes in the Annex to Council Regulation (EEC) No 2658/87 on the Tariff and Statistical Nomenclature and on the Common Customs Tariff; The Comission of the European Communities: Brussels, Belgium, 2002.
- García-González, A.; Velasco, J.; Velasco, L.; Ruiz-Méndez, M.V. An Analytical Simplification for Faster Determination of Fatty Acid Composition and Phytosterols in Seed Oils. Food Anal. Methods 2018, 11, 1234–1242. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Romero-Sánchez, I.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Ability of Selenium Species to Inhibit Metal-Induced Aβ Aggregation Involved in the Development of Alzheimer’s Disease. Anal. Bioanal. Chem. 2020, 412, 6485–6497. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C.; Liu, J. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
| Sample | Seed Oil (%, w) | Defatted Seed (%, w) |
|---|---|---|
| PBF | (37.6 ± 0.4) a | (62 ± 1) a |
| PAF | (45.2 ± 0.1) b | (47 ± 4) b |
| PAFD | (68 ± 1) c | (30 ± 2) c |
| Fatty Acids | PBF Oil (%) | PAF Oil (%) | PAFD Oil (%) |
|---|---|---|---|
| Palmitic acid (C16:0) | (5.60 ± 0.05) a | (5.52 ± 0.01) a | (5.15 ± 0.01) b |
| Palmitoleic acid (C16:1n7) | (0.765 ± 0.004) a | (0.586 ± 0.006) b | (0.59 ± 0.03) b |
| Margaric acid (C17:0) | (0.0439 ± 0.0008) a | (0.04 ± 0.01) a | (0.048 ± 0.002) a |
| Cis-10-heptadecenoic acid (C17:1n10c) | (0.082 ± 0.004) a | (0.092 ± 0.001) b | (0.0879 ± 0.0009) b |
| Stearic acid (C18:0) | (1.9 ± 0.1) a,b | (1.85 ± 0.06) a | (2.13 ± 0.04) b |
| Oleic acid (C18:1n9c) | (75.56 ± 0.04) a | (74 ± 1) a,b | (72 ± 1) b |
| Linoleic acid (C18:2n6c) | (15.83 ± 0.02) a | (18.1 ± 0.9) a,b | (20 ± 1) b |
| Arachidic acid (C20:0) | (0.176 ± 0.001) a | (0.174 ± 0.001) a,b | (0.157 ± 0.003) b |
| (7.76 ± 0.07) a | (7.58 ± 0.09) b | (7.49 ± 0.06) b | |
| (92.24 ± 0.07) a | (92.42 ± 0.09) b | (92.51 ± 0.06) b | |
| (76.40 ± 0.05) a | (74 ± 1) a,b | (72 ± 1) b | |
| (15.83 ± 0.02) a | (18.0 ± 0.9) a,b | (20 ± 1) b | |
| PUFA/SFA ratio | (2.04 ± 0.02) a | (2.38 ± 0.09) a | (2.7 ± 0.2) b |
| Lipid Indexes | PBF Oil | PAF Oil | PAFD Oil |
|---|---|---|---|
| Desirable fatty acid (DFA) (%) | (94.18 ± 0.05) a | (94.27 ± 0.03) a | (94.64 ± 0.01) c |
| Atherogenicity (AI) | (0.0607 ± 0.0005) a | (0.0597 ± 0.0002) b | (0.0557 ± 0.0002) c |
| Hypocholesterolemic/Hypercholesterolemic (H/H) | (16.3 ± 0.1) a | (16.62 ± 0.05) b | (17.8 ± 0.1) c |
| Oleic acid/linoleic acid ratio (O/L) | (4.772 ± 0.002) a | (4.1 ± 0.3) a,b | (3.6 ± 0.3) b |
| Compound | LOD/LOQ (mg·L−1) | Linear Range (mg·L−1) | Calibration Curve | CV (%) Intraday (n = 3) | CV (%) Interday (N = 9) | ||||
|---|---|---|---|---|---|---|---|---|---|
| a | b (L·mg−1) | R2 | k | Area | k | Area | |||
| α | 0.17 */0.5 ** | 0.5–20 *; 20–100 ** | (0 ± 2)·103 *; (0 ± 5)·104 ** | (115 ± 2)·102 *; (132 ± 7)·102 ** | 0.9990 *; 0.9942 ** | 1.39 *; 2.10 ** 0.56 *; 1.20 ** 1.75 *; 2.12 ** | 4.3 *; 2.3 ** 3.7 *; 2.4 ** 3.8 *; 2.5 ** | 6.4 *; 3.9 ** | 3.7 *; 2.6 ** |
| γ | 0.17 */0.5 ** | 0.5–20 *; 20–100 ** | (3 ± 4)·103 *; (0 ± 5)·104 ** | (129 ± 4)·102 *; (146 ± 7)·102 ** | 0.9975 *; 0.9957 ** | 1.30 *; 1.79 ** 0.58 *; 1.24 ** 2.42 *; 2.22 ** | 5.2 *; 2.0 ** 3.6 *; 3.2 ** 2.9 *; 2.7 ** | 6.9 *; 4.0 ** | 4.3 *; 2.9 ** |
| δ | 0.10 */0.3 ** | 0.3–20 *; 20–100 ** | (7 ± 5)·103 *; (0 ± 1)·104 ** | (88 ± 4)·102 *; (117 ± 2)·102 ** | 0.9923 *; 0.9996 ** | 1.02 *; 1.94 ** 0.50 *; 1.37 ** 2.93 *; 2.45 ** | 3.5 *; 1.9 ** 4.5 *; 3.1 ** 3.3 *; 2.2 ** | 6.7 *; 4.1 ** | 4.1 *; 2.6 ** |
| Compound | PBF (µg·g−1) | PAF (µg·g−1) | PAFD (µg·g−1) |
|---|---|---|---|
| 2,3-Dihydroxybenzoic acid A | 27.3 ± 0.1 a | 86 ± 4 b | 35 ± 2 c |
| Neochlorogenic acid B | 217 ± 1 ab | 368 ± 98 b | 90 ± 3 a |
| Chlorogenic acid B | 36.1 ± 0.7 a | 117 ± 22 b | 51 ± 14 a |
| Vanillic acid A | n.q. | 0.9 ± 0.1 a | n.q. |
| Caffeic acid B | 3.6 ± 0.3 a | 14 ± 2 a | 64 ± 10 b |
| Syringic acid A | n.q. | n.d. | n.q. |
| p-Coumaric acid C | 2.3 ± 0.9 a | 2.301 ± 0.009 a | 6.1 ± 0.3 b |
| trans-Ferulic acid D | 18.5 ± 0.6 a | n.d. | 3.4 ± 0.2 b |
| Kaempferol 3-rutinoside E | n.q. | n.d. | n.q. |
| Isorhamnetin 3-rutinoside F | 1.86 ± 0.08 a | n.q. | n.q. |
| Quercetin F | 15.1 ± 0.7 a | 28 ± 2 a | 15 ± 8 a |
| Kaempferol E | 1.38 ± 0.02 a | 0.9 ± 0.3 a | 19 ± 12 a |
| Gallic acid G | 8.9 ± 0.1 a | 34 ± 2 b | n.q. |
| Hesperidin H | n.d. | n.q. | n.q. |
| Catechin I | n.d. | n.d. | n.q. |
| Epicatechin J | n.d. | n.d. | n.q. |
| Protocatechuate A | 31.80 ± 0.08 a | 100 ± 11 b | 39 ± 7 a |
| Total phenolic acids | 346 ± 1 a | 722 ± 123 b | 289 ± 12 a |
| Total flavonoids | 18.4 ± 0.7 a | 29 ± 2 a | 34 ± 19 a |
| Total phenolics | 364.2 ± 0.7 a | 752 ± 121 b | 324 ± 0.7 a |
| Plum Seed Oils | DPPH IC50 (mg·mL−1 of Oil) |
|---|---|
| PBF | (20 ± 3) a |
| PAF | (21 ± 1) a |
| PAFD | (36 ± 2) b |
| Plum Seed Oils | Intercept (mg·mL−1) | Slope (h−1) | t1/2 (h) | R2 |
|---|---|---|---|---|
| PBF | (−4.2 ± 0.1) × 10−3 | (4.05 ± 0.04) | 1732 (72 days) | 0.78 |
| PAF | (−2.0 ± 0.5) × 10−4 | (4.03± 0.01) | 3465 (144 days) | 0.64 |
| PAFD | (−9 ± 3) × 10−3 | (3.7 ± 0.2) | 81 (3 days) | 0.86 |
| Defatted Seed Phenolic Extracts | DPPH IC50 (mg·g−1) | TBARS IC50 (mg·g−1) |
|---|---|---|
| PBF | (1.0 ± 0.2) a | (2.1 ± 0.1) b |
| PAF | (0.9 ± 0.2) a | (5.0 ± 0.6) b |
| PAFD | (1.9 ± 0.1) b | (1.3 ± 0.1) a |
| Phenolic Extracts | Gram-Negative Bacteria Escherichia coli MIC (mg·mL−1) | Gram-Positive Bacteria Staphylococcus aureus MIC (mg·mL−1) |
|---|---|---|
| PBF | >20 | >20 |
| PAF | 20 | >20 |
| PAFD | 20 | 20 |
| Streptomycin antibiotic-positive standard | 20 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Blázquez, S.; Pedrera-Cajas, L.; Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Rodríguez-Bencomo, J.J.; Miranda, R. The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties. Int. J. Mol. Sci. 2024, 25, 1236. https://doi.org/10.3390/ijms25021236
Rodríguez-Blázquez S, Pedrera-Cajas L, Gómez-Mejía E, Vicente-Zurdo D, Rosales-Conrado N, León-González ME, Rodríguez-Bencomo JJ, Miranda R. The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties. International Journal of Molecular Sciences. 2024; 25(2):1236. https://doi.org/10.3390/ijms25021236
Chicago/Turabian StyleRodríguez-Blázquez, Sandra, Laura Pedrera-Cajas, Esther Gómez-Mejía, David Vicente-Zurdo, Noelia Rosales-Conrado, María Eugenia León-González, Juan José Rodríguez-Bencomo, and Ruben Miranda. 2024. "The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties" International Journal of Molecular Sciences 25, no. 2: 1236. https://doi.org/10.3390/ijms25021236
APA StyleRodríguez-Blázquez, S., Pedrera-Cajas, L., Gómez-Mejía, E., Vicente-Zurdo, D., Rosales-Conrado, N., León-González, M. E., Rodríguez-Bencomo, J. J., & Miranda, R. (2024). The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties. International Journal of Molecular Sciences, 25(2), 1236. https://doi.org/10.3390/ijms25021236