The Progress of the Biotechnological Production of Class IIa Bacteriocins in Various Cell Factories and Its Future Challenges
Abstract
1. Introduction
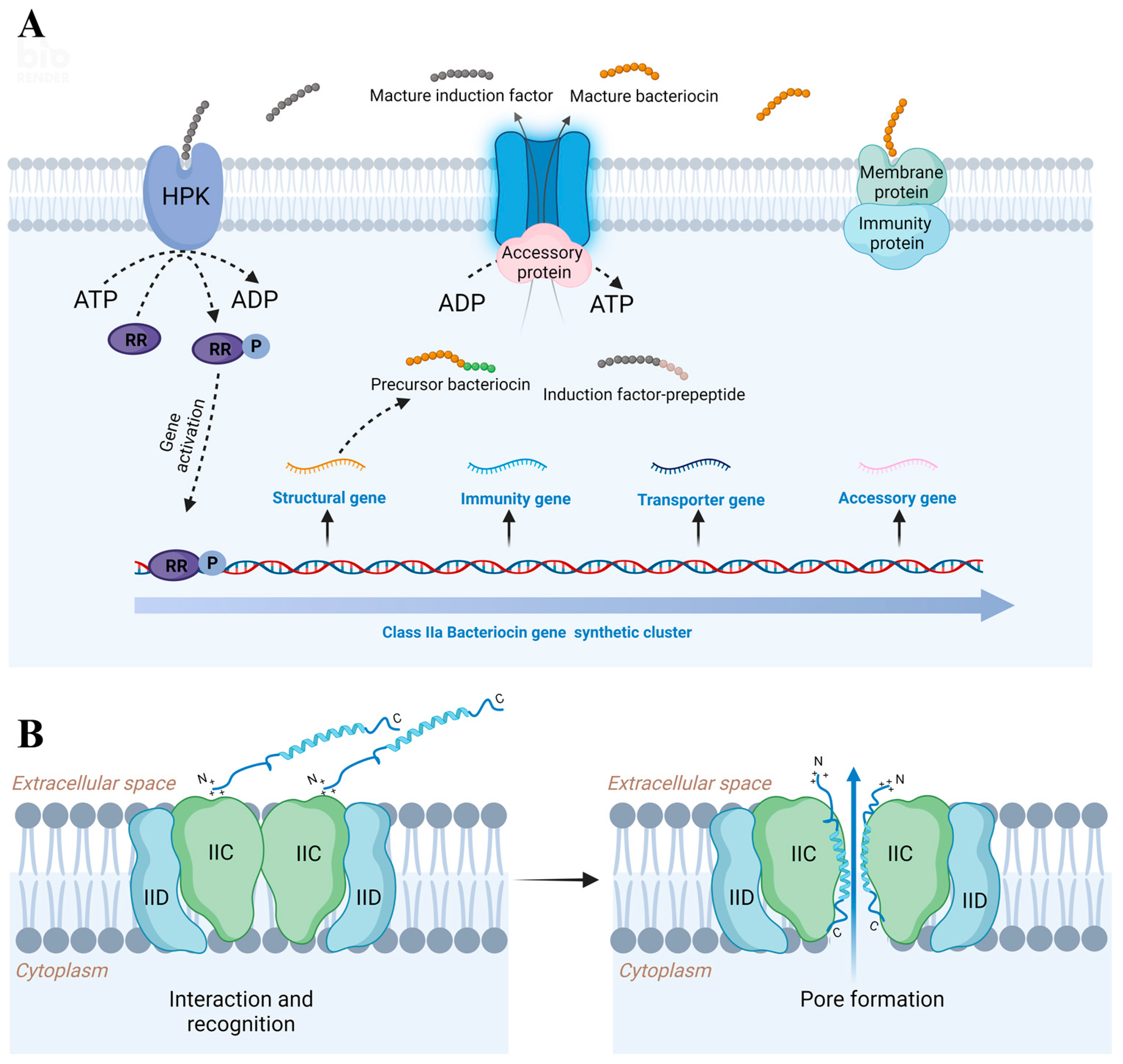
2. General Properties of Class IIa Bacteriocins
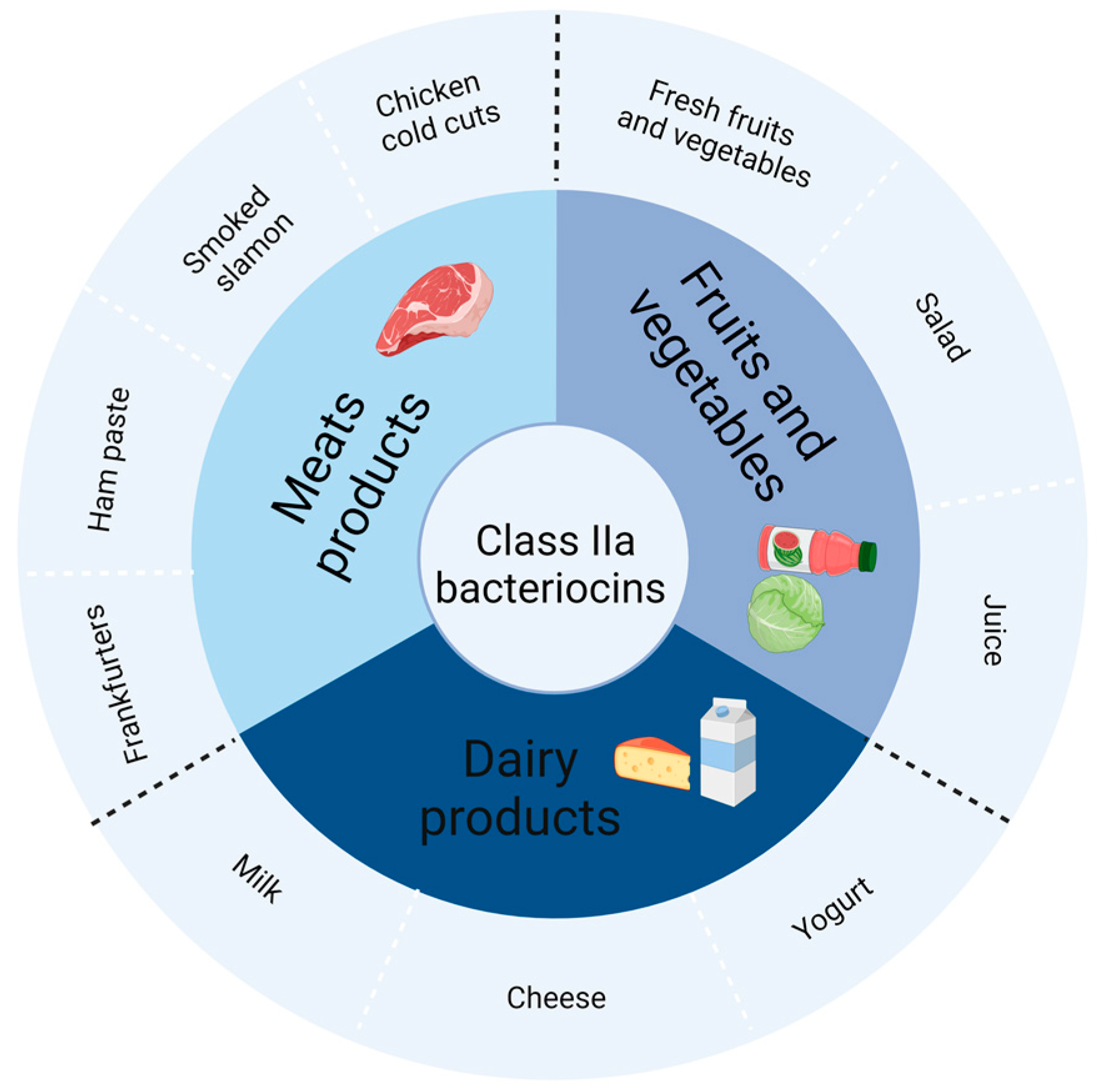
3. Potential Applications of Class IIa Bacteriocins
4. The Production of Class IIa Bacteriocins by Different Cell Factories
4.1. Recombinant Class IIa Bacteriocin Expression Using E. coli as Cell Factories
4.2. Recombinant Class IIa Bacteriocin Expression Using LAB as Cell Factories
4.3. Recombinant Class IIa Bacteriocin Expression Using Yeast as Cell Factories
4.4. The Other Cell Factories for Recombinant Class IIa Bacteriocins Expression
5. Challenges
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lü, X. Current status and potentiality of class II bacteriocins from lactic acid bacteria: Structure, mode of action and applications in the food industry. Trends Food Sci. Technol. 2022, 120, 387–401. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A. Ribosomally synthesized bacteriocins of lactic acid bacteria: Simplicity yet having wide potentials—A review. Int. J. Biol. Macromol. 2024, 256, 128325. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.H.; Zhang, C.; Wang, Y.F.; Shi, J.; Zhang, L.W.; Ding, Z.Q.; Qu, X.J.; Cui, H.Y. Class IIa Bacteriocins: Diversity and New Developments. Int. J. Mol. Sci. 2012, 13, 16668–16703. [Google Scholar] [CrossRef]
- Fernandes, S.; Gomes, I.B.; Simoes, M.; Simoes, L.C. Novel chemical-based approaches for biofilm cleaning and disinfection. Curr. Opin. Food Sci. 2024, 55, 101124. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, L.; Wang, X.; Lu, Y.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lu, X. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 863–899. [Google Scholar] [CrossRef]
- Garsa, A.K.; Kumariya, R.; Sood, S.K.; Kumar, A.; Kapila, S. Bacteriocin Production and Different Strategies for Their Recovery and Purification. Probiotics Antimicrob. Proteins 2014, 6, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Gao, C.; Song, W.; Wei, W.; Wu, J.; Liu, L.; Chen, X. Engineering status of protein for improving microbial cell factories. Biotechnol. Adv. 2024, 70, 108282. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Drider, D.; Elmorjani, K.; Marion, D.; Prevost, H. Heterologous expression and purification of active divercin V41, a class IIa bacteriocin encoded by a synthetic gene in Escherichia coli. J. Bacteriol. 2004, 186, 4276–4284. [Google Scholar] [CrossRef]
- Gibbs, G.M.; Davidson, B.E.; Hillier, A.J. Novel expression system for large-scale production and purification of recombinant class IIa bacteriocins and its application to piscicolin 126. Appl. Environ. Microbiol. 2004, 70, 3292–3297. [Google Scholar] [CrossRef]
- Vermeulen, R.R.; Van Staden, A.D.P.; Dicks, L. Heterologous Expression of the Class IIa Bacteriocins, Plantaricin 423 and Mundticin ST4SA, in Escherichia coli Using Green Fluorescent Protein as a Fusion Partner. Front. Microbiol. 2020, 11, 1634. [Google Scholar] [CrossRef] [PubMed]
- Olejnik-Schmidt, A.K.; Schmidt, M.T.; Sip, A.; Szablewski, T.; Grajek, W. Expression of bacteriocin divercin AS7 in Escherichia coli and its functional analysis. Ann. Microbiol. 2014, 64, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, F.; Li, S.; Xie, Y.; Zhang, H.; Chen, W. Cloning and heterologous expression of a bacteriocin sakacin P from Lactobacillus sakei in Escherichia coli. Appl. Microbiol. Biotechnol. 2012, 94, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pereira, B.; O’Connor, P.M.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Controlled functional expression of the bacteriocins pediocin PA-1 and bactofencin A in Escherichia coli. Sci. Rep. 2017, 7, 3069. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhao, H.; Zhang, C.; Lu, F.; Bie, X.; Lu, Z. Expression of a novel bacteriocin-the plantaricin Pln1-in Escherichia coli and its functional analysis. Protein Expr. Purif. 2016, 119, 85–93. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, W.; Ma, Q.; Yu, Z.; Zhang, R. Production of bacteriocin E50–52 by small ubiquitin-related modifier fusion in Escherichia coli. Pol. J. Microbiol. 2013, 62, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, X.; Tian, F.; Song, Y.; Chen, Y.Q.; Zhang, H.; Chen, W. Cloning, expression, and identification of a novel class IIa bacteriocin in the Escherichia coli cell-free protein expression system. Biotechnol. Lett. 2012, 34, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.J.; Diep, D.B.; Borrero, J.; Gutiez, L.; Arbulu, S.; Nes, I.F.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Cloning strategies for heterologous expression of the bacteriocin enterocin A by Lactobacillus sakei Lb790, Lb. plantarum NC8 and Lb. casei CECT475. Microb. Cell Fact. 2015, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Gutierrez, J.; Criado, R.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Cloning, production and expression of the bacteriocin enterocin A produced by Enterococcus faecium PLBC21 in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2007, 76, 667–675. [Google Scholar] [CrossRef]
- Liu, G.R.; Wang, H.F.; Griffiths, M.W.; Li, P.L. Heterologous extracellular production of enterocin P in Lactococcus lactis by a food-grade expression system. Eur. Food Res. Technol. 2011, 233, 123–129. [Google Scholar] [CrossRef]
- Sanchez, J.; Borrero, J.; Gomez-Sala, B.; Basanta, A.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Cloning and heterologous production of Hiracin JM79, a Sec-dependent bacteriocin produced by Enterococcus hirae DCH5, in lactic acid bacteria and Pichia pastoris. Appl. Environ. Microbiol. 2008, 74, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Li, R.Q.; Saris, P.E.J.; Takala, T.M. Genetic characterisation and heterologous expression of leucocin C, a class IIa bacteriocin from Leuconostoc carnosum 4010. Appl. Microbiol. Biotechnol. 2013, 97, 3509–3518. [Google Scholar] [CrossRef]
- Schoeman, H.; Vivier, M.A.; Du Toit, M.; Dicks, L.M.; Pretorius, I.S. The development of bactericidal yeast strains by expressing the Pediococcus acidilactici pediocin gene (pedA) in Saccharomyces cerevisiae. Yeast 1999, 15, 647–656. [Google Scholar] [CrossRef]
- Van Reenen, C.A.; Chikindas, M.L.; Van Zyl, W.H.; Dicks, L.M. Characterization and heterologous expression of a class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum 423, in Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 81, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Criado, R.; Martin, M.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Production of enterocin P, an antilisterial pediocin-like bacteriocin from Enterococcus faecium P13, in Pichia pastoris. Antimicrob. Agents Chemother. 2005, 49, 3004–3008. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Groleau, D.; Miguez, C.B.; Jette, J.F.; Aomari, H.; Subirade, M. Production of pediocin PA-1 in the methylotrophic yeast Pichia pastoris reveals unexpected inhibition of its biological activity due to the presence of collagen-like material. Protein Expr. Purif. 2005, 43, 111–125. [Google Scholar] [CrossRef]
- Arbulu, S.; Jimenez, J.J.; Gutiez, L.; Feito, J.; Cintas, L.M.; Herranz, C.; Hernandez, P.E. Cloning and expression of synthetic genes encoding native, hybrid- and bacteriocin-derived chimeras from mature class IIa bacteriocins, by Pichia pastoris (syn. Komagataella spp.). Food Res. Int. 2019; 121, 888–899. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Borrero, J.; Gutiez, L.; Arbulu, S.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Use of synthetic genes for cloning, production and functional expression of the bacteriocins enterocin A and bacteriocin E 50-52 by Pichia pastoris and Kluyveromyces lactis. Mol. Biotechnol. 2014, 56, 571–583. [Google Scholar] [CrossRef]
- Borrero, J.; Kunze, G.; Jimenez, J.J.; Boer, E.; Gutiez, L.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Cloning, production, and functional expression of the bacteriocin enterocin A, produced by Enterococcus faecium T136, by the yeasts Pichia pastoris, Kluyveromyces lactis, Hansenula polymorpha, and Arxula adeninivorans. Appl. Environ. Microbiol. 2012, 78, 5956–5961. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Borrero, J.; Diep, D.B.; Gutiez, L.; Nes, I.F.; Herranz, C.; Cintas, L.M.; Hernandez, P.E. Cloning, production, and functional expression of the bacteriocin sakacin A (SakA) and two SakA-derived chimeras in lactic acid bacteria (LAB) and the yeasts Pichia pastoris and Kluyveromyces lactis. J. Ind. Microbiol. Biotechnol. 2013, 40, 977–993. [Google Scholar] [CrossRef]
- Li, R.; Wan, X.; Takala, T.M.; Saris, P.E.J. Heterologous Expression of the Leuconostoc Bacteriocin Leucocin C in Probiotic Yeast Saccharomyces boulardii. Probiotics Antimicrob. Proteins 2021, 13, 229–237. [Google Scholar] [CrossRef]
- Rossouw, M.; Cripwell, R.A.; Vermeulen, R.R.; van Staden, A.D.; van Zyl, W.H.; Dicks, L.M.T.; Viljoen-Bloom, M. Heterologous Expression of Plantaricin 423 and Mundticin ST4SA in Saccharomyces cerevisiae. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef] [PubMed]
- Goldbeck, O.; Desef, D.N.; Ovchinnikov, K.V.; Perez-Garcia, F.; Christmann, J.; Sinner, P.; Crauwels, P.; Weixler, D.; Cao, P.; Becker, J.; et al. Establishing recombinant production of pediocin PA-1 in Corynebacterium glutamicum. Metab. Eng. 2021, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, Z.; Zhang, X.; Wu, H.; Bai, X.; Zhang, H.; Hu, R.; Han, S.; Pang, Y.; Gao, Z.; et al. Heterologous expression of pediocin/papA in Bacillus subtilis. Microb. Cell Fact. 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Tanhaeian, A.; Damavandi, M.S.; Mansury, D.; Ghaznini, K. Expression in eukaryotic cells and purification of synthetic gene encoding enterocin P: A bacteriocin with broad antimicrobial spectrum. AMB Express 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: The state of the art toward improved bacteriocins. J. Control Release 2020, 321, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.; Dar, R.A.; Fomda, B.A.; Nazir, R. Combating food spoilage and pathogenic microbes via bacteriocins: A natural and eco-friendly substitute to antibiotics. Food Control 2023, 149, 109710. [Google Scholar] [CrossRef]
- Drider, D.; Fimland, G.; Hechard, Y.; McMullen, L.M.; Prevost, H. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 2006, 70, 564–582. [Google Scholar] [CrossRef]
- Unal, M.A.; Kaymaz, O.; Gunes Altuntas, E.; Juneja, V.K.; Elmali, A. Effect of Disulfide Bonds on the Thermal Stability of Pediocin: In-silico Screening Using Molecular Dynamics Simulation. J. Food Prot. 2023, 86, 100107. [Google Scholar] [CrossRef] [PubMed]
- Kareb, O.; Aider, M. Quorum Sensing Circuits in the Communicating Mechanisms of Bacteria and Its Implication in the Biosynthesis of Bacteriocins by Lactic Acid Bacteria: A Review. Probiotics Antimicrob. Proteins 2020, 12, 5–17. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Li, L.; Jiang, X.; Chen, Z.; Zhao, F.; Yi, Y. Biosynthesis and Production of Class II Bacteriocins of Food-Associated Lactic Acid Bacteria. Fermentation 2022, 8, 217. [Google Scholar] [CrossRef]
- Zheng, S.; Sonomoto, K. Diversified transporters and pathways for bacteriocin secretion in gram-positive bacteria. Appl. Microbiol. Biotechnol. 2018, 102, 4243–4253. [Google Scholar] [CrossRef] [PubMed]
- Jeckelmann, J.M.; Erni, B. The mannose phosphotransferase system (Man-PTS)—Mannose transporter and receptor for bacteriocins and bacteriophages. Biochim. Biophys. Acta—Biomembr. 2020, 1862, 183412. [Google Scholar] [CrossRef] [PubMed]
- Kjos, M.; Salehian, Z.; Nes, I.F.; Diep, D.B. An extracellular loop of the mannose phosphotransferase system component IIC is responsible for specific targeting by class IIa bacteriocins. J. Bacteriol. 2010, 192, 5906–5913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, J.; Wang, C.; Wang, J. Structural Basis of Pore Formation in the Mannose Phosphotransferase System by Pediocin PA-1. Appl. Environ. Microbiol. 2022, 88, e0199221. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xiong, T.; Huang, T.; Xu, X.; Fan, P.; Qiao, B.; Xie, M. Factors affecting production and effectiveness, performance improvement and mechanisms of action of bacteriocins as food preservative. Crit. Rev. Food Sci. Nutr. 2023, 63, 12294–12307. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef] [PubMed]
- Porto, M.C.; Kuniyoshi, T.M.; Azevedo, P.O.; Vitolo, M.; Oliveira, R.P. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef]
- Gu, Q.; Yan, J.; Lou, Y.; Zhang, Z.; Li, Y.; Zhu, Z.; Liu, M.; Wu, D.; Liang, Y.; Pu, J.; et al. Bacteriocins: Curial guardians of gastrointestinal tract. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13292. [Google Scholar] [CrossRef] [PubMed]
- Bartram, E.; Asai, M.; Gabant, P.; Wigneshweraraj, S. Enhancing the antibacterial function of probiotic Escherichia coli Nissle: When less is more. Appl. Environ. Microbiol. 2023, 89, e0097523. [Google Scholar] [CrossRef]
- Geldart, K.G.; Kommineni, S.; Forbes, M.; Hayward, M.; Dunny, G.M.; Salzman, N.H.; Kaznessis, Y.N. Engineered E. coli Nissle 1917 for the reduction of vancomycin-resistant Enterococcus in the intestinal tract. Bioeng. Transl. Med. 2018, 3, 197–208. [Google Scholar] [CrossRef]
- Ismael, M.; Qayyum, N.; Gu, Y.; Zhezhe, Y.; Cui, Y.; Zhang, Y.; Lu, X. Protective effect of plantaricin bio-LP1 bacteriocin on multidrug-resistance Escherichia coli infection by alleviate the inflammation and modulate of gut-microbiota in BALB/c mice model. Int. J. Biol. Macromol. 2023, 246, 125700. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.; Pang, X.; Bian, Z.; Zhu, J.; Hao, Y.; Zhang, H.; Xie, Y. Plantaricin BM-1 decreases viability of SW480 human colorectal cancer cells by inducing caspase-dependent apoptosis. Front. Microbiol. 2022, 13, 1103600. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Suar, M.; Panda, S.K. Nanotechnological interventions in bacteriocin formulations—Advances, and scope for challenging food spoilage bacteria and drug-resistant foodborne pathogens. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.M.; Samir, S.; Okasha, H. Interdisciplinary gene manipulation, molecular cloning, and recombinant expression of modified human growth hormone isoform-1 in E. coli system. Int. J. Biol. Macromol. 2024, 257, 128637. [Google Scholar] [CrossRef]
- Bedard, F.; Hammami, R.; Zirah, S.; Rebuffat, S.; Fliss, I.; Biron, E. Synthesis, antimicrobial activity and conformational analysis of the class IIa bacteriocin pediocin PA-1 and analogs thereof. Sci. Rep. 2018, 8, 9029. [Google Scholar] [CrossRef] [PubMed]
- Incir, I.; Kaplan, O. Escherichia coli as a versatile cell factory: Advances and challenges in recombinant protein production. Protein Expr. Purif. 2024, 219, 106463. [Google Scholar] [CrossRef]
- Peirotén, Á.; Landete, J.M. Natural and engineered promoters for gene expression in Lactobacillus species. Appl. Microbiol. Biotechnol. 2020, 104, 3797–3805. [Google Scholar] [CrossRef]
- Singh, S.K.; Tiendrebeogo, R.W.; Chourasia, B.K.; Kana, I.H.; Singh, S.; Theisen, M. Lactococcus lactis provides an efficient platform for production of disulfide-rich recombinant proteins from Plasmodium falciparum. Microb. Cell Fact. 2018, 17, 55. [Google Scholar] [CrossRef]
- De Brabander, P.; Uitterhaegen, E.; Delmulle, T.; De Winter, K.; Soetaert, W. Challenges and progress towards industrial recombinant protein production in yeasts: A review. Biotechnol. Adv. 2023, 64, 108121. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Y.; Hu, Z.; Shi, T.; Wang, Y.; Ye, C.; Huang, H. Bacillus sp. as a microbial cell factory: Advancements and future prospects. Biotechnol. Adv. 2023, 69, 108278. [Google Scholar] [CrossRef]
| Cell Factories | Producing Bacteria | Bacteriocin | Expression Plasmid | Expression Form | Antimicrobial Activity/Indicator Bacteria | Reference |
|---|---|---|---|---|---|---|
| Escherichia coli | ||||||
| E. coli Origami (DE3) (pLysS) | Carnobacterium divergens V41 | divercin V41 | pET-32b | intracellular | 842.8 AU μg−1/Listeria innocua F | [9] |
| E. coli AD494 (DE3) | Carnobacterium piscicola JG126 | piscicolin 126 | pET32a | intracellular | 247,584 AU mg−1/L. monocytogenes 4A | [10] |
| E. coli BL21 (DE3) | Lactobacillus plantarum 423 | plantaricin 423 | pRSFDuet-1 | intracellular | 83.33 BU mL−1/L. monocytogenes EDG-e | [11] |
| E. coli BL21 (DE3) | Enterococcus mundtii ST4SA | mundticin ST4SA | pRSFDuet-1 | intracellular | 1600 BU mL−1/L. monocytogenes EDG-e | [11] |
| E. coli Origami (DE3) (pLysS) | Carnobacterium divergens AS7 | divercin AS7 | pET28b+ | intracellular | NE | [12] |
| E. coli BL21 (DE3) | Lactobacillus sakei | sakacin P | pET28a (+) | intracellular | NE | [13] |
| E. coli TunerTM (DE3) | Pediococcus acidilactici LMG2351 | pediocin PA-1 | pETcoco-2 | extracellular | 640 BU mL−1/Listeria innocua DPC3572 | [14] |
| E. coli BL21 (DE3) | L. plantarum 163 | plantaricin Pln1 | pET32a | intracellular | NE | [15] |
| Escherichia coli BL21 (DE3) | synthesised | bacteriocin E 50-52 | pET SUMO | intracellular | NE | [16] |
| E. coli cell-free system | synthesised | bacteriocin NB-C1 | pIVEX 2.4d | intracellular | NE | [17] |
| Lactobacillus | ||||||
| Lactobacillus casei CECT475 | Enterococcus faecium T136 | enterocin A | pSIP411UAI) | extracellular | 255,191 BU mg−1/L. monocytogenes CECT911 | [18] |
| L. lactis IL1403 | E. faecium PLBC21 | enterocin A | pMPA15 | extracellular | NE | [19] |
| L. lactis MG1614 | E. faecium LM-2 | enterocin P | pLEB590 | extracellular | 7.24 × 104 AU mg−1/L. monocytogenes 54002 | [20] |
| L. lactis NZ9000 | E. faecium PLBC21 | enterocin A | pMPA15 | extracellular | NE | [19] |
| L. lactis NZ9000 | Enterococcus hirae DCH5 | hiracin JM79 | pMG36c pNZ8048 | extracellular | 26,480 BU mg−1/E. faecium T136 | [21] |
| L. lactis NZ9000 | Leuconostoc carnosum 4010 | leucocin C | pLEB690 | extracellular | NE | [22] |
| Yeast | ||||||
| Saccharomyces cerevisiae Y294 | P. acidilactici | pediocin PA-1 | YEp352 | extracellular | NE | [23] |
| S. cerevisiae L5366h | L. plantarum 423 | plantaricin 423 | YEp352 | extracellular | NE | [24] |
| P. pastoris X-33 | E. faecium P13 | enterocin P | pPICZαA | extracellular | 10,240 BU mL−1/E. faecium T136 | [25] |
| P. pastoris X-33 | P. acidilactici | pediocin PA-1 | pPICZαA | extracellular | NE | [26] |
| P. pastoris X-33 | Enterococcus hirae DCH5 | hiracin JM79 | pPICZαA | extracellular | 5217 BU mg−1/E. faecium T136 | [21] |
| P. pastoris X-33 | E. faecium | enterocin HF and | pPICZαA | extracellular | 11,129 BU mL−1/Pediococcus damnosus CECT4797 | [27] |
| P. pastoris X-33 | E. faecium | enterocin CRL35 | pPICZαA | extracellular | 2720 BU mL−1/P. damnosus CECT4797 | [27] |
| P. pastoris X-33 | E. faecium T136; | enterocin A | pPICZαA | extracellular | 371,200 BU μg−1/L. monocytogenes 4031 | [28] |
| Kluyveromyces. lactis GG799EA | E. faecium T136; | enterocin A | pKLAC2 | extracellular | 343 BU μg−1/L. monocytogenes 4031 | [28] |
| P. pastoris X-33 | E. faecium NRRL B-32746 | bacteriocin E 50-52 | pPICZαA | extracellular | 75 BU μg−1/L. seeligeri 917 | [28] |
| Kluyveromyces. lactis GG799EA | E. faecium NRRL B-32746 | bacteriocin E 50-52 | pKLAC2 | extracellular | 1312 BU μg−1/L. ivanovii 913 | [28] |
| P. pastoris X-33 | E. faecium T136 | enterocin A | pPICZαA; YRC-ALEU2m | extracellular | 5528 BU μg−1/L. monocytogenes CECT | [29] |
| K. lactis GG799EA | E. faecium T136 | enterocin A | pKLAC2; | extracellular | 3252 BU μg−1/L. monocytogenes | [29] |
| H. polymorpha KL8-1EA | E. faecium T136 | enterocin A | pBTEA | extracellular | 213 BU μg−1/L. monocytogenes | [29] |
| K. lactis GG799 | Lactobacillus sakei Lb706 | sakacin A | pKLAC2 | extracellular | 32.5 BU ml−1/E. faecium T136 | [30] |
| Saccharomyces boulardii CNCM I-745 | Leuconostoc carnosum 4010 | leucocin C | pSF-Blast | extracellular | NE | [31] |
| Saccharomyces cerevisiae Y294 | Lactobacillus plantarum 423 | plantaricin 423 | yBBH1-MFα1 | extracellular | 320 AU mL−1/L. monocytogenes EDG-e | [32] |
| S. cerevisiae Y294 | Enterococcus mundtii ST4SA | mundticin ST4SA | yBBH1-MFα1 | extracellular | 533 AU mL−1/L. monocytogenes EDG-e | [32] |
| The other cell factories | ||||||
| Corynebacterium glutamicum CR099 | Pediococcus acidilactici 347 | pediocin PA-1 | pEKEx2 | extracellular | 20,480 BU mL−1/L. monocytogenes EGDe:pIMK2 | [33] |
| Bacillus subtilis WB800N | Lactobacillus plantarum Zhang-LL | pediocin/PapA | pHT43 | extracellular | NE | [34] |
| Chinese hamster ovary cells | Enterococcus spp. | enterocin P | pcDNA™3.1(+) | extracellular | NE | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shang, N.; Huang, Y.; Gao, B.; Li, P. The Progress of the Biotechnological Production of Class IIa Bacteriocins in Various Cell Factories and Its Future Challenges. Int. J. Mol. Sci. 2024, 25, 5791. https://doi.org/10.3390/ijms25115791
Wang Y, Shang N, Huang Y, Gao B, Li P. The Progress of the Biotechnological Production of Class IIa Bacteriocins in Various Cell Factories and Its Future Challenges. International Journal of Molecular Sciences. 2024; 25(11):5791. https://doi.org/10.3390/ijms25115791
Chicago/Turabian StyleWang, Yu, Nan Shang, Yueying Huang, Boya Gao, and Pinglan Li. 2024. "The Progress of the Biotechnological Production of Class IIa Bacteriocins in Various Cell Factories and Its Future Challenges" International Journal of Molecular Sciences 25, no. 11: 5791. https://doi.org/10.3390/ijms25115791
APA StyleWang, Y., Shang, N., Huang, Y., Gao, B., & Li, P. (2024). The Progress of the Biotechnological Production of Class IIa Bacteriocins in Various Cell Factories and Its Future Challenges. International Journal of Molecular Sciences, 25(11), 5791. https://doi.org/10.3390/ijms25115791






