Map-Based Cloning and Characterization of a Major QTL Gene, FfR1, Which Confers Resistance to Rice Bakanae Disease
Abstract
1. Introduction
2. Results
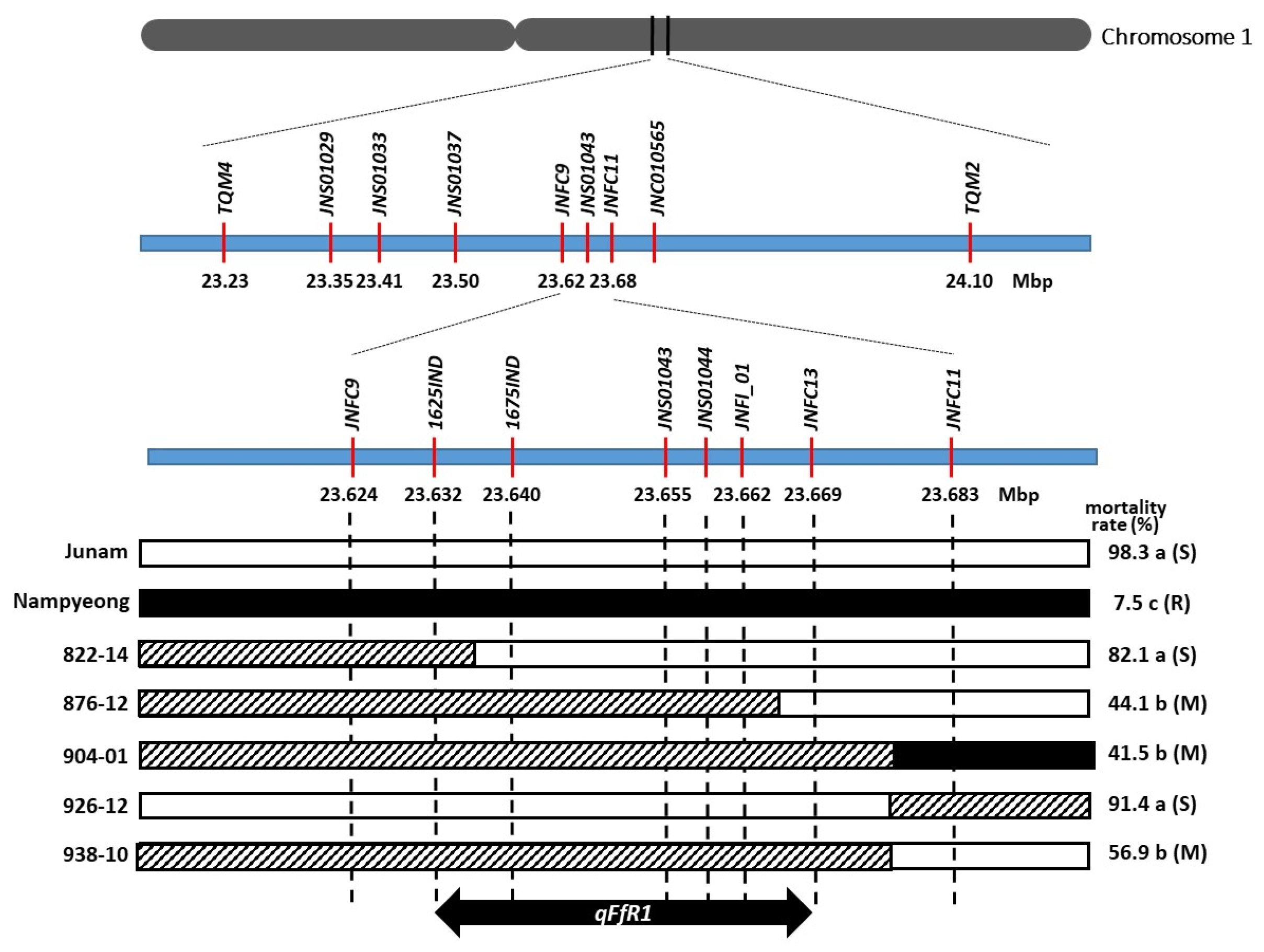
2.1. Fine-Mapping and Identification of qFfR1
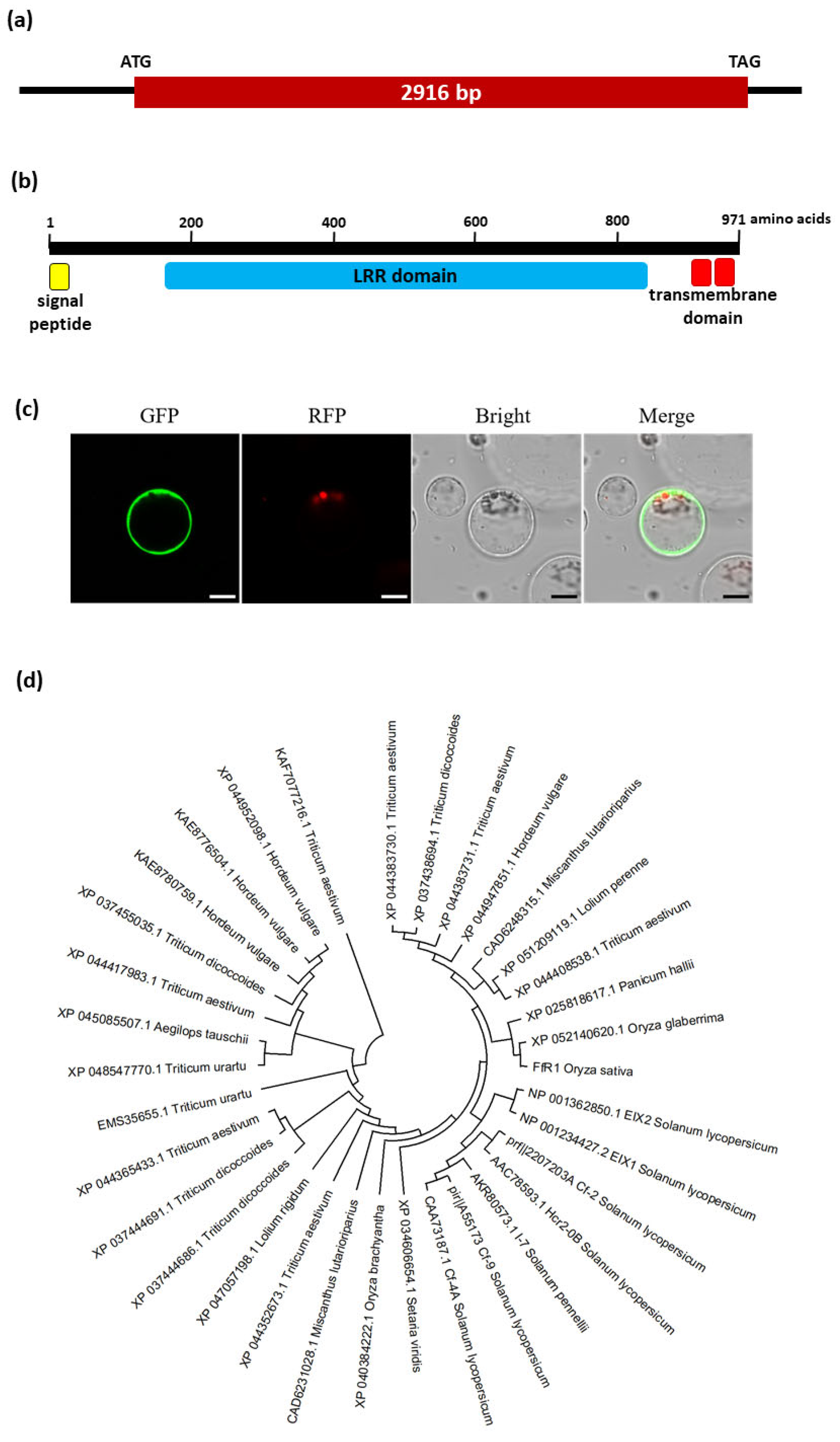
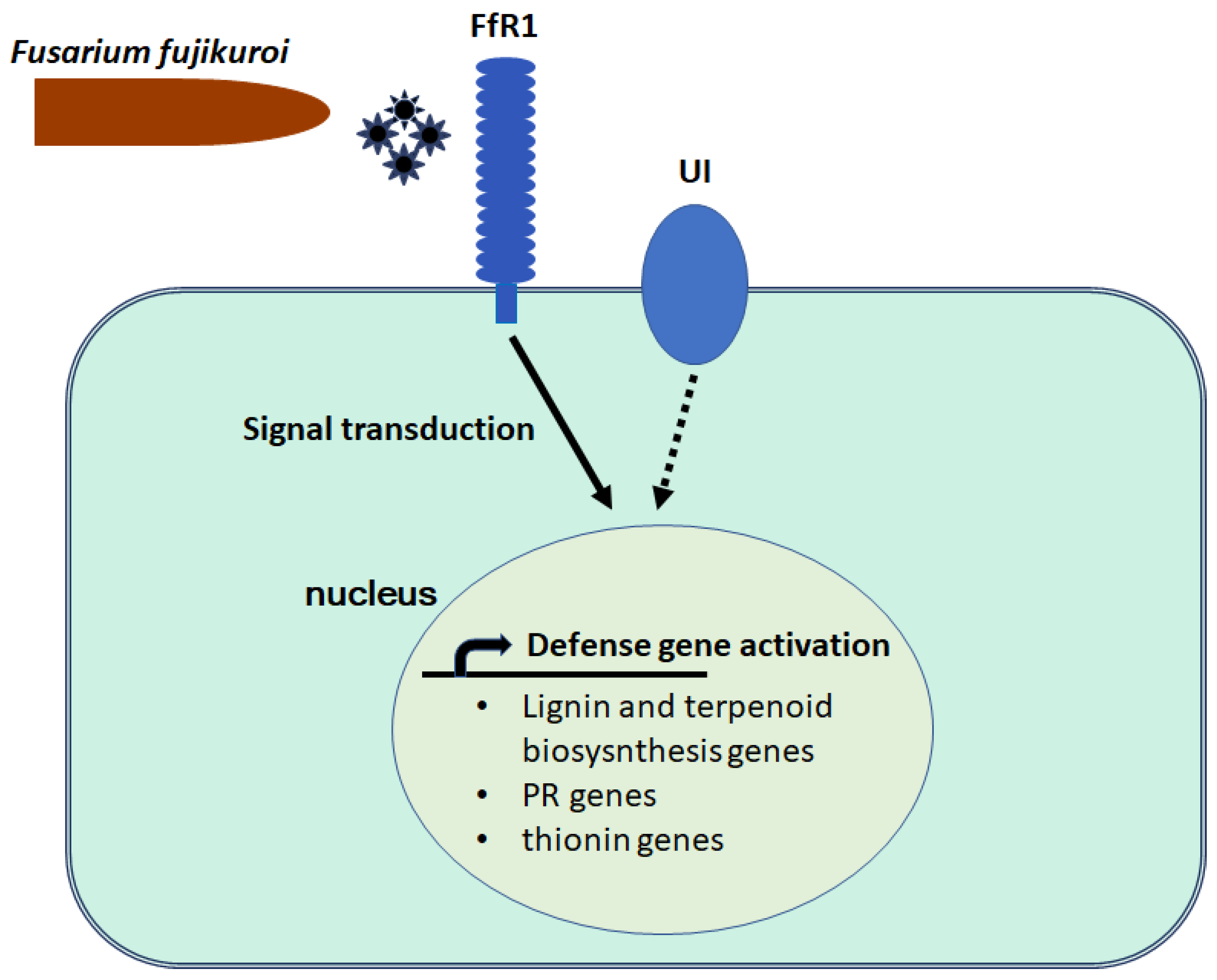
2.2. The FfR1 Gene and Protein Structures, Subcellular Localization, and Gene Expression Profile
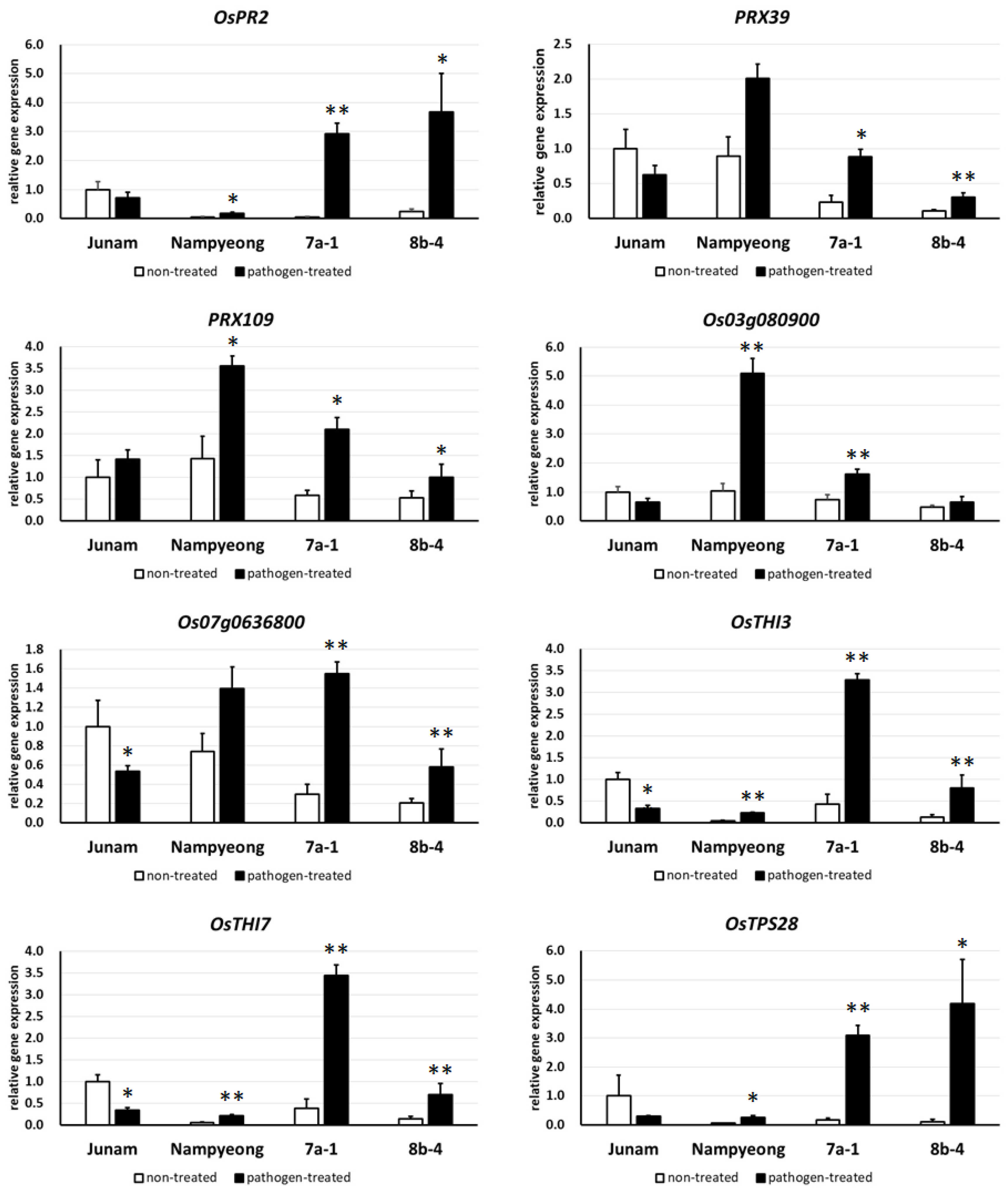
2.3. RNA Sequencing Analysis
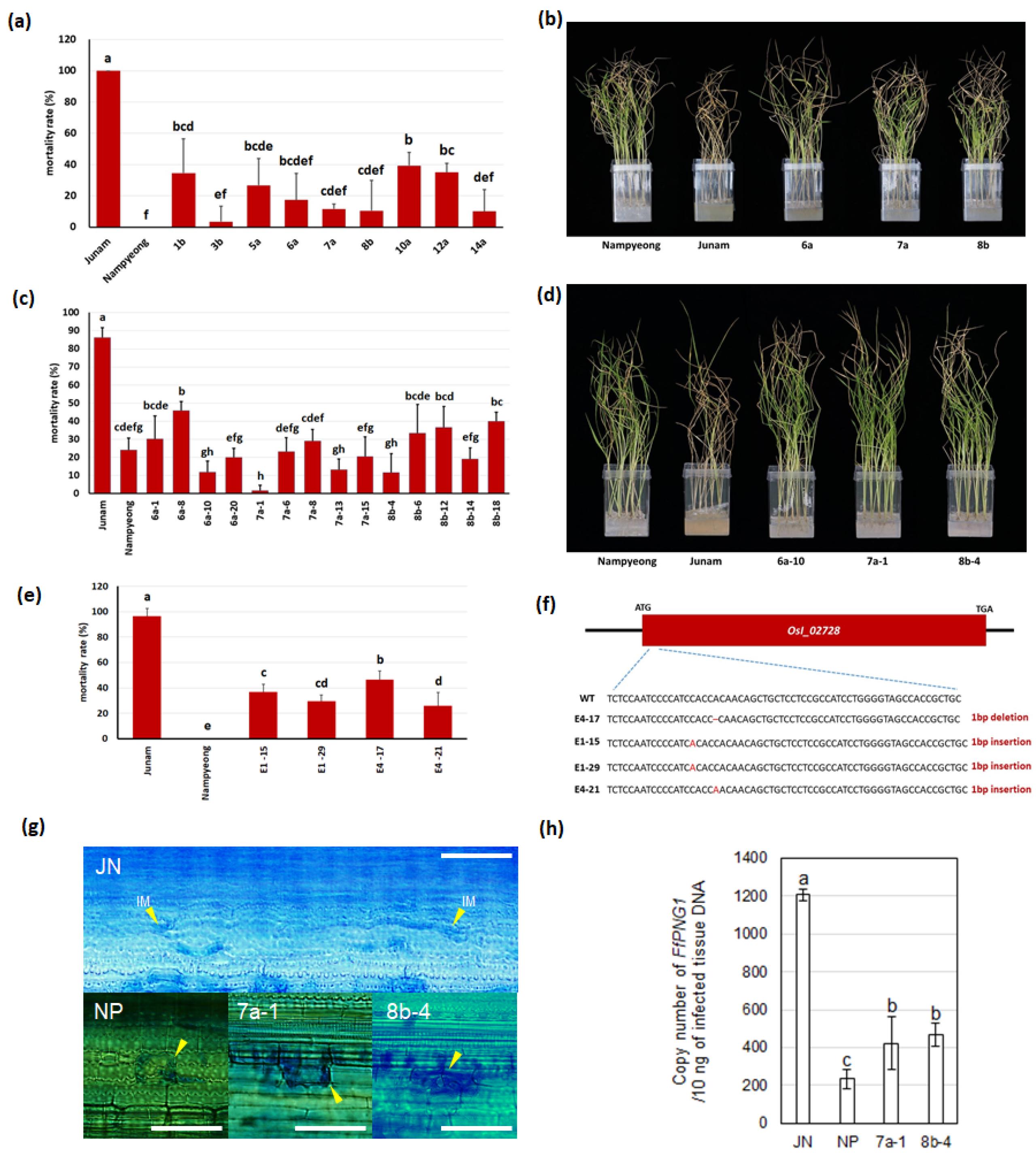
2.4. Development of the FfR1 Selection Marker
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Fine-Mapping
4.2. Isolation and Transformation of the Candidate Gene
4.3. Evaluation of the Pathogen’s Ramification in Planta
4.4. Subcellular Localization
4.5. Phylogenetic and Statistical Analysis
4.6. RNA-Seq Analysis and qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.K.; Solanki, I.S.; Bashyal, B.M.; Singh, Y.; Srivastava, K. Bakanae of rice—An emerging disease in Asia. J. Anim. Plant Sci. 2015, 25, 1499–1514. [Google Scholar]
- Amatulli, M.T.; Spadaro, D.; Gullino, M.L.; Garibaldi, A. Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol. 2010, 59, 839–844. [Google Scholar] [CrossRef]
- Carter, L.L.; Leslie, J.F.; Webster, R.K. Population structure of Fusarium fujikuroi from California rice and water grass. Phytopathology 2008, 98, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Manandhar, H.K.; Plattner, R.D.; Manandhar, G.G.; Poling, S.M.; Maragos, C.M. Fusarium species from nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl. Environ. Microbiol. 2000, 66, 1020–1025. [Google Scholar] [CrossRef]
- Jeon, Y.A.; Yu, S.H.; Lee, Y.Y.; Park, H.J.; Lee, S.; Sung, J.S.; Kim, Y.G.; Lee, H.S. Incidence, molecular characteristics and pathogenicity of Gibberella fujikuroi species complex associated with rice seeds from Asian countries. Mycobiology 2013, 41, 225–233. [Google Scholar] [CrossRef]
- Nicolli, C.P.; Haidukowski, M.; Susca, A.; Gomes, L.B.; Logrieco, A.; Stea, G.; Del Ponte, E.M.; Moretti, A.; Pfenning, L.H. Fusarium fujikuroi species complex in Brazilian rice: Unveiling increased phylogenetic diversity and toxigenic potential. Int. J. Food Microbiol. 2020, 330, 108667. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Lu, Y.; He, D.; Lee, Y.W.; Ji, F.; Xu, J.; Shi, J. Fusarium fujikuroi Species Complex Associated with Rice, Maize, and Soybean from Jiangsu Province, China: Phylogenetic, Pathogenic, and Toxigenic Analysis. Plant Dis. 2020, 104, 2193–2201. [Google Scholar] [CrossRef]
- Scherm, B.; Balmas, V.; Infantino, A.; Aragona, M.; Valente, M.T.; Desiderio, F.; Marcello, A.; Phanthavong, S.; Burgess, L.W.; Rau, D. Clonality, spatial structure, and pathogenic variation in Fusarium fujikuroi from rain-fed rice in southern Laos. PLoS ONE 2019, 14, e0226556. [Google Scholar] [CrossRef]
- Tadasanahaller, P.S.; Bashyal, B.M.; Yadav, J.; Krishnan Subbaiyan, G.; Ellur, R.K.; Aggarwal, R. Identification and characterization of Fusarium fujikuroi pathotypes responsible for an emerging bakanae disease of Rice in India. Plants 2023, 12, 1303. [Google Scholar] [CrossRef]
- Wulff, E.G.; Sorensen, J.L.; Lubeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. associated with rice bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef]
- Shin, S.; Ryu, H.; Jung, J.-Y.; Yoon, Y.-J.; Kwon, G.; Lee, N.; Kim, N.H.; Lee, R.; Oh, J.; Baek, M.; et al. Past and future epidemiological perspectives and integrated management of rice bakanae in Korea. Plant Pathol. J. 2023, 39, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, T.; Liang, S.; Liu, K.; Zhou, M.; Chen, C. Molecular mechanism of resistance of Fusarium fujikuroi to benzimidazole fungicides. FEMS Microbiol. Lett. 2014, 357, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, T.; Duan, Y.; Yang, Y.; Wu, J.; Zhao, D.; Xiao, X.; Pan, X.; Chen, W.; Wang, J.; et al. Evaluation of phenamacril and ipconazole for control of rice bakanae disease caused by Fusarium fujikuroi. Plant Dis. 2018, 102, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.P.; Li, J.S.; Wang, J.X.; Wu, L.Y.; Wang, Y.F.; Chen, C.J.; Zhou, M.G.; Hou, Y.P. Effects of the dinitroaniline fungicide fluazinam on Fusarium fujikuroi and rice. Pestic. Biochem. Physiol. 2018, 152, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-R.; Lee, S.-W.; Lee, S.-W.; Kim, I.-S. Morphological changes of fungal cell wall and ABC transporter as resistance responses of rice Bakanae disease pathogen Fusarium fujikuroi CF337 to prochloraz. Korean J. Environ. Agric. 2012, 31, 20–36. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lai, M.H.; Chu, Y.L.; Wu, D.H.; Tung, C.W.; Chen, Y.J.; Chung, C.L. Identification of qBK2.1, a novel QTL controlling rice resistance against Fusarium fujikuroi. Bot. Stud. 2023, 64, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lai, M.H.; Tung, C.W.; Wu, D.H.; Chang, F.Y.; Lin, T.C.; Chung, C.L. Genome-wide association mapping of gene loci affecting disease resistance in the rice-Fusarium fujikuroi pathosystem. Rice 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Fiyaz, R.A.; Krishnan, S.G.; Rajashekara, H.; Yadav, A.K.; Bashyal, B.M.; Bhowmick, P.K.; Singh, N.K.; Prabhu, K.V.; Singh, A.K. Development of high throughput screening protocol and identification of novel sources of resistance against bakanae disease in rice (Oryza sativa L.). Indian J. Genet. Plant Breed. 2014, 74, 414–422. [Google Scholar] [CrossRef]
- Hur, Y.J.; Lee, S.B.; Shin, D.J.; Kim, T.H.; Cho, J.H.; Han, S.I.; Oh, S.H.; Lee, J.Y.; Son, Y.B.; Lee, J.H.; et al. Screening of rice germplasm for bakanae disease resistance in rice. Korean J. Breed. Sci. 2016, 48, 22–28. [Google Scholar] [CrossRef]
- Kim, M.H.; Hur, Y.J.; Lee, S.B.; Kwon, T.; Hwang, U.H.; Park, S.K.; Yoon, Y.N.; Lee, J.H.; Cho, J.H.; Shin, D.; et al. Large-scale screening of rice accessions to evaluate resistance to bakanae disease. J. Gen. Plant Pathol. 2014, 80, 408–414. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, M.J.; Choi, H.W.; Kim, S.T.; Park, J.W.; Myung, I.S.; Park, K.; Lee, S.W. Development of in vitro seedling screening method for selection of resistant rice against bakanae disease. Res. Plant Dis. 2011, 17, 288–294. [Google Scholar] [CrossRef]
- Cheon, K.-S.; Jeong, Y.-M.; Lee, Y.-Y.; Oh, J.; Kang, D.-Y.; Oh, H.; Kim, S.L.; Kim, N.; Lee, E.; Baek, J.; et al. Kompetitive allele-specific PCR marker development and quantitative trait locus mapping for bakanae disease resistance in Korean Japonica rice varieties. Plant Breed. Biotechnol. 2019, 7, 208–219. [Google Scholar] [CrossRef]
- Fiyaz, R.A.; Yadav, A.K.; Krishnan, S.G.; Ellur, R.K.; Bashyal, B.M.; Grover, N.; Bhowmick, P.K.; Nagarajan, M.; Vinod, K.K.; Singh, N.K.; et al. Mapping quantitative trait loci responsible for resistance to bakanae disease in rice. Rice 2016, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.J.; Lee, S.B.; Kim, T.H.; Kwon, T.; Lee, J.H.; Shin, D.J.; Park, S.K.; Hwang, U.H.; Cho, J.H.; Yoon, Y.N.; et al. Mapping of qBK1, a major QTL for bakanae disease resistance in rice. Mol. Breed. 2015, 35, 78. [Google Scholar] [CrossRef]
- Kang, D.Y.; Cheon, K.S.; Oh, J.; Oh, H.; Kim, S.L.; Kim, N.; Lee, E.; Choi, I.; Baek, J.; Kim, K.H.; et al. Rice genome resequencing reveals a major quantitative trait locus for resistance to bakanae disease caused by Fusarium fujikuroi. Int. J. Mol. Sci. 2019, 20, 2598. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Hur, Y.-J.; Cho, J.-H.; Lee, J.-H.; Kim, T.-H.; Cho, S.-M.; Song, Y.-C.; Seo, Y.-S.; Lee, J.; Kim, T.-S.; et al. Molecular mapping of qBK1WD, a major QTL for bakanae disease resistance in rice. Rice 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Lee, J.-Y.; Kang, J.-W.; Mang, H.; Kabange, N.R.; Seong, G.-U.; Kwon, Y.; Lee, S.-M.; Shin, D.; Lee, J.-H.; et al. A novel locus for bakanae disease resistance, qBK4T, identified in rice. Agronomy 2022, 12, 2567. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, N.; Jo, S.; Hur, Y.J.; Lee, J.Y.; Cho, J.H.; Lee, J.H.; Kang, J.W.; Song, Y.C.; Bombay, M.; et al. Mapping of a major QTL, qBK1(Z), for bakanae disease resistance in rice. Plants 2021, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-D.; Guo, L.-B.; Li, X.-M.; Ji, Z.-J.; Ma, L.-Y.; Qian, Q. Analysis of QTLs for resistance to rice bakanae disease. Chin. J. Rice Sci. 2006, 20, 657–659. [Google Scholar]
- Volante, A.; Tondelli, A.; Aragona, M.; Valente, M.T.; Biselli, C.; Desiderio, F.; Bagnaresi, P.; Matic, S.; Gullino, M.L.; Infantino, A.; et al. Identification of bakanae disease resistance loci in japonica rice through genome wide association study. Rice 2017, 10, 29. [Google Scholar] [CrossRef]
- Son, S.; Kim, H.; Lee, K.S.; Kim, S.; Park, S.R. Rice glutaredoxin GRXS15 confers broad-spectrum resistance to Xanthomonas oryzae pv. oryzae and Fusarium fujikuroi. Biochem. Biophys. Res. Commun. 2020, 533, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Son, S.; Nam, S.; Suh, E.J.; Lee, S.I.; Park, S.R. OsWRKY114 Is a player in rice immunity against Fusarium fujikuroi. Int. J. Mol. Sci. 2023, 24, 6604. [Google Scholar] [CrossRef]
- Matic, S.; Bagnaresi, P.; Biselli, C.; Orru, L.; Amaral Carneiro, G.; Siciliano, I.; Vale, G.; Gullino, M.L.; Spadaro, D. Comparative transcriptome profiling of resistant and susceptible rice genotypes in response to the seedborne pathogen Fusarium fujikuroi. BMC Genom. 2016, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, B.M.; Rawat, K.; Parmar, P.; Gupta, A.K.; Gupta, S.; Krishnan, S.G.; Choudhary, R.; Ercisli, S.; Kovacevic, A.; Aggarwal, R. Transcriptomic analysis of bakanae disease resistant and susceptible rice genotypes in response to infection by Fusarium fujikuroi. Mol. Biol. Rep. 2022, 49, 11959–11972. [Google Scholar] [CrossRef]
- Ji, H.; Kim, T.H.; Lee, G.S.; Kang, H.J.; Lee, S.B.; Suh, S.C.; Kim, S.L.; Choi, I.; Baek, J.; Kim, K.H. Mapping of a major quantitative trait locus for bakanae disease resistance in rice by genome resequencing. Mol. Genet. Genom. 2018, 293, 579–586. [Google Scholar] [CrossRef]
- Ji, H.; Shin, Y.; Lee, C.; Oh, H.; Yoon, I.S.; Baek, J.; Cha, Y.S.; Lee, G.S.; Kim, S.L.; Kim, K.H. Genomic variation in Korean Japonica rice varieties. Genes 2021, 12, 1749. [Google Scholar] [CrossRef]
- Dixon, M.S.; Jones, D.A.; Keddie, J.S.; Thomas, C.M.; Harrison, K.; Jones, J.D.G. The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell 1996, 84, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Jones, D.A.; Parniske, M.; Harrison, K.; Balint-Kurti, P.J.; Hatzixanthis, K.; Jones, J.D. Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell 1997, 9, 2209–2224. [Google Scholar] [CrossRef]
- Dixon, M.S.; Hatzixanthis, K.; Jones, D.A.; Harrison, K.; Jones, J.D.G. The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number. Plant Cell 1998, 10, 1915–1925. [Google Scholar] [CrossRef]
- Jones, D.A.; Thomas, C.M.; Hammond-Kosack, K.E.; Balint-Kurti, P.J.; Jones, J.D. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 1994, 266, 789–793. [Google Scholar] [CrossRef]
- Gonzalez-Cendales, Y.; Catanzariti, A.M.; Baker, B.; McGrath, D.J.; Jones, D.A. Identification of I-7 expands the repertoire of genes for resistance to Fusarium wilt in tomato to three resistance gene classes. Mol. Plant Pathol. 2016, 17, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Ron, M.; Avni, A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 2004, 16, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budinska, E.; et al. Dirigent proteins in plants: Modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Gheysen, G.; Ullah, C.; Verbeek, R.; Shang, C.; De Vleesschauwer, D.; Hofte, M.; Kyndt, T. The role of thionins in rice defence against root pathogens. Mol. Plant Pathol. 2015, 16, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Lei, L.; Liu, Z.; Zhou, S.; Yang, C.; Zhu, X.; Guo, H.; Zhang, F.; Peng, M.; Zhang, M.; et al. Selection of a subspecies-specific diterpene gene cluster implicated in rice disease resistance. Nat. Plants 2020, 6, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Wulff, B.B.; Chakrabarti, A.; Jones, D.A. Recognitional specificity and evolution in the tomato-Cladosporium fulvum pathosystem. Mol. Plant Microbe Interact. 2009, 22, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Kawchuk, L.M.; Hachey, J.; Lynch, D.R.; Kulcsar, F.; van Rooijen, G.; Waterer, D.R.; Robertson, A.; Kokko, E.; Byers, R.; Howard, R.J.; et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 6511–6515. [Google Scholar] [CrossRef]
- Rooney, H.C.E.; van ’t Klooster, J.W.; van der Hoorn, R.A.L.; Joosten, M.H.A.J.; Jones, J.D.G.; de Wit, P.J.G.M. Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2–dependent disease resistance. Science 2005, 308, 1783–1786. [Google Scholar] [CrossRef]
- Zhang, W.; Fraiture, M.; Kolb, D.; Loffelhardt, B.; Desaki, Y.; Boutrot, F.F.; Tor, M.; Zipfel, C.; Gust, A.A.; Brunner, F. Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell 2013, 25, 4227–4241. [Google Scholar] [CrossRef] [PubMed]
- Liebrand, T.W.H.; van den Burg, H.A.; Joosten, M.H.A.J. Two for all: Receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci. 2014, 19, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Liebrand, T.W.H.; van den Berg, G.C.M.; Zhang, Z.; Smit, P.; Cordewener, J.H.G.; America, A.H.P.; Sklenar, J.; Jones, A.M.E.; Tameling, W.I.L.; Robatzek, S.; et al. Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc. Natl. Acad. Sci. USA 2013, 110, 10010–10015. [Google Scholar] [CrossRef] [PubMed]
- Fradin, E.F.; Zhang, Z.; Ayala, J.C.J.; Castroverde, C.D.M.; Nazar, R.N.; Robb, J.; Liu, C.-M.; Thomma, B.P.H.J. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Garibaldi, A.; Gullino, M.L. Combined and single effects of elevated CO2 and temperatures on rice bakanae disease under controlled conditions in phytotrons. Plant Pathol. 2021, 70, 815–826. [Google Scholar] [CrossRef]
- Jahan, Q.S.A.; Sultana, Z.; Ud-Daula, M.A.; Ashikuzzaman, M.; Reja, M.S.; Rahman, M.M.; Khaton, A.; Tang, M.A.K.; Rahman, M.S.; Faruquee, H.M.; et al. Optimization of green silver nanoparticles as nanofungicides for management of rice bakanae disease. Heliyon 2024, 10, e27579. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.V.; Hechanova, S.L.; Hernandez, J.E.; Li, C.P.; Tulek, A.; Ahn, E.K.; Jairin, J.; Choi, I.R.; Sundaram, R.M.; Jena, K.K.; et al. Available cloned genes and markers for genetic improvement of biotic stress resistance in rice. Front. Plant Sci. 2023, 14, 1247014. [Google Scholar] [CrossRef] [PubMed]
- Alisaac, E.; Mahlein, A.-K. Fusarium Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.H.; Haas, M.; Huang, Y.; Tandukar, Z.; Muehlbauer, G.; Smith, K.P.; Steffenson, B.J. Meta-analysis of the genetics of resistance to Fusarium head blight and deoxynivalenol accumulation in barley and considerations for breeding. Plant Breed. 2024, 143, 2–25. [Google Scholar] [CrossRef]
- Hu, C.; Chen, P.; Zhou, X.; Li, Y.; Ma, K.; Li, S.; Liu, H.; Li, L. Arms Race between the Host and Pathogen Associated with Fusarium Head Blight of Wheat. Cells 2022, 11, 2275. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bae, S.C.; Kim, J.-S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Minkenberg, B.; Yang, Y. Targeted gene mutation in rice using CRISPR-Cas9 system. Bio-Protocol 2014, 4, e1225. [Google Scholar] [CrossRef]
- Hwang, I.S.; Kang, W.R.; Hwang, D.J.; Bae, S.C.; Yun, S.H.; Ahn, I.P. Evaluation of bakanae disease progression caused by Fusarium fujikuroi in Oryza sativa L. J. Microbiol. 2013, 51, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Song, G.; Im, J.H. OsWRKY7 contributes to pattern-triggered immunity against Xanthomonas oryzae pv. oryzae. Biochem. Biophys. Res. Commun. 2024, 700, 149568. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Cheon, K.-S.; Shin, Y.; Lee, C.; Son, S.; Oh, H.; Yoon, D.-K.; Lee, S.; Cho, M.; Jun, S.; et al. Map-Based Cloning and Characterization of a Major QTL Gene, FfR1, Which Confers Resistance to Rice Bakanae Disease. Int. J. Mol. Sci. 2024, 25, 6214. https://doi.org/10.3390/ijms25116214
Ji H, Cheon K-S, Shin Y, Lee C, Son S, Oh H, Yoon D-K, Lee S, Cho M, Jun S, et al. Map-Based Cloning and Characterization of a Major QTL Gene, FfR1, Which Confers Resistance to Rice Bakanae Disease. International Journal of Molecular Sciences. 2024; 25(11):6214. https://doi.org/10.3390/ijms25116214
Chicago/Turabian StyleJi, Hyeonso, Kyeong-Seong Cheon, Yunji Shin, Chaewon Lee, Seungmin Son, Hyoja Oh, Dong-Kyung Yoon, Seoyeon Lee, Mihyun Cho, Soojin Jun, and et al. 2024. "Map-Based Cloning and Characterization of a Major QTL Gene, FfR1, Which Confers Resistance to Rice Bakanae Disease" International Journal of Molecular Sciences 25, no. 11: 6214. https://doi.org/10.3390/ijms25116214
APA StyleJi, H., Cheon, K.-S., Shin, Y., Lee, C., Son, S., Oh, H., Yoon, D.-K., Lee, S., Cho, M., Jun, S., Lee, G.-S., Baek, J., Kim, S. L., Ahn, I.-P., Oh, J.-H., Yoon, H.-J., Cha, Y.-S., & Kim, K.-H. (2024). Map-Based Cloning and Characterization of a Major QTL Gene, FfR1, Which Confers Resistance to Rice Bakanae Disease. International Journal of Molecular Sciences, 25(11), 6214. https://doi.org/10.3390/ijms25116214






