Deleting Mitochondrial Superoxide Dismutase 2 in Salivary Gland Ductal Epithelial Cells Recapitulates Non-Sjögren’s Sicca Syndrome
Abstract
1. Introduction
2. Results
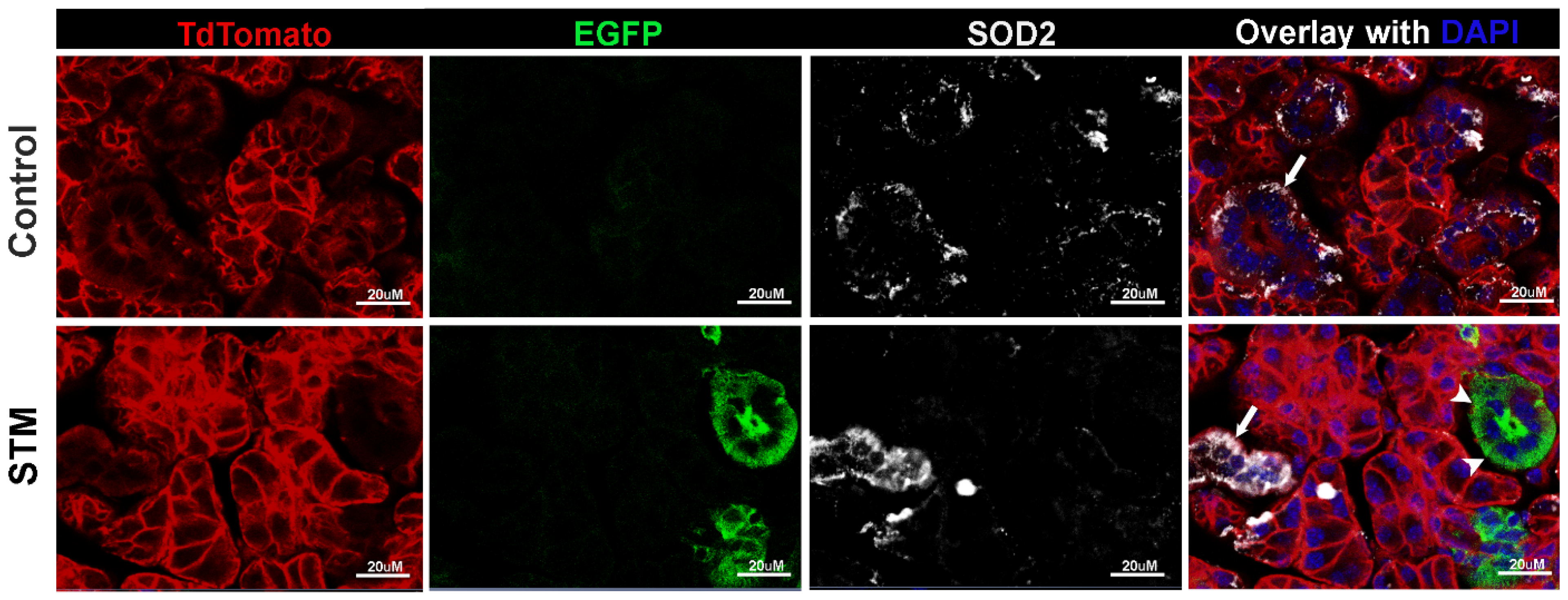
2.1. Sod2 Expression Is Reduced in Salivary Glands of i-sg-Sod2 KO Mice
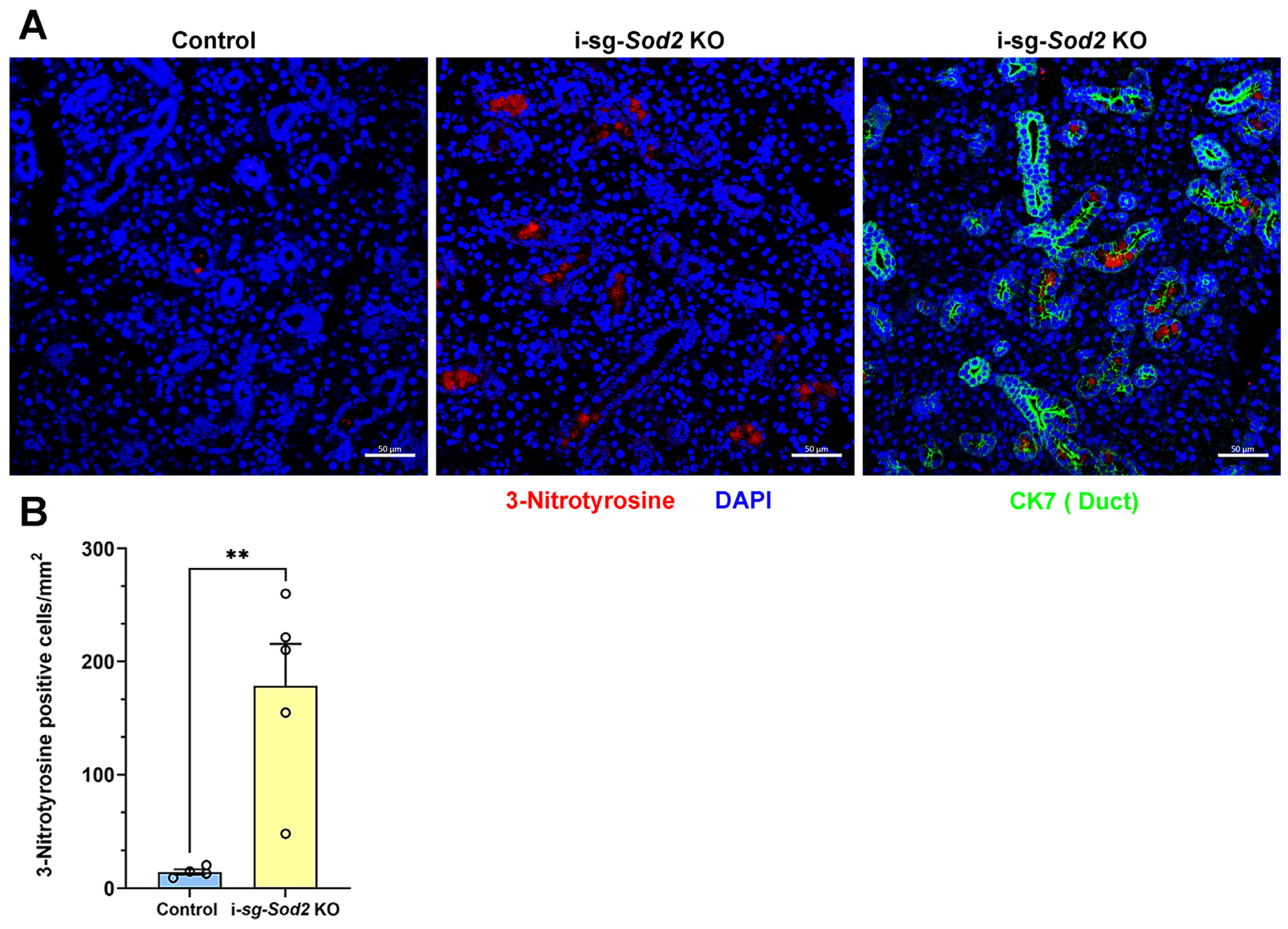
2.2. Deletion of Sod2 in the Ductal Epithelial Cells Leads to an Increase in Oxidative Stress
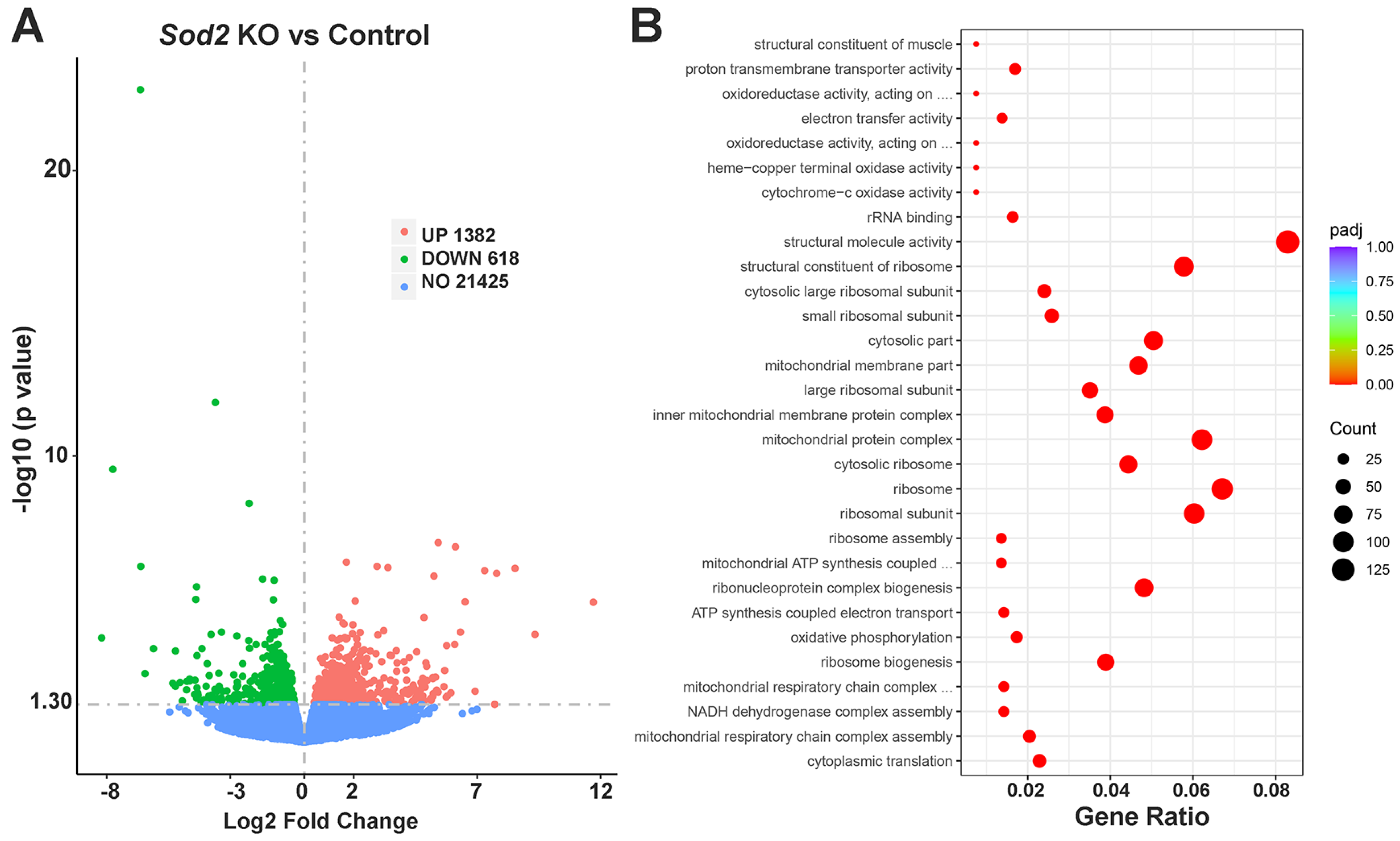
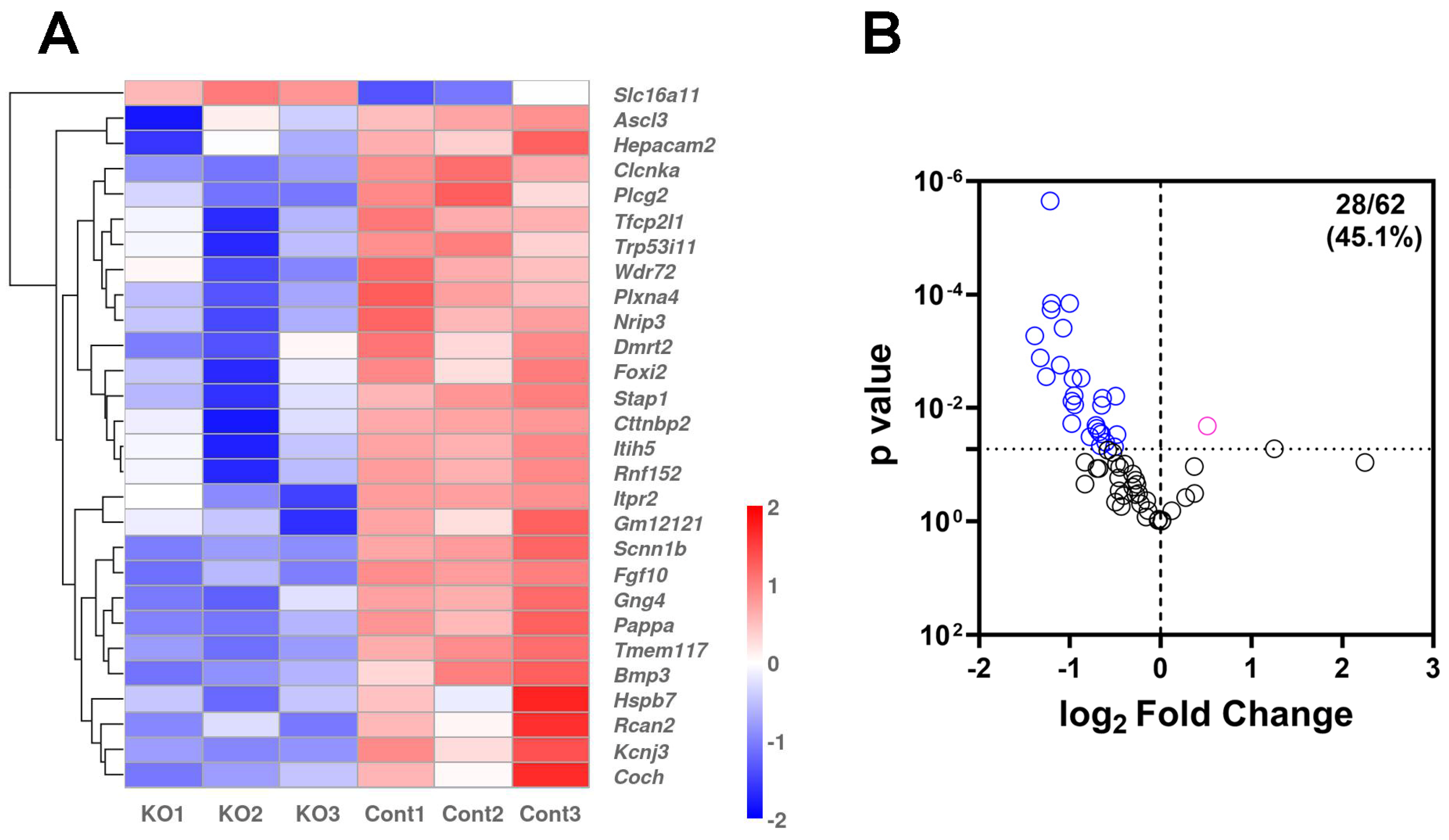
2.3. RNA-Seq Analysis of i-sg-Sod2 KO Salivary Glands Shows Significant Alterations in Gene Expression
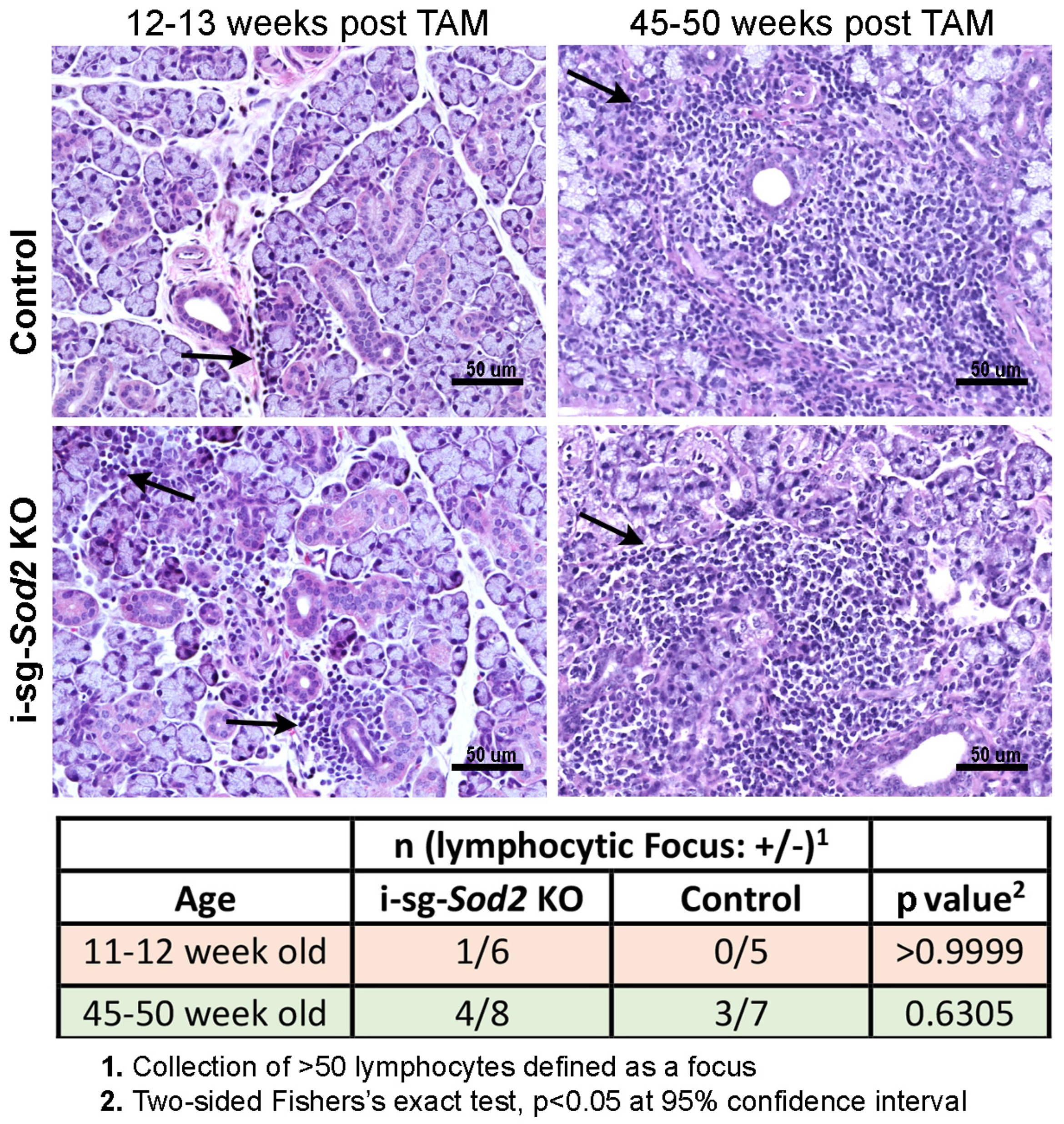
2.4. I-sg-Sod2 KO Mice Develop Salivary Gland Dysfunction without Significant Differences in Tissue Pathology
3. Discussion
4. Materials and Methods
4.1. Mouse Models
4.2. Generation of Sod2fl/fl; Tfcp2l1cre/ERT2 Mice
4.3. Generation of Sod2fl/fl; Tfcp2l1cre/ERT2; Rosa26R-mT/mG+/− Mice
4.4. Tamoxifen Treatment
4.5. Saliva Collection
4.6. Immunohistochemistry and Immunofluorescence
4.7. Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions and Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kreisel, D.; Goldstein, D.R. Processes of Sterile Inflammation. J. Immunol. 2013, 191, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef] [PubMed]
- Norheim, K.B.; Jonsson, G.; Harboe, E.; Hanasand, M.; Gøransson, L.; Omdal, R. Oxidative Stress, as Measured by Protein Oxidation, Is Increased in Primary Sjøgren’s Syndrome. Free Radic. Res. 2012, 46, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.N.; Gemcioglu, E.; Baser, S.; Erten, S.; Erel, O. Thiol/Disulfide Homeostasis Impaired in Patients with Primary Sjögren’s Syndrome. J. Med. Biochem. 2021, 40, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, C.; Kawano, S.; Tsuji, G.; Hatachi, S.; Jikimoto, T.; Sugiyama, D.; Kasagi, S.; Komori, T.; Nakamura, H.; Yodoi, J.; et al. Thioredoxin May Exert a Protective Effect against Tissue Damage Caused by Oxidative Stress in Salivary Glands of Patients with Sjögren’s Syndrome. J. Rheumatol. 2007, 34, 2035–2043. [Google Scholar]
- Ryo, K.; Yamada, H.; Nakagawa, Y.; Tai, Y.; Obara, K.; Inoue, H.; Mishima, K.; Saito, I. Possible Involvement of Oxidative Stress in Salivary Gland of Patients with Sjogren’s Syndrome. Pathobiology 2006, 73, 252–260. [Google Scholar] [CrossRef]
- Wakamatsu, T.H.; Dogru, M.; Matsumoto, Y.; Kojima, T.; Kaido, M.; Ibrahim, O.M.A.; Sato, E.A.; Igarashi, A.; Ichihashi, Y.; Satake, Y.; et al. Evaluation of Lipid Oxidative Stress Status in Sjögren Syndrome Patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 201–210. [Google Scholar] [CrossRef]
- Cejková, J.; Ardan, T.; Jirsová, K.; Jechová, G.; Malec, J.; Simonová, Z.; Cejka, C.; Filipec, M.; Dotrelová, D.; Brunová, B. The Role of Conjunctival Epithelial Cell Xanthine Oxidoreductase/Xanthine Oxidase in Oxidative Reactions on the Ocular Surface of Dry Eye Patients with Sjögren’s Syndrome. Histol. Histopathol. 2007, 22, 997–1003. [Google Scholar] [CrossRef]
- Cejková, J.; Ardan, T.; Simonová, Z.; Cejka, C.; Malec, J.; Jirsová, K.; Filipec, M.; Dotrelová, D.; Brůnová, B. Nitric Oxide Synthase Induction and Cytotoxic Nitrogen-Related Oxidant Formation in Conjunctival Epithelium of Dry Eye (Sjögren’s Syndrome). Nitric Oxide 2007, 17, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cejková, J.; Ardan, T.; Simonová, Z.; Cejka, C.; Malec, J.; Dotrelová, D.; Brunová, B. Decreased Expression of Antioxidant Enzymes in the Conjunctival Epithelium of Dry Eye (Sjögren’s Syndrome) and Its Possible Contribution to the Development of Ocular Surface Oxidative Injuries. Histol. Histopathol. 2008, 23, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Baldini, C.; Bootsma, H.; Bowman, S.J.; Jonsson, R.; Mariette, X.; Sivils, K.; Theander, E.; Tzioufas, A.; Ramos-Casals, M. Sjögren Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16047. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.E.; Cox, D.; Shiboski, C.H.; Schiødt, M.; Wu, A.; Lanfranchi, H.; Umehara, H.; Zhao, Y.; Challacombe, S.; Lam, M.Y.; et al. Associations between Salivary Gland Histopathologic Diagnoses and Phenotypic Features of Sjögren’s Syndrome among 1,726 Registry Participants. Arthritis Rheum. 2011, 63, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Price, E.J.; Venables, P.J.W. Dry Eyes and Mouth Syndrome—A Subgroup of Patients Presenting with Sicca Symptoms. Rheumatology 2002, 41, 416–422. [Google Scholar] [CrossRef]
- Pucino, V.; Turner, J.D.; Nayar, S.; Kollert, F.; Rauz, S.; Richards, A.; Higham, J.; Poveda-Gallego, A.; Bowman, S.J.; Barone, F.; et al. Sjögren’s and Non-Sjögren’s Sicca Share a Similar Symptom Burden but with a Distinct Symptom-Associated Proteomic Signature. RMD Open 2022, 8, e002119. [Google Scholar] [CrossRef]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef]
- Garrett, J.R. The Proper Role of Nerves in Salivary Secretion: A Review. J. Dent. Res. 1987, 66, 387–397. [Google Scholar] [CrossRef]
- Proctor, G.B.; Carpenter, G.H. Salivary Secretion: Mechanism and Neural Regulation. Monogr. Oral. Sci. 2014, 24, 14–29. [Google Scholar] [CrossRef]
- Kim, Y.-J. Xerostomia and Its Cellular Targets. Int. J. Mol. Sci. 2023, 24, 5358. [Google Scholar] [CrossRef] [PubMed]
- Sangalli, L.; Eldomiaty, W.; Miller, C.S. Xerogenic Medications May Contribute to Decreased Unstimulated Salivary Flow in Patients with Oral Burning and/or Gastro-Esophageal Reflux Disease. Front. Dent. Med. 2023, 4, 1047235. [Google Scholar] [CrossRef]
- Ikegami, T.; Suzuki, Y.; Shimizu, T.; Isono, K.; Koseki, H.; Shirasawa, T. Model Mice for Tissue-Specific Deletion of the Manganese Superoxide Dismutase (MnSOD) Gene. Biochem. Biophys. Res. Commun. 2002, 296, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, E.O.; Aure, M.H.; Xie, X.; Myal, Y.; Gan, L.; Ovitt, C.E. Cell-Specific Cre Strains For Genetic Manipulation in Salivary Glands. PLoS ONE 2016, 11, e0146711. [Google Scholar] [CrossRef]
- Nojima, K.; Miyazaki, H.; Hori, T.; Vargova, L.; Oohashi, T. Assessment of Possible Contributions of Hyaluronan and Proteoglycan Binding Link Protein 4 to Differential Perineuronal Net Formation at the Calyx of Held. Front. Cell Dev. Biol. 2021, 9, 730550. [Google Scholar] [CrossRef] [PubMed]
- Shunmoogam, N.; Naidoo, P.; Chilton, R. Paraoxonase (PON)-1: A Brief Overview on Genetics, Structure, Polymorphisms and Clinical Relevance. Vasc. Health Risk Manag. 2018, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Culp, D.J.; Latchney, L.R.; Fallon, M.A.; Denny, P.A.; Denny, P.C.; Couwenhoven, R.I.; Chuang, S. The Gene Encoding Mouse Muc19: cDNA, Genomic Organization and Relationship to Smgc. Physiol. Genom. 2004, 19, 303–318. [Google Scholar] [CrossRef]
- Das, B.; Cash, M.N.; Hand, A.R.; Shivazad, A.; Culp, D.J. Expression of Muc19/Smgc Gene Products during Murine Sublingual Gland Development: Cytodifferentiation and Maturation of Salivary Mucous Cells. J. Histochem. Cytochem. 2009, 57, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Cash, M.N.; Hand, A.R.; Shivazad, A.; Grieshaber, S.S.; Robinson, B.; Culp, D.J. Tissue Distibution of Murine Muc19/Smgc Gene Products. J. Histochem. Cytochem. 2010, 58, 141–156. [Google Scholar] [CrossRef]
- Nakata, H.; Terakawa, J.; Horike, S.-I.; Daikoku, T.; Iseki, S. The Lack of Terminal Tubule Cells in the Submandibular Gland of Mice Deficient in Submandibular Gland Protein C. Cell Tissue Res. 2020, 381, 229–237. [Google Scholar] [CrossRef]
- NIDCR Salivary Gland Atlas. Available online: https://sgmap.nidcr.nih.gov/sgmap/sgexp.html (accessed on 5 April 2024).
- Rugel-Stahl, A.; Elliott, M.E.; Ovitt, C.E. Ascl3 Marks Adult Progenitor Cells of the Mouse Salivary Gland. Stem Cell Res. 2012, 8, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mauduit, O.; Aure, M.H.; Delcroix, V.; Basova, L.; Srivastava, A.; Umazume, T.; Mays, J.W.; Bellusci, S.; Tucker, A.S.; Hajihosseini, M.K.; et al. A Mesenchymal to Epithelial Switch in Fgf10 Expression Specifies an Evolutionary-Conserved Population of Ionocytes in Salivary Glands. Cell Rep. 2022, 39, 110663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nojiri, H.; Shirasawa, T. Tissue-Specific Deletion of Manganese Superoxide Dismutase (Mn-SOD) in Mice. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 475–487. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Carlson, E.; Melov, S.; Ursell, P.; Olson, T.; Noble, L.; Yoshimura, M.; Berger, C.; Chan, P.; et al. Dilated Cardiomyopathy and Neonatal Lethality in Mutant Mice Lacking Manganese Superoxide-Dismutase. Nat. Genet. 1995, 11, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Lynn, S.; Huang, E.J.; Elchuri, S.; Naeemuddin, M.; Nishinaka, Y.; Yodoi, J.; Ferriero, D.M.; Epstein, C.J.; Huang, T.-T. Selective Neuronal Vulnerability and Inadequate Stress Response in Superoxide Dismutase Mutant Mice. Free Radic. Biol. Med. 2005, 38, 817–828. [Google Scholar] [CrossRef]
- Zhuang, A.; Yang, C.; Liu, Y.; Tan, Y.; Bond, S.T.; Walker, S.; Sikora, T.; Laskowski, A.; Sharma, A.; de Haan, J.B.; et al. SOD2 in Skeletal Muscle: New Insights from an Inducible Deletion Model. Redox Biol. 2021, 47, 102135. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Irigoyen, O.; Bovenga, F.; Piglionica, M.; Piccinin, E.; Cariello, M.; Arconzo, M.; Peres, C.; Corsetto, P.A.; Rizzo, A.M.; Ballanti, M.; et al. Enterocyte Superoxide Dismutase 2 Deletion Drives Obesity. iScience 2022, 25, 103707. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Kumar, G.; Thadathil, N.; Piekarz, K.M.; Mohammed, S.; Lopez, S.D.; Qaisar, R.; Walton, D.; Brown, J.L.; Murphy, A.; et al. Neuronal Deletion of MnSOD in Mice Leads to Demyelination, Inflammation and Progressive Paralysis That Mimics Phenotypes Associated with Progressive Multiple Sclerosis. Redox Biol. 2023, 59, 102550. [Google Scholar] [CrossRef]
- Papinska, J.; Bagavant, H.; Gmyrek, G.B.; Sroka, M.; Tummala, S.; Fitzgerald, K.A.; Deshmukh, U.S. Activation of Stimulator of Interferon Genes (STING) and Sjögren Syndrome. J. Dent. Res. 2018, 97, 893–900. [Google Scholar] [CrossRef]
- Wahl, A.M.; Takano, T.; Su, S.; Warner, B.M.; Perez, P.; Sneyd, J.; Yule, D.I. Structural and Functional Analysis of Salivary Intercalated Duct Cells Reveals a Secretory Phenotype. J. Physiol. 2023, 601, 4539–4556. [Google Scholar] [CrossRef]
- Tajiri, A.; Higuchi, H.; Miyawaki, T. Hyperoxia Reduces Salivary Secretion by Inducing Oxidative Stress in Mice. Arch. Oral. Biol. 2019, 98, 38–46. [Google Scholar] [CrossRef]
- Bagavant, H.; Durslewicz, J.; Pyclik, M.; Makuch, M.; Papinska, J.A.; Deshmukh, U.S. Age-Associated B Cell Infiltration in Salivary Glands Represents a Hallmark of Sjögren’s-like Disease in Aging Mice. GeroScience 2024. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 ACR-EULAR Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.R.; Motta, A.C.F.; Módulo, C.M.; Garcia, D.M.; Chiorini, J.A.; Louzada-Junior, P.; Rocha, E.M. Clinical and Laboratory Evaluation of Sicca Complaints: Distinctive Aspects of Primary, Secondary and Non-Sjogren Syndrome. Adv. Rheumatol. 2022, 62, 23. [Google Scholar] [CrossRef] [PubMed]
- Tashbayev, B.; Garen, T.; Palm, Ø.; Chen, X.; Herlofson, B.B.; Young, A.; Hove, L.H.; Rykke, M.; Singh, P.B.; Aqrawi, L.A.; et al. Patients with Non-Sjögren’s Sicca Report Poorer General and Oral Health-Related Quality of Life than Patients with Sjögren’s Syndrome: A Cross-Sectional Study. Sci. Rep. 2020, 10, 2063. [Google Scholar] [CrossRef]
- Charras, A.; Arvaniti, P.; Le Dantec, C.; Dalekos, G.N.; Zachou, K.; Bordron, A.; Renaudineau, Y. JAK Inhibitors and Oxidative Stress Control. Front. Immunol. 2019, 10, 2814. [Google Scholar] [CrossRef]
- Luzzi, R.; Belcaro, G.; Hu, S.; Feragalli, B.; Hosoi, M.; Dugall, M.; Ledda, A. Efficacy of Pycnogenol® Supplementation in Remission Phases of Sjögren Syndrome. Minerva Cardioangiol. 2018, 66, 543–546. [Google Scholar] [CrossRef]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A Global Double-Fluorescent Cre Reporter Mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef]
- Bagavant, H.; Trzeciak, M.; Papinska, J.; Biswas, I.; Dunkleberger, M.L.; Sosnowska, A.; Deshmukh, U.S. A Method for the Measurement of Salivary Gland Function in Mice. J. Vis. Exp. 2018, 131, 57203. [Google Scholar] [CrossRef]
- Papinska, J.; Bagavant, H.; Gmyrek, G.B.; Deshmukh, U.S. Pulmonary Involvement in a Mouse Model of Sjögren’s Syndrome Induced by STING Activation. Int. J. Mol. Sci. 2020, 21, 4512. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Nandula, S.-R.; Scindia, Y.M.; Dey, P.; Bagavant, H.; Deshmukh, U.S. Activation of Innate Immunity Accelerates Sialoadenitis in a Mouse Model for Sjögren’s Syndrome-like Disease. Oral Dis. 2011, 17, 801–807. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papinska, J.A.; Durślewicz, J.; Bagavant, H.; Deshmukh, U.S. Deleting Mitochondrial Superoxide Dismutase 2 in Salivary Gland Ductal Epithelial Cells Recapitulates Non-Sjögren’s Sicca Syndrome. Int. J. Mol. Sci. 2024, 25, 5983. https://doi.org/10.3390/ijms25115983
Papinska JA, Durślewicz J, Bagavant H, Deshmukh US. Deleting Mitochondrial Superoxide Dismutase 2 in Salivary Gland Ductal Epithelial Cells Recapitulates Non-Sjögren’s Sicca Syndrome. International Journal of Molecular Sciences. 2024; 25(11):5983. https://doi.org/10.3390/ijms25115983
Chicago/Turabian StylePapinska, Joanna A., Justyna Durślewicz, Harini Bagavant, and Umesh S. Deshmukh. 2024. "Deleting Mitochondrial Superoxide Dismutase 2 in Salivary Gland Ductal Epithelial Cells Recapitulates Non-Sjögren’s Sicca Syndrome" International Journal of Molecular Sciences 25, no. 11: 5983. https://doi.org/10.3390/ijms25115983
APA StylePapinska, J. A., Durślewicz, J., Bagavant, H., & Deshmukh, U. S. (2024). Deleting Mitochondrial Superoxide Dismutase 2 in Salivary Gland Ductal Epithelial Cells Recapitulates Non-Sjögren’s Sicca Syndrome. International Journal of Molecular Sciences, 25(11), 5983. https://doi.org/10.3390/ijms25115983






