B-Cell Activation Biomarkers in Salivary Glands Are Related to Lymphomagenesis in Primary Sjögren’s Disease: A Pilot Monocentric Exploratory Study
Abstract
1. Introduction
2. Results
2.1. Baseline Demographic and Clinical Characteristics of Primary Sjögren’s Disease and Sicca Disease Cohorts
2.2. Primary Sjögren’s Disease and Sicca Disease Patients Showed Different Histological Features in Terms of Labial Salivary Glands’ CD20pos and CD138pos Cell Enrichment
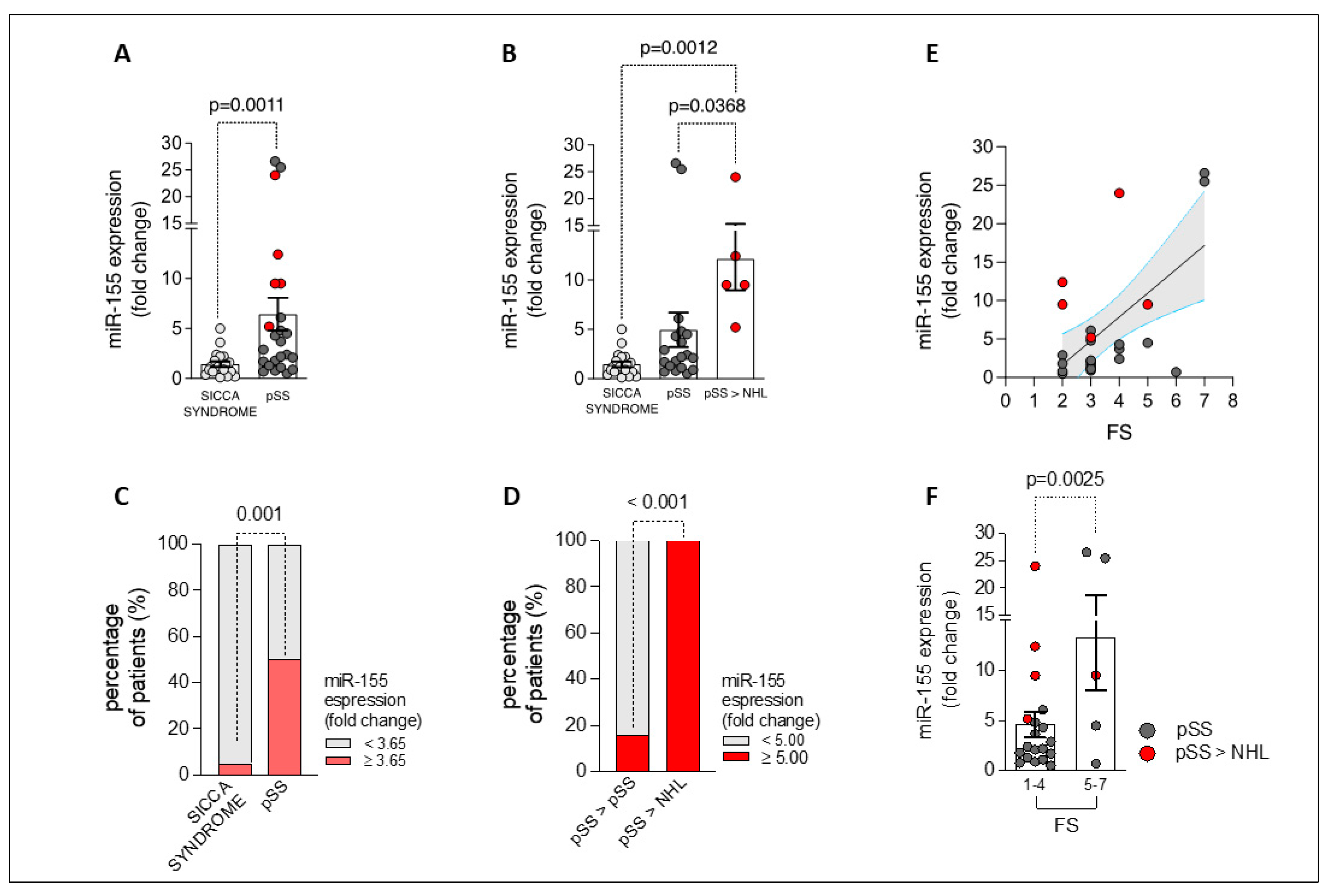
2.3. miR-155 Is Overexpressed in the Labial Salivary Glands of Patients with Primary Sjögren’s Disease, Being Related to Lymphomagenesis and Degree of Focal Inflammation
2.4. BAFF-R and IL-6R Expression Are Upregulated in Labial Salivary Glands of Patients with Primary Sjögren’s Disease, Being Related to NHL Development and Directly Correlating with miR-155 Levels
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Minor Salivary Glands Biopsy
4.3. Clinical and Immunological Assessment of Patient Cohorts
4.4. Immunohistochemistry of CD20 and CD138 in Labial Salivary Gland Tissue
4.5. miR-155, BAFF-R, and IL-6R Expression in Labial Salivary Gland Biopsy by qPCR
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebastian, A.; Szachowicz, A.; Wiland, P. Classification criteria for secondary Sjögren’s disease. Curr. State Knowl. Reumatol. 2019, 57, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Carsons, S.E.; Patel, B.C. Sjogren Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vitali, C.; Dolcino, M.; Del Papa, N.; Minniti, A.; Pignataro, F.; Maglione, W.; Lunardi, C.; Puccetti, A. Gene Expression Profiles in Primary Sjögren’s Disease With and Without Systemic Manifestations. ACR Open Rheumatol. 2019, 1, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Goules, A.V.; Tzioufas, A.G. Primary Sjögren’s disease: Clinical phenotypes, outcome and the development of biomarkers. Autoimmun. Rev. 2016, 15, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. Sjögren Disease-associated lymphomas: An update on pathogenesis and management. Br. J. Haematol. 2015, 168, 317–327. [Google Scholar] [CrossRef]
- Zhan, Q.; Zhang, J.; Lin, Y.; Chen, W.; Fan, X.; Zhang, D. Pathogenesis and treatment of Sjogren’s disease: Review and update. Front. Immunol. 2023, 14, 1127417. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Silina, K.; van den Broek, M.; Hirahara, K.; Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 2023, 19, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Manfrè, V.; Chatzis, L.G.; Cafaro, G.; Fonzetti, S.; Calvacchi, S.; Fulvio, G.; Garcia, I.C.N.; La Rocca, G.; Ferro, F.; Perricone, C.; et al. Sjögren’s disease: One year in review 2022. Clin. Exp. Rheumatol. 2022, 40, 2211–2224. [Google Scholar] [PubMed]
- Alivernini, S.; Kurowska-Stolarska, M.; Tolusso, B.; Benvenuto, R.; Elmesmari, A.; Canestri, S.; Petricca, L.; Mangoni, A.; Fedele, A.L.; Di Mario, C.; et al. MicroRNA-155 influences B-cell function through PU.1 in rheumatoid arthritis. Nat. Commun. 2016, 7, 12970. [Google Scholar] [CrossRef]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155-at the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2018, 8, 1932. [Google Scholar] [CrossRef]
- Pauley, K.M.; Stewart, C.M.; Gauna, A.E.; Dupre, L.C.; Kuklani, R.; Chan, A.L.; Pauley, B.A.; Reeves, W.H.; Chan, E.K.L.; Cha, S. Altered miR-146a expression in Sjögren’s disease and its functional role in innate immunity. Eur. J. Immunol. 2011, 41, 2029–2039. [Google Scholar] [CrossRef]
- Kamounah, S.; Sembler-Møller, M.L.; Nielsen, C.H.; Pedersen, A.M.L. Sjögren’s disease: Novel insights from proteomics and miRNA expression analysis. Front. Immunol. 2023, 14, 1183195. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Mona, M.; Lee, K.E.; Kim, D.H.; Han, K. MicroRNAs in Autoimmune Sjögren’s Disease. Genom. Inform. 2018, 16, e19. [Google Scholar] [CrossRef] [PubMed]
- Bedewy, A.M.L.; Elmaghraby, S.M.; Shehata, A.A.; Kandil, N.S. Prognostic Value of miRNA-155 Expression in B-Cell Non-Hodgkin Lymphoma. Turk. J. Hematol. 2017, 34, 207–212. [Google Scholar]
- Shabgah, A.G.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. The role of BAFF and APRIL in rheumatoid arthritis. J. Cell Physiol. 2019, 234, 17050–17063. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Morand, E.F.; Schneider, P.; Mackay, F. The BAFF/APRIL system in SLE pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Kuley, R.; Draves, K.E.; Elkon, K.B.; Giltiay, N.V.; Clark, E.A. B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease. Front. Immunol. 2023, 14, 1050528. [Google Scholar] [CrossRef] [PubMed]
- Felten, R.; Devauchelle-Pensec, V.; Seror, R.; Duffau, P.; Saadoun, D.; Hachulla, E.; Yves, H.P.; Salliot, C.; Perdriger, A.; Morel, J.; et al. Interleukin 6 receptor inhibition in primary Sjögren syndrome: A multicentre double-blind randomised placebo-controlled trial. Ann. Rheum. Dis. 2021, 80, 329–338. [Google Scholar] [CrossRef]
- Gong, Y.Z.; Nititham, J.; Taylor, K.; Miceli-Richard, C.; Sordet, C.; Wachsmann, D.; Bahram, S.; Georgel, P.; Criswell, L.A.; Sibilia, J.; et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren’s syndrome. J. Autoimmun. 2014, 51, 57–66. [Google Scholar] [CrossRef]
- Navarro-Mendoza, E.P.; Aguirre-Valencia, D.; Posso-Osorio, I.; Correa-Forero, S.V.; Torres-Cutiva, D.F.; Loaiza, D.; Tobón, G.J. Cytokine markers of B lymphocytes in minor salivary gland infiltrates in Sjögren’s syndrome. Autoimmun. Rev. 2018, 17, 709–714. [Google Scholar] [CrossRef]
- De Vita, S.; Gandolfo, S. Predicting lymphoma development in patients with Sjögren’s disease. Expert. Rev. Clin. Immunol. 2019, 15, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yuan, F. Hypocomplementemia in Primary Sjogren’s Syndrome: A Retrospective Study of 120 Treatment-Naive Chinese Patients. Int. J. Gen. Med. 2022, 15, 359–366. [Google Scholar] [CrossRef]
- Traianos, E.Y.; Locke, J.; Lendrem, D.; Bowman, S.; Hargreaves, B.; Macrae, V.; UK Primary Sjögren’s Syndrome Registry; Tarn, J.R.; Ng, W.-F. Serum CXCL13 levels are associated with lymphoma risk and lymphoma occurrence in primary Sjögren’s disease. Rheumatol. Int. 2020, 40, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Le Dantec, C.; Varin, M.M.; Brooks, W.H.; Pers, J.O.; Youinou, P.; Renaudineau, Y. Epigenetics and Sjögren’s disease. Curr. Pharm. Biotechnol. 2012, 13, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius, G.E.; Björk, A.; Wahren-Herlenius, M. Genetics and epigenetics of primary Sjögren disease: Implications for future therapies. Nat. Rev. Rheumatol. 2023, 19, 288–306. [Google Scholar] [CrossRef]
- Kapsogeorgou, E.K.; Tzioufas, A.G. Interaction of Human Salivary Gland Epithelial Cells with B Lymphocytes: Implications in the Pathogenesis of Sjögren’s Disease. Mediterr. J. Rheumatol. 2020, 31, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Han, M.; Zhu, X.; Xiao, F.; Huang, E.; Che, N.; Tang, X.; Zou, H.; Jiang, Q.; Lu, L. The Multiple Roles of B Cells in the Pathogenesis of Sjögren’s Disease. Front. Immunol. 2021, 12, 684999. [Google Scholar] [CrossRef] [PubMed]
- Wang-Renault, S.F.; Boudaoud, S.; Nocturne, G.; Roche, E.; Sigrist, N.; Daviaud, C.; Tinggaard, A.B.; Renault, V.; Deleuze, J.-F.; Mariette, X.; et al. Deregulation of microRNA expression in purified T and B lymphocytes from patients with primary Sjögren’s disease. Ann. Rheum. Dis. 2018, 77, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, L.Y.; Zhang, P.; Yu, C.Q. miR-146a and miR-155 expression in PBMCs from patients with Sjögren’s syndrome. J. Oral Pathol. Med. 2014, 43, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Papp, G.; Póliska, S.; Szabó, K.; Tarr, T.; Bálint, B.L.; Szodoray, P.; Zeher, M. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s disease. PLoS ONE 2017, 12, e0174585. [Google Scholar] [CrossRef]
- Ibrahem, H.M. B cell dysregulation in primary Sjögren’s disease: A review. Jpn. Dent. Sci. Rev. 2019, 55, 139–144. [Google Scholar] [CrossRef]
- Cornec, D.; Devauchelle-Pensec, V.; Tobón, G.J.; Pers, J.O.; Jousse-Joulin, S.; Saraux, A. B cells in Sjögren’s disease: From pathophysiology to diagnosis and treatment. J. Autoimmun. 2012, 39, 161–167. [Google Scholar] [CrossRef]
- Della Bella, C.; Soluri, M.F.; Puccio, S.; Benagiano, M.; Grassi, A.; Bitetti, J.; Cianchi, F.; Sblattero, D.; Peano, C.; D’elios, M.M. The Helicobacter pylori CagY Protein Drives Gastric Th1 and Th17 Inflammation and B Cell Proliferation in Gastric MALT Lymphoma. Int. J. Mol. Sci. 2021, 22, 9459. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-H.; Yeh, K.-H.; Chen, L.-T.; Lin, C.-W.; Hsu, P.-N.; Hsu, C.; Wu, M.-S.; Tzeng, Y.-S.; Tsai, H.-J.; Wang, H.-P.; et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: A distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014, 4, e220. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Karanti, S.; Jung, I.; Dahia, P.L.M.; Aguiar, R.C.T. Coordinated Expression of MicroRNA-155 and Predicted Target Genes in Diffuse Large B-cell Lymphoma. Cancer Genet. Cytogenet. 2008, 181, 8. [Google Scholar] [CrossRef]
- Rai, D.; Kim, S.W.; McKeller, M.R.; Dahia, P.L.M.; Aguiar, R.C.T. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3111–3116. [Google Scholar] [CrossRef]
- Zhu, F.-Q.; Zeng, L.; Tang, N.; Tang, Y.-P.; Zhou, B.-P.; Li, F.-F.; Wu, W.-G.; Zeng, X.-B.; Peng, S.-S. MicroRNA-155 Downregulation Promotes Cell Cycle Arrest and Apoptosis in Diffuse Large B-Cell Lymphoma. Oncol. Res. 2016, 24, 415–427. [Google Scholar] [CrossRef]
- Mackay, F.; Browning, J.L. BAFF: A fundamental survival factor for B cells. Nat. Rev. Immunol. 2002, 2, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ballesteros, F.J.; Palafox-Sánchez, C.A.; Franco-Topete, R.A.; Muñoz-Valle, J.F.; Orozco-Barocio, G.; Martínez-Bonilla, G.E.; Gómez-López, C.E.; Marín-Rosales, M.; López-Villalobos, E.F.; Luquin, S.; et al. Expression of BAFF and BAFF receptors in primary Sjögren’s disease patients with ectopic germinal center-like structures. Clin. Exp. Med. 2020, 20, 615–626. [Google Scholar] [CrossRef]
- Varin, M.M.; Le Pottier, L.; Youinou, P.; Saulep, D.; Mackay, F.; Pers, J.O. B-cell tolerance breakdown in Sjögren’s disease: Focus on BAFF. Autoimmun. Rev. 2010, 9, 604–608. [Google Scholar] [CrossRef]
- Groom, J.; Kalled, S.L.; Cutler, A.H.; Olson, C.; Woodcock, S.A.; Schneider, P.; Tschopp, J.; Cachero, T.G.; Batten, M.; Wheway, J.; et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s disease. J. Clin. Investig. 2002, 109, 59–68. [Google Scholar] [CrossRef]
- Chen, M.; Lin, X.; Liu, Y.; Li, Q.; Deng, Y.; Liu, Z.; Brand, D.; Guo, Z.; He, X.; Ryffel, B.; et al. The function of BAFF on T helper cells in autoimmunity. Cytokine Growth Factor Rev. 2014, 25, 301–305. [Google Scholar] [CrossRef]
- Smulski, C.R.; Eibel, H. BAFF and BAFF-Receptor in B Cell Selection and Survival. Front. Immunol. 2018, 9, 2285. [Google Scholar] [CrossRef]
- Kapsogeorgou, E.K.; Voulgarelis, M.; Tzioufas, A.G. Predictive markers of lymphomagenesis in Sjögren’s disease: From clinical data to molecular stratification. J. Autoimmun. 2019, 104, 102316. [Google Scholar] [CrossRef] [PubMed]
- Kapsogeorgou, E.K.; Papageorgiou, A.; Protogerou, A.D.; Voulgarelis, M.; Tzioufas, A.G. Low miR200b-5p levels in minor salivary glands: A novel molecular marker predicting lymphoma development in patients with Sjögren’s disease. Ann. Rheum. Dis. 2018, 77, 1200–1207. [Google Scholar] [CrossRef]
- Ittah, M.; Miceli-Richard, C.; Eric Gottenberg, J.; Lavie, F.; Lazure, T.; Ba, N.; Sellam, J.; Lepajolec, C.; Mariette, X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren’s disease. Arthritis Res. Ther. 2006, 8, R51. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial-immune cell interplay in primary Sjögren disease salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, J.Y.; Xu, W. Role of BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit. Rev. Oncol. Hematol. 2014, 91, 113–122. [Google Scholar] [CrossRef]
- Takahata, H.; Ohara, N.; Ichimura, K.; Tanaka, T.; Sato, Y.; Morito, T.; Takata, K.; Kojima, M.; Kobata, T.; Yoshino, T. BAFF-R is expressed on B-cell lymphomas depending on their origin, and is related to proliferation index of nodal diffuse large B-cell lymphomas. J. Clin. Exp. Hematop. 2010, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, T.; Metsärinne, K.; Teppo, A.M.; Fyhrquist, F. Immunoreactive interleukin-6 in serum of patients with B-lymphoproliferative diseases. J. Intern. Med. 1992, 232, 439–442. [Google Scholar] [CrossRef]
- Grisius, M.M.; Bermudez, D.K.; Fox, P.C. Salivary and serum interleukin 6 in primary Sjögren’s syndrome. J. Rheumatol. 1997, 24, 1089–1091. [Google Scholar]
- Tishler, M.; Yaron, I.; Geyer, O.; Shirazi, I.; Naftaliev, E.; Yaron, M. Elevated tear interleukin-6 levels in patients with Sjögren syndrome. Ophthalmology 1998, 105, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Zhou, D.; Zhu, L.; Qian, W.; Ye, X. Increased serum level of interleukin-6 correlates with negative prognostic factors in extranodal NK/T-cell lymphoma. Transl. Cancer Res. 2020, 9, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, W.S.; Park, C. Interleukin-6 mediates resistance to PI3K-pathway-targeted therapy in lymphoma. BMC Cancer 2019, 19, 936. [Google Scholar] [CrossRef] [PubMed]
- Hashwah, H.; Bertram, K.; Stirm, K.; Stelling, A.; Wu, C.T.; Kasser, S.; Manz, M.G.; Theocharides, A.P.; Tzankov, A.; Müller, A. The IL-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol. Med. 2019, 11, e10576. [Google Scholar] [CrossRef]
- Burger, R. Impact of interleukin-6 in hematological malignancies. Transfus. Med. Hemother. 2013, 40, 336–343. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjögren’s disease: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, S.; Shiboski, C.; Criswell, L.; Baer, A.; Challacombe, S.; Lanfranchi, H.; Schiødt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. American College of Rheumatology Classification Criteria for Sjögren’s Disease: A Data-Driven, Expert Consensus Approach in the SICCA Cohort. Arthritis Care Res. 2012, 64, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Disease: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Keogh, R.H.; Cox, D.R. Case-Control Studies. In Institute of Mathematical Statistics Monographs; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Daniels, T.E. Labial salivary gland biopsy in Sjögren’s disease. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984, 27, 147–156. [Google Scholar] [CrossRef]
- Greenspan, J.S.; Daniels, T.E.; Talal, N.; Sylvester, R.A. The histopathology of Sjögren’s disease in labial salivary gland biopsies. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 217–229. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Sisó-Almirall, A.; Bosch, X.; Tzioufas, A.G. Topical and systemic medications for the treatment of primary Sjögren’s disease. Nat. Rev. Rheumatol. 2012, 8, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Tzioufas, A.G.; Stone, J.H.; Sisó, A.; Bosch, X. Treatment of primary Sjögren disease: A systematic review. JAMA 2010, 304, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Vivino, F.B.; Carsons, S.E.; Foulks, G.; Daniels, T.E.; Parke, A.; Brennan, M.T.; Forstot, S.L.; Scofield, R.H.; Hammitt, K.M. New Treatment Guidelines for Sjögren’s Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Ravaud, P.; Bowman, S.J.; Baron, G.; Tzioufas, A.; Theander, E.; Gottenberg, J.-E.; Bootsma, H.; Mariette, X.; Vitali, C.; et al. EULAR Sjogren’s disease disease activity index: Development of a consensus systemic disease activity index for primary Sjogren’s disease. Ann. Rheum. Dis. 2010, 69, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Bootsma, H.; Saraux, A.; Bowman, S.J.; Theander, E.; Brun, J.G.; Baron, G.; Le Guern, V.; Devauchelle-Pensec, V.; Ramos-Casals, M.; et al. Defining disease activity states and clinically meaningful improvement in primary Sjögren’s disease with EULAR primary Sjögren’s disease disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann. Rheum. Dis. 2016, 75, 382–389. [Google Scholar] [CrossRef]
- Alivernini, S.; Tolusso, B.; Petricca, L.; Bui, L.; Di Sante, G.; Peluso, G.; Benvenuto, R.; Fedele, A.L.; Federico, F.; Ferraccioli, G.; et al. Synovial features of patients with rheumatoid arthritis and psoriatic arthritis in clinical and ultrasound remission differ under anti-TNF therapy: A clue to interpret different chances of relapse after clinical remission? Ann. Rheum. Dis. 2017, 76, 1228–1236. [Google Scholar] [CrossRef]
- Alivernini, S.; Tolusso, B.; Gessi, M.; Gigante, M.R.; Mannocci, A.; Petricca, L.; Perniola, S.; Di Mario, C.; Bui, L.; Fedele, A.L.; et al. Inclusion of Synovial Tissue-Derived Characteristics in a Nomogram for the Prediction of Treatment Response in Treatment-Naive Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2021, 73, 1601–1613. [Google Scholar] [CrossRef]
- Alivernini, S.; Bruno, D.; Tolusso, B.; Bui, L.; Petricca, L.; Gigante, M.R.; Birra, D.; Fedele, A.L.; Peluso, G.; Federico, F.; et al. Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 116. [Google Scholar] [CrossRef]


| Variable | Sicca Disease (n = 20) | pSS Cohort (n = 24) | p | pSS without NHL Evolution (n = 19) | pSS with NHL Evolution (n = 5) | p * | p ** | p *** |
|---|---|---|---|---|---|---|---|---|
| Gender, female (%) | 19 (95.0) | 21 (87.5) | 0.39 | 17 (89.4) | 5 (100) | 0.45 | 0.52 | 0.62 |
| Age (years) | 52.5 (40.3–57.3) | 54.5 (47.3–64.8) | 0.10 | 53.0 (46.0–61.0) | 67.0 (54.5–77.0) | 0.04 | 0.33 | 0.02 |
| Disease duration (years) | - | 10.5 ± 3.4 | - | 12.3 ± 1.4 | 7.3 ± 3.1 | 0.13 | - | - |
| Lymphocytic focus score (foci/4 mm2) | 0 | 3.5 ± 0.3 | <0.0001 | 3.6 ± 0.4 | 3.2 ± 0.6 | 0.64 | <0.0001 | <0.0001 |
| ANA | 4 (20) | 24 (100) | <0.0001 | 19 (100) | 5 (100) | 1 | <0.0001 | <0.0001 |
| Anti-Ro/SSA, n (%) | 0 (0) | 22 (92) | <0.0001 | 18 (95) | 4 (75) | 0.18 | <0.0001 | <0.0001 |
| Anti-La/SSB, n (%) | 0 (0) | 20 (83) | <0.0001 | 17 (89) | 3 (60) | 0.13 | <0.0001 | 0.0003 |
| RF-IgApos, n (%) | 0 (0) | 1 (0.04) | 0.93 | 1 (0.05) | 0 (0) | 0.96 | 0.92 | 1 |
| RF-IgMpos, n (%) | 0 (0) | 3 (0.13) | 0.87 | 3 (0.16) | 0 (0) | 0.93 | 0.86 | 1 |
| IgG (mg/dL) | 1147.7 ± 59.8 | 1247.7 ± 79.6 | 0.34 | 1184.6 ± 60.7 | 1468.8 ± 291.2 | 0.14 | 0.67 | 0.09 |
| IgM (mg/dL) | 138.2 ± 12.6 | 119.5 ± 11.9 | 0.29 | 132.6 ± 12.8 | 69.4 ± 16.7 | 0.03 | 0.76 | 0.02 |
| IgA (mg/dL) | 193.9 ± 20.4 | 228.8 ± 26.7 | 0.32 | 188.9 ± 20.1 | 380.6 ± 73.9 | 0.002 | 0.86 | 0.002 |
| Gamma-globulin (%) | 14.3 ± 0.6 | 17.1 ± 0.9 | 0.02 | 15.9 ± 0.8 | 21.4 ± 1.8 | 0.006 | 0.12 | 0.0001 |
| ALC (×109/L) | 2319.9 ± 304.3 | 1928.1 ± 77.4 | 0.18 | 1685.6 ± 90.8 | 1889.6 ± 126.7 | 0.29 | 0.06 | 0.49 |
| C3 (mg/dL) | 109.3 ± 4.3 | 111.9 ± 3.8 | 0.65 | 112.4 ± 4.7 | 109.5 ± 4.1 | 0.76 | 0.63 | 0.98 |
| C4 (mg/dL) | 21.7 ± 1.6 | 25.0 ± 2.2 | 0.25 | 22.6 ± 1.8 | 24.5 ± 6.7 | 0.70 | 0.71 | 0.54 |
| CRP (mg/L) | 2.0 ± 0.6 | 1.9 ± 0.4 | 0.89 | 1.7 ± 0.3 | 3.1 ± 2.7 | 0.34 | 0.66 | 0.54 |
| ESR (mm/1st h) | 14.1 ± 3.1 | 21.0 ± 2.8 | 0.11 | 19.3 ± 3.1 | 26.5 ± 6.8 | 0.31 | 0.24 | 0.09 |
| ESSDAI | - | 2.8 ± 0.3 | - | 6.5 ± 2.7 | 8.7 ± 4.2 | 0.70 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, D.; Tolusso, B.; Lugli, G.; Di Mario, C.; Petricca, L.; Perniola, S.; Bui, L.; Benvenuto, R.; Ferraccioli, G.; Alivernini, S.; et al. B-Cell Activation Biomarkers in Salivary Glands Are Related to Lymphomagenesis in Primary Sjögren’s Disease: A Pilot Monocentric Exploratory Study. Int. J. Mol. Sci. 2024, 25, 3259. https://doi.org/10.3390/ijms25063259
Bruno D, Tolusso B, Lugli G, Di Mario C, Petricca L, Perniola S, Bui L, Benvenuto R, Ferraccioli G, Alivernini S, et al. B-Cell Activation Biomarkers in Salivary Glands Are Related to Lymphomagenesis in Primary Sjögren’s Disease: A Pilot Monocentric Exploratory Study. International Journal of Molecular Sciences. 2024; 25(6):3259. https://doi.org/10.3390/ijms25063259
Chicago/Turabian StyleBruno, Dario, Barbara Tolusso, Gianmarco Lugli, Clara Di Mario, Luca Petricca, Simone Perniola, Laura Bui, Roberta Benvenuto, Gianfranco Ferraccioli, Stefano Alivernini, and et al. 2024. "B-Cell Activation Biomarkers in Salivary Glands Are Related to Lymphomagenesis in Primary Sjögren’s Disease: A Pilot Monocentric Exploratory Study" International Journal of Molecular Sciences 25, no. 6: 3259. https://doi.org/10.3390/ijms25063259
APA StyleBruno, D., Tolusso, B., Lugli, G., Di Mario, C., Petricca, L., Perniola, S., Bui, L., Benvenuto, R., Ferraccioli, G., Alivernini, S., & Gremese, E. (2024). B-Cell Activation Biomarkers in Salivary Glands Are Related to Lymphomagenesis in Primary Sjögren’s Disease: A Pilot Monocentric Exploratory Study. International Journal of Molecular Sciences, 25(6), 3259. https://doi.org/10.3390/ijms25063259







