POSS Engineering of Multifunctional Nanoplatforms for Chemo-Mild Photothermal Synergistic Therapy
Abstract
1. Introduction
2. Results and Discussion
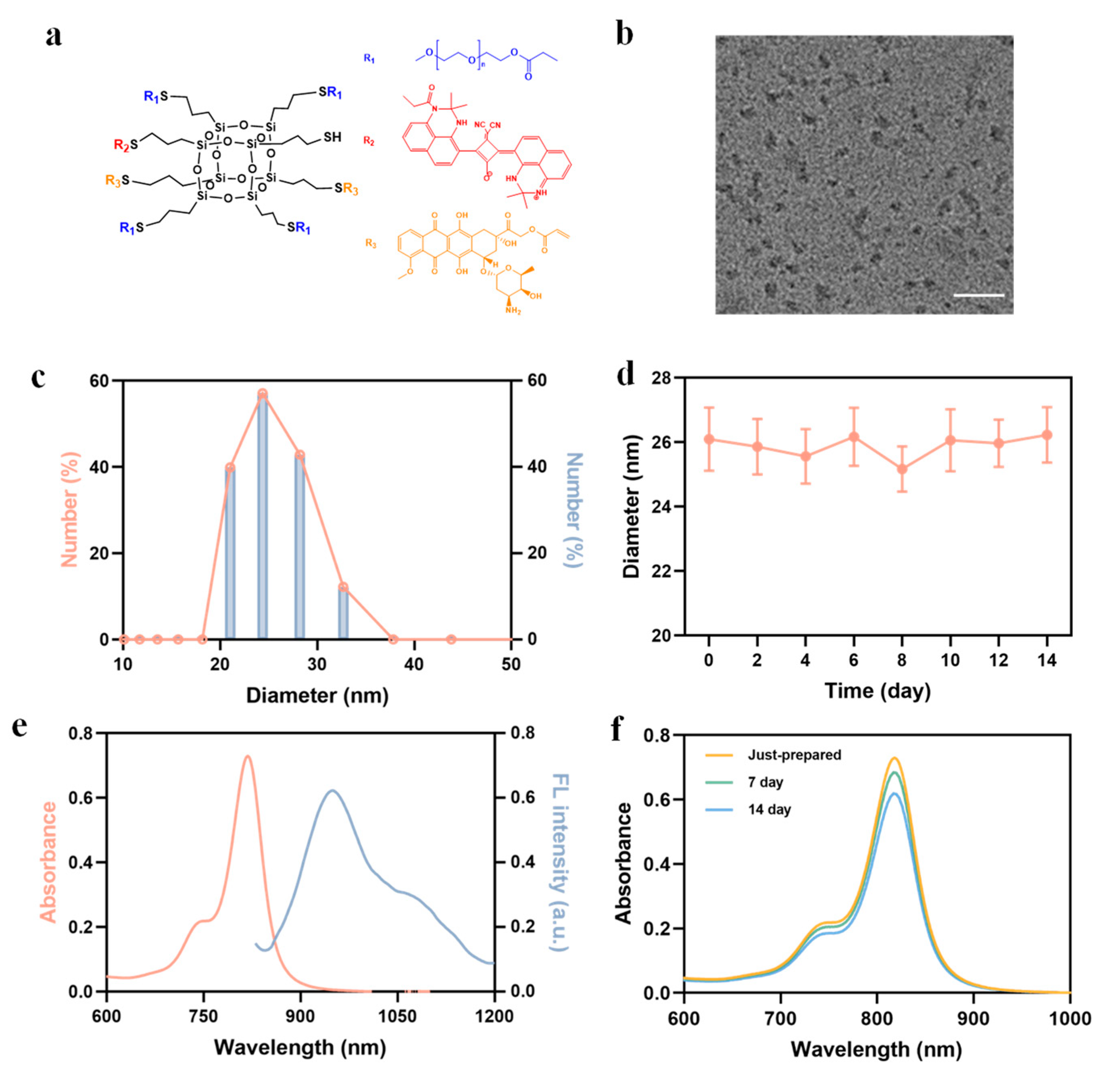
2.1. Preparation and Characterization of POSS-SQ-DOX NPs
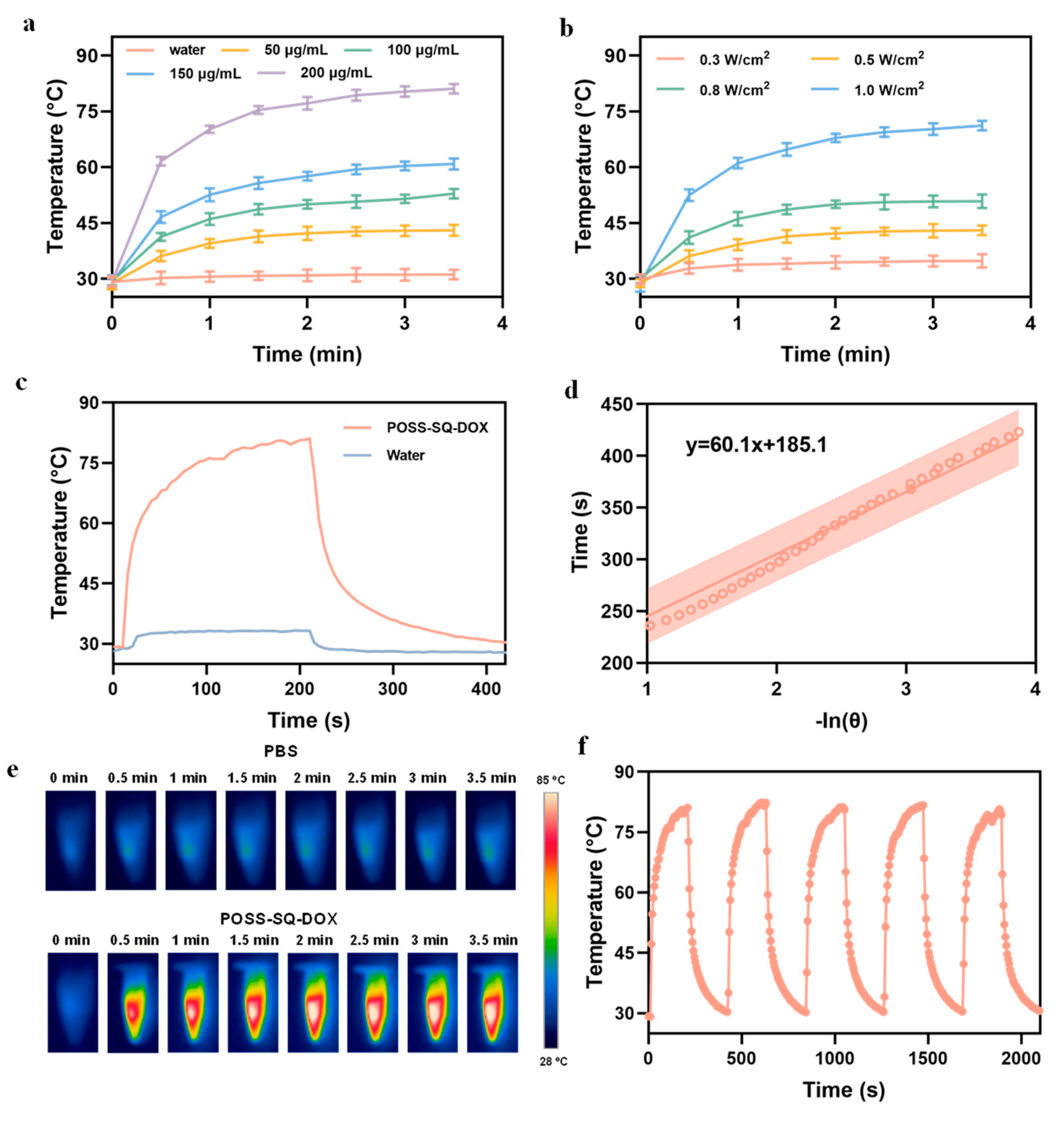
2.2. Photothermal Performance of POSS-SQ-DOX NPs
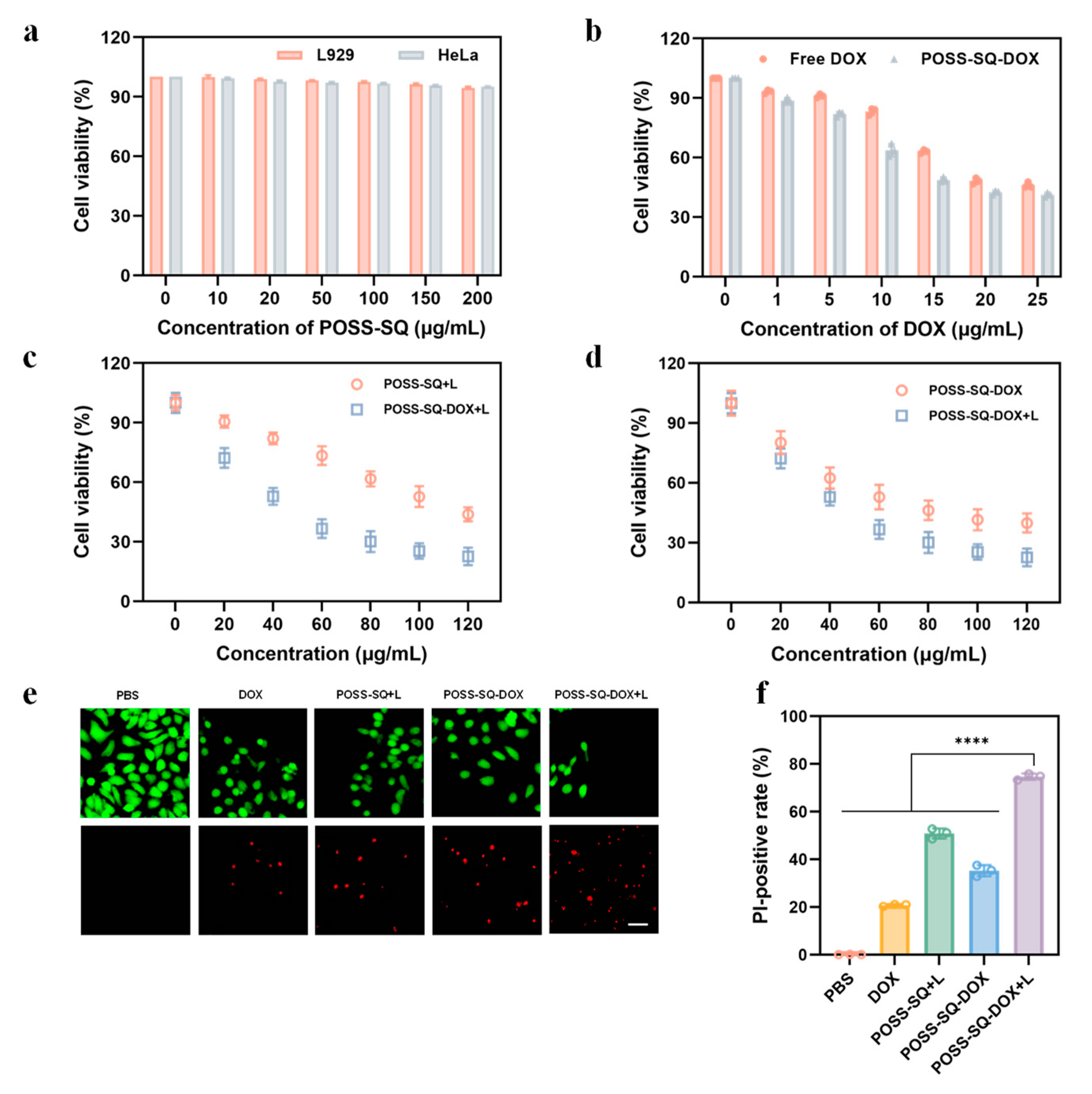
2.3. Evaluation Synergies Effect In Vitro
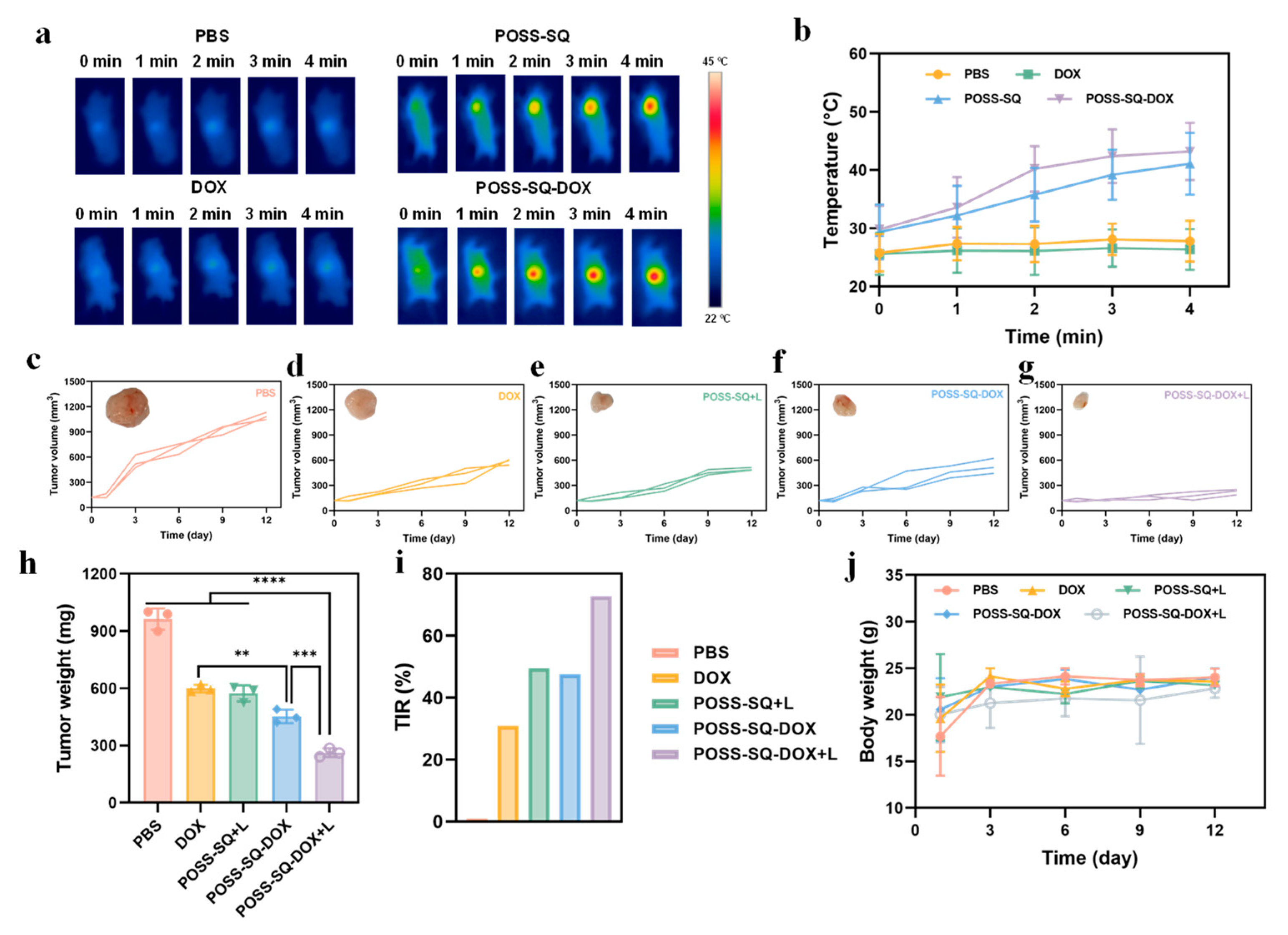
2.4. In Vitro Tumor Therapy
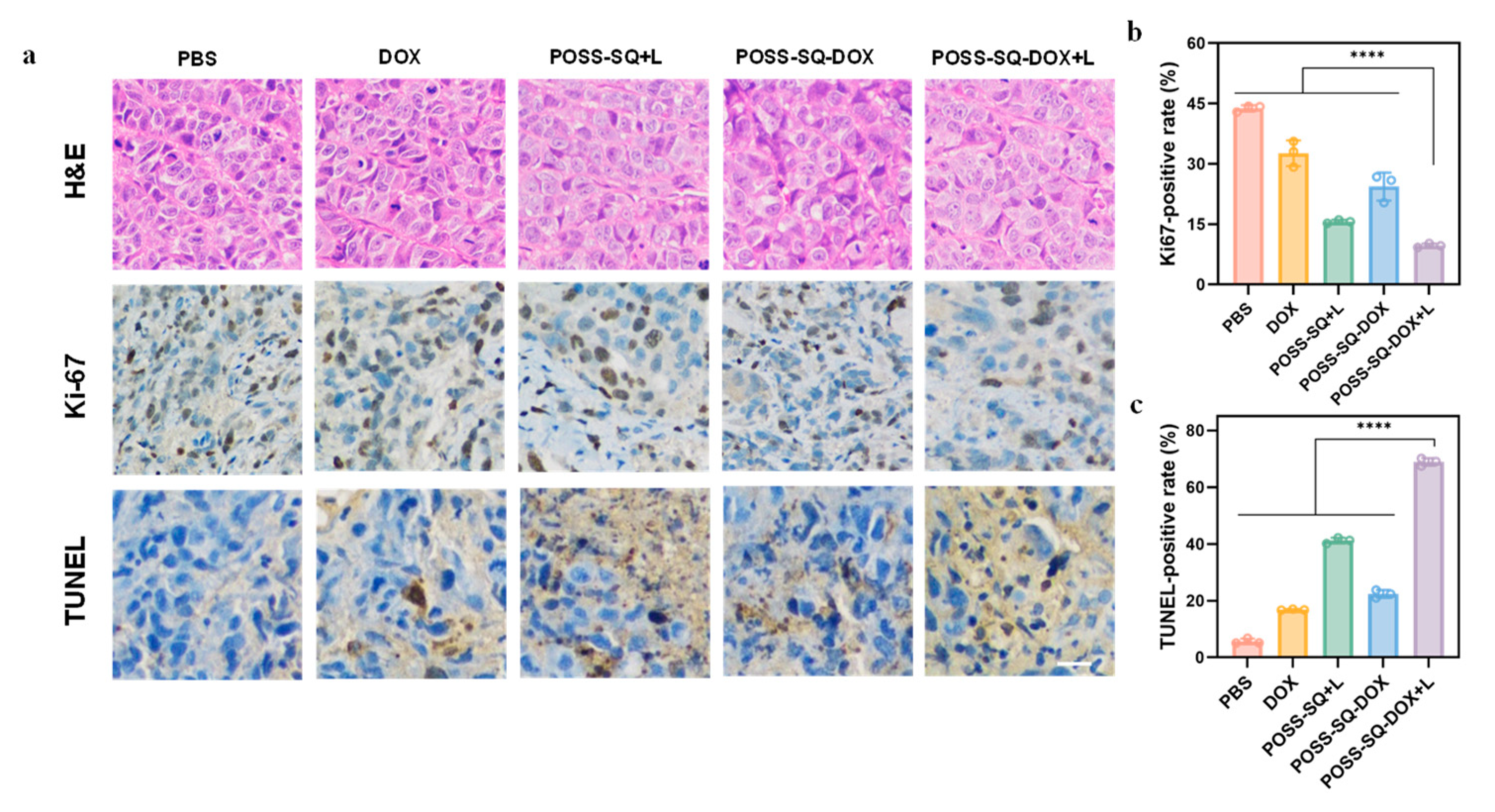
2.5. Anti-Tumor Therapy In Vivo
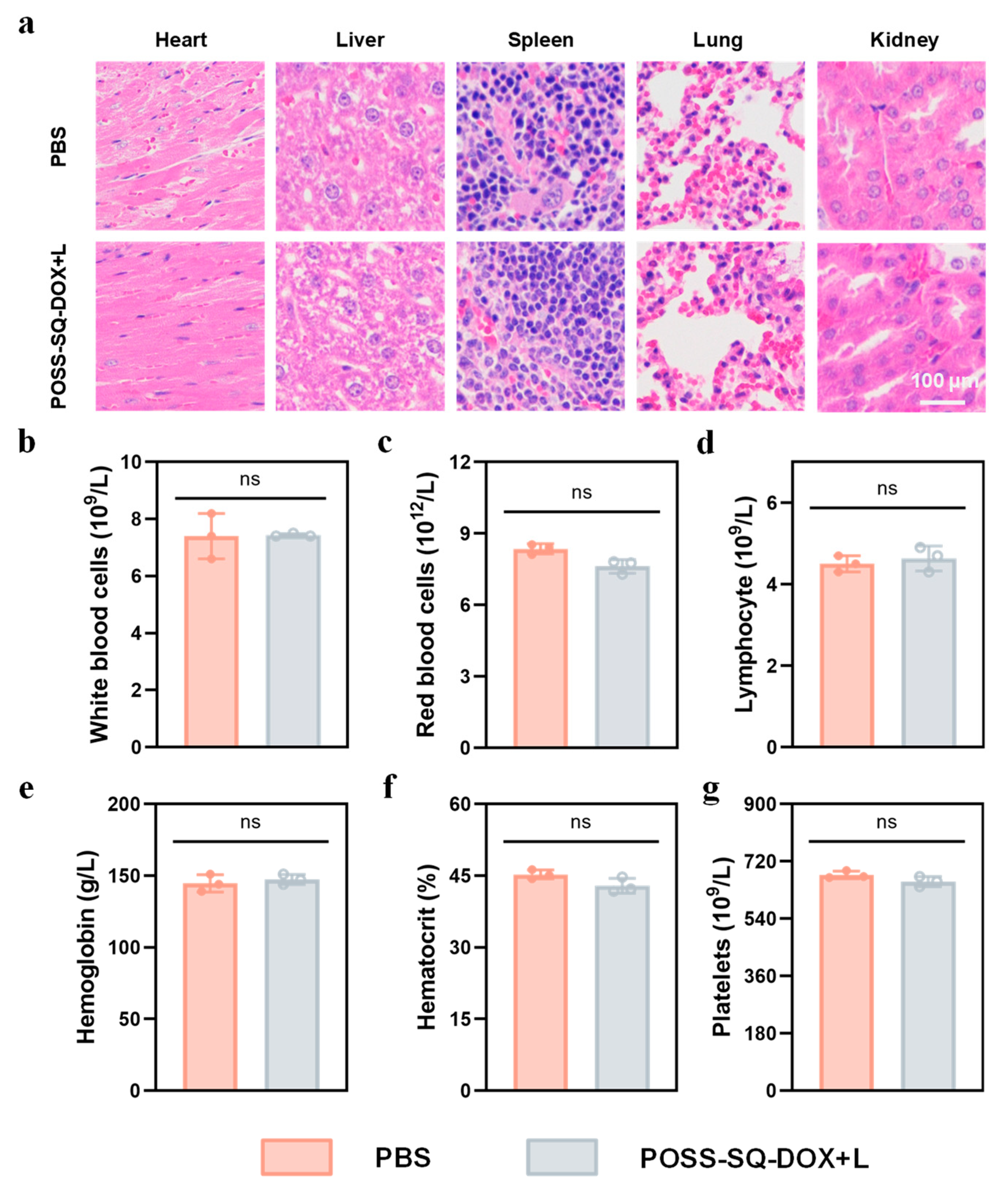
2.6. In Vivo Safety Evaluation
3. Materials and Methods
3.1. General Methods
3.1.1. Materials
3.1.2. Synthesis of DOX-AC
3.1.3. Synthesis of POSS-SQ NPs
3.1.4. Synthesis of POSS-SQ-DOX NPs
3.2. Characterization of POSS-SQ-DOX NPs
3.3. Stability of POSS-SQ-DOX NPs
3.4. Optical Performance of POSS-SQ-DOX NPs
3.5. Cytotoxicity and Combination Index Evaluation
3.6. Live/Dead Cell Double-Staining Assay
3.7. In Vivo Synergistic Anti-Tumor Effects
3.8. Histological Studies
3.9. Blood Routine Assay
3.10. Ethical Statement
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Yong, Y.; Li, J.; Gu, Z.; Zhao, Y. High-Throughput Synthesis of Single-Layer MoS2 Nanosheets as a Near-Infrared Photothermal-Triggered Drug Delivery for Effective Cancer Therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, J.; Wu, Y.; Feng, L.; Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016, 320, 100–117. [Google Scholar] [CrossRef]
- Zhao, R.; Han, X.; Li, Y.; Wang, H.; Ji, T.; Zhao, Y.; Nie, G. Photothermal Effect Enhanced Cascade-Targeting Strategy for Improved Pancreatic Cancer Therapy by Gold Nanoshell@Mesoporous Silica Nanorod. ACS Nano 2017, 11, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wei, R.; Cui, B.; Liu, X.; Chen, Y.; Pan, W.; Li, N.; Tang, B. Ultrathin functionalized covalent organic framework nanosheets for tumor-targeted photodynamic therapy. Chem. Commun. 2021, 57, 6082–6085. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.; Zhou, Q.; Zhang, J.; Yu, Y.; Sun, T. Mild Chemo-Photothermal Synergistic Therapy for Tumors Based on Gold-Nanoparticles Coupled with Metformin. ACS Appl. Nano Mater. 2023, 6, 5729–5736. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Bian, W.Q.; Wang, Y.K.; Pan, Z.X.; Chen, N.P.; Li, X.J.; Wong, W.-L.; Liu, X.J.; He, Y.; Zhang, K.; Lu, Y.-J. Review of Functionalized Nanomaterials for Photothermal Therapy of Cancers. ACS Appl. Nano Mater. 2021, 4, 11353–11385. [Google Scholar] [CrossRef]
- Ma, H.; Xue, M.Q. Recent Advances in the Photothermal Applications of Two-Dimensional Nanomaterials: Photothermal Therapy and Beyond. J. Mater. Chem. A 2021, 9, 17569–17591. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef]
- Nasseri, B.; Alizadeh, E.; Bani, F.; Davaran, S.; Akbarzadeh, A.; Rabiee, N.; Bahadori, A.; Ziaei, M.; Bagherzadeh, M.; Saeb, M.R.; et al. Nanomaterials for Photothermal and Photodynamic Cancer Therapy. Appl. Phys. Rev. 2022, 9, 11317. [Google Scholar] [CrossRef]
- Wang, Z.T.; Wang, M.L.; Wang, X.X.; Hao, Z.K.; Han, S.B.; Wang, T.; Zhang, H.Y. Photothermal-Based Nanomaterials and Photothermal-Sensing: An Overview. Biosens. Bioelectron. 2023, 220, 114883. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.F.; Hu, Y.L.; Zhao, Y.; Tang, K.Y.; Zhang, Z.J.; Liu, Z.L.; Wang, Y.; Guo, H.Y.; Miao, Y.C.; Du, H.D.; et al. Nanomaterials for Photothermal Cancer Therapy. RSC Adv. 2023, 13, 14443–14460. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, C.; Ruan, Z.; Xu, P.; Yang, X.; Yuan, P.; Wang, Q.; Yan, L. Polypeptide-Conjugated Second Near-Infrared Organic Fluorophore for Image-Guided Photothermal Therapy. ACS Nano 2019, 13, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Y.; Du, Y.; Zhang, Y.; Wang, X.; Ding, Y.; Yang, X.; Meng, F.; Tu, J.; Luo, L.; et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019, 10, 4871. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, Y.; Huang, F.; Wang, X.; Lu, J.; Jia, F.; Pan, Z.; Cui, X.; Ge, G.; Deng, X.; et al. Complementary autophagy inhibition and glucose metabolism with rattle-structured polydopamine@mesoporous silica nanoparticles for augmented low-temperature photothermal therapy and in vivo photoacoustic imaging. Theranostics 2020, 10, 7273–7286. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Adv. Mater. 2017, 29, 1703588–1703599. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, X.; Hu, X.; Xiao, L.; Zhang, L.; Song, L.; Xu, M.; Zou, Y.; Chen, L.; Chen, Z.; et al. Simultaneous Application of Photothermal Therapy and an Anti-inflammatory Prodrug using Pyrene-Aspirin-Loaded Gold Nanorod Graphitic Nanocapsules. Angew. Chem. Int. Edit. 2018, 57, 177–181. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, X.; Gao, P.; Li, N.; Tang, B. An anti-inflammatory nanoagent for tumor-targeted photothermal therapy. Chem. Commun. 2019, 55, 9645–9648. [Google Scholar] [CrossRef]
- Li, B.; Hao, G.; Sun, B.; Gu, Z.; Xu, Z.P. Engineering a Therapy-Induced “Immunogenic Cancer Cell Death” Amplifier to Boost Systemic Tumor Elimination. Adv. Funct. Mater. 2020, 30, 1909745–1909756. [Google Scholar] [CrossRef]
- Yi, X.; Duan, Q.-Y.; Wu, F.-G. Low-Temperature Photothermal Therapy: Strategies and Applications. Research 2021, 2021, 9816594. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Sun, X.; Liang, G. Nanoagent-Promoted Mild-Temperature Photothermal Therapy for Cancer Treatment. Adv. Funct. Mater. 2021, 31, 2100738–2100751. [Google Scholar] [CrossRef]
- Deng, X.; Guan, W.; Qing, X.; Yang, W.; Que, Y.; Tan, L.; Liang, H.; Zhang, Z.; Wang, B.; Liu, X.; et al. Ultrafast Low-Temperature Photothermal Therapy Activates Autophagy and Recovers Immunity for Efficient Antitumor Treatment. ACS Appl. Mater. Interfaces 2020, 12, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, L.; An, Y.; Li, Y.; Liu, Y.; Wang, L.; Guo, J.; Wang, J.; Zhou, J. Thermoresponsive Nanogel-Encapsulated PEDOT and HSP70 Inhibitor for Improving the Depth of the Photothermal Therapeutic Effect. Adv. Funct. Mater. 2016, 26, 4749–4759. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Hu, K.; Zou, Y.; Li, Y.; Ma, R.; Zhang, Q.; Cheng, Y. Autophagy inhibition enabled efficient photothermal therapy at a mild temperature. Biomaterials 2017, 141, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cen, D.; Ren, Z.; Wang, Y.; Cai, X.; Huang, J.; Di Silvio, L.; Li, X.; Han, G. Bismuth embedded silica nanoparticles loaded with autophagy suppressant to promote photothermal therapy. Biomaterials 2019, 221, 119419. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef]
- Ma, Z.; Han, K.; Dai, X.; Han, H. Precisely Striking Tumors without Adjacent Normal Tissue Damage via Mitochondria-Templated Accumulation. ACS Nano 2018, 12, 6252–6262. [Google Scholar] [CrossRef]
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem. Inter. Edit. 2019, 58, 1057–1061. [Google Scholar] [CrossRef]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Boosting Cancer Therapy with Organelle-Targeted Nanomaterials. ACS Appl. Mater. Interfaces 2019, 11, 26529–26558. [Google Scholar] [CrossRef]
- Yin, H.; Guan, X.; Lin, H.; Pu, Y.; Fang, Y.; Yue, W.; Zhou, B.; Wang, Q.; Chen, Y.; Xu, H. Nanomedicine-Enabled Photonic Thermogaseous Cancer Therapy. Adv. Sci. 2019, 7, 1901954–1901965. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, T.; Xu, M.; Yang, L.; Song, Y.; Li, N. SO2 prodrug doped nanorattles with extra-high drug payload for “collusion inside and outside” photothermal/pH triggered—Gas therapy. Biomaterials 2020, 257, 120236–120249. [Google Scholar] [CrossRef]
- You, C.; Li, Y.; Dong, Y.; Ning, L.; Zhang, Y.; Yao, L.; Wang, F. Low-Temperature Trigger Nitric Oxide Nanogenerators for Enhanced Mild Photothermal Therapy. ACS Biomater. Sci. Eng. 2020, 6, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, J.; Jiang, K.; Tang, Z.; Wang, Y.; Li, Z.; Liu, C.; Wu, A.; Lin, H. Ce6-Modified Carbon Dots for Multimodal-Imaging-Guided and Single-NIR-Laser-Triggered Photothermal/Photodynamic Synergistic Cancer Therapy by Reduced Irradiation Power. ACS Appl. Mater. Interfaces 2019, 11, 5791–5803. [Google Scholar] [CrossRef] [PubMed]
- She, D.; Peng, S.; Liu, L.; Huang, H.; Zheng, Y.; Lu, Y.; Geng, D.; Yin, B. Biomimic FeS2 nanodrug with hypothermal photothermal effect by clinical approved NIR-II light for augmented chemodynamic therapy. Chem. Eng. J. 2020, 400, 125933–125943. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.W.; Guo, Y.; Jia, H.R.; Cheng, X.; Deng, Y.; Yu, X.W.; Zhu, Y.X.; Guo, H.Y.; Sun, W.; et al. Enzyme-Mediated Tumor Starvation and Phototherapy Enhance Mild-Temperature Photothermal Therapy. Adv. Funct. Mater. 2020, 30, 1909391–1909403. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Chen, S.; Wang, Y.; Zhang, Y.; Wang, Y.; Wei, D.; Zhang, L.; Rong, G.; Weng, Y.; et al. Near-Infrared Light Irradiation Induced Mild Hyperthermia Enhances Glutathione Depletion and DNA Interstrand Cross-Link Formation for Efficient Chemotherapy. ACS Nano 2020, 14, 14831–14845. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Y.; Zhang, J.; Yang, X.; Ding, D.; Shi, Y.; Xu, H.; Gao, X. Azulene-Containing Squaraines for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 19192–19203. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- He, J.; Jo, Y.J.; Sun, X.; Qiao, W.; Ok, J.; Kim, T.i.; Li, Z.A. Squaraine Dyes for Photovoltaic and Biomedical Applications. Adv. Funct. Mater. 2020, 31, 2008201–2008233. [Google Scholar] [CrossRef]
- Grande, V.; Doria, F.; Freccero, M.; Wurthner, F. An Aggregating Amphiphilic Squaraine: A Light-up Probe That Discriminates Parallel G-Quadruplexes. Angew. Chem. Int. Ed. Engl. 2017, 56, 7520–7524. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Ma, X.; Zhao, Y. Graphene Oxide Wrapping on Squaraine-Loaded Mesoporous Silica Nanoparticles for Bioimaging. J. Am. Chem. Soc. 2012, 134, 17346–17349. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.X.; Qiao, Z.Y.; Mayerhoffer, U.; Spenst, P.; Li, X.J.; Wurthner, F.; Wang, H. Nano-confined squaraine dye assemblies: New photoacoustic and near-infrared fluorescence dual-modular imaging probes in vivo. Bioconjug. Chem. 2014, 25, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Saneesh Babu, P.S.; Manu, P.M.; Dhanya, T.J.; Tapas, P.; Meera, R.N.; Surendran, A.; Aneesh, K.A.; Vadakkancheril, S.J.; Ramaiah, D.; Nair, S.A.; et al. Bis(3,5-diiodo-2,4,6-trihydroxyphenyl)squaraine photodynamic therapy disrupts redox homeostasis and induce mitochondria-mediated apoptosis in human breast cancer cells. Sci. Rep. 2017, 7, 42126. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, Y.; Zou, R.; Bian, K.; Liu, P.; Shen, S.; Yang, W.; Zhang, B.; Wang, D. Molecular Engineered Squaraine Nanoprobe for NIR-II/Photoacoustic Imaging and Photothermal Therapy of Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4276–4284. [Google Scholar] [CrossRef] [PubMed]
- Dimitriev, O.P.; Dimitriyeva, A.P.; Tolmachev, A.I.; Kurdyukov, V.V. Solvent-induced organization of squaraine dyes in solution capillary layers and adsorbed films. J. Phys. Chem. B 2005, 109, 4561–4567. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Wang, X.; Hu, Y.; Hu, H.; Wu, D.C.; Xu, F.J. Bioreducible POSS-cored star-shaped polycation for efficient gene delivery. ACS Appl. Mater. Interfaces 2014, 6, 1044–1052. [Google Scholar] [CrossRef]
- Pang, B.; Liu, Y.; Cao, R.; Zhang, W. Hybrid Nanoscale Vesicles of Polyhedral Oligomeric Silsesquioxane-Based Star Block Copolymers for Thermal Insulation Applications. ACS Appl. Nano Mater. 2022, 5, 7042–7050. [Google Scholar] [CrossRef]
- Chi, H.; Wang, M.; Li, J.; Tian, H.; Chong, Y.T.; Lim, S.H.; Wang, Y.; Wang, F. Polyhedral Oligomeric Silsesquioxane as a Polarity Mediator and Reinforced Nanofiller for Fabricating Robust and Hierarchical Porous Film for Cell Bioengineering. ACS Appl. Polym. Mater. 2022, 4, 5882–5890. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Gao, Y.; Yang, D.; Wang, F.; Zhang, L.; Bao, B.; Wang, L. Nanoscale Organic-Inorganic Hybrid Photosensitizers for Highly Effective Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 248–255. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, K.; Gu, Y.; Guang, S.; Feng, J.; Xu, H. Novel multifunctional nano-hybrid polyhedral oligomeric silsesquioxane-based molecules with high cell permeability: Molecular design and application for diagnosis and treatment of tumors. Nanoscale 2021, 13, 2982–2994. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Gu, Y.; Lin, N.; Ning, X.; Lu, Y.; Zhao, G.; Guang, S.; Feng, J.; Xu, H. Autonomous Bionanorobots via a Cage-Shaped Silsesquioxane Vehicle for In Vivo Heavy Metal Detoxification. ACS Appl. Mater. Interfaces 2022, 14, 29238–29249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Han, Z.; Lu, Z.R. A targeted nanoglobular contrast agent from host-guest self-assembly for MR cancer molecular imaging. Biomaterials 2016, 85, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, L.; Li, L.; Zhou, Z.; Huang, Y. Subcellular co-delivery of two different site-oriented payloads for tumor therapy. Nanoscale 2017, 9, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, X.; Cao, Q.; Yang, Y.; Wu, D. POSS-based supramolecular amphiphilic zwitterionic complexes for drug delivery. Biomater. Sci. 2019, 7, 1984–1994. [Google Scholar] [CrossRef]
- John, L. Selected developments and medical applications of organic-inorganic hybrid biomaterials based on functionalized spherosilicates. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Abudukelimu, S.; Wei, G.; Huang, J.; Zhao, G.; Wei, L.; Cui, W.; Lu, M.; Yao, W. Polyhedral oligomeric silsesquioxane (POSS)-based hybrid nanocomposite for synergistic chemo-photothermal therapy against pancreatic cancer. Chem. Eng. J. 2022, 442, 136124–136134. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, C.; Chechetka, S.A.; Miyako, E.; Pu, K. Semiconducting Polymer Nanobioconjugates for Targeted Photothermal Activation of Neurons. J. Am. Chem. Soc. 2016, 138, 9049–9052. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, J.; Jiang, Y.; Zhen, X.; Wang, T.; Qiu, S.; Lou, X.; Gao, M.; Pu, K. Enhancing Both Biodegradability and Efficacy of Semiconducting Polymer Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2018, 12, 1801–1810. [Google Scholar] [CrossRef]
- Yun, K.; Guo, J.; Zhu, R.; Wang, T.; Zhang, X.; Pan, H.; Pan, W. Design of ROS-Responsive Hyaluronic Acid–Methotrexate Conjugates for Synergistic Chemo-Photothermal Therapy for Cancer. Mol. Pharm. 2022, 19, 3323–3335. [Google Scholar] [CrossRef]


| Treatment | IC50 (μg/mL) | CI | |
|---|---|---|---|
| DOX | SQ-N +L | ||
| Free DOX | 19.94 | ||
| Free SQ-N +L | 17.45 | ||
| DOX:SQ-N +L (1:3) | 4.15 | 12.01 | 0.99 |
| DOX:SQ-N +L (1:2) | 5.43 | 10.68 | 0.88 |
| DOX:SQ-N +L (1:1) | 7.62 | 8.07 | 0.84 |
| DOX:SQ-N +L (2:1) | 9.32 | 4.33 | 0.72 |
| DOX:SQ-N +L (3:1) | 11.70 | 3.75 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Geng, X.; Guang, S.; Xu, H. POSS Engineering of Multifunctional Nanoplatforms for Chemo-Mild Photothermal Synergistic Therapy. Int. J. Mol. Sci. 2024, 25, 1012. https://doi.org/10.3390/ijms25021012
Gu Z, Geng X, Guang S, Xu H. POSS Engineering of Multifunctional Nanoplatforms for Chemo-Mild Photothermal Synergistic Therapy. International Journal of Molecular Sciences. 2024; 25(2):1012. https://doi.org/10.3390/ijms25021012
Chicago/Turabian StyleGu, Zhengye, Xiaochuan Geng, Shanyi Guang, and Hongyao Xu. 2024. "POSS Engineering of Multifunctional Nanoplatforms for Chemo-Mild Photothermal Synergistic Therapy" International Journal of Molecular Sciences 25, no. 2: 1012. https://doi.org/10.3390/ijms25021012
APA StyleGu, Z., Geng, X., Guang, S., & Xu, H. (2024). POSS Engineering of Multifunctional Nanoplatforms for Chemo-Mild Photothermal Synergistic Therapy. International Journal of Molecular Sciences, 25(2), 1012. https://doi.org/10.3390/ijms25021012





