Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction
Abstract
1. Introduction
2. Results
2.1. Statistical Analyses
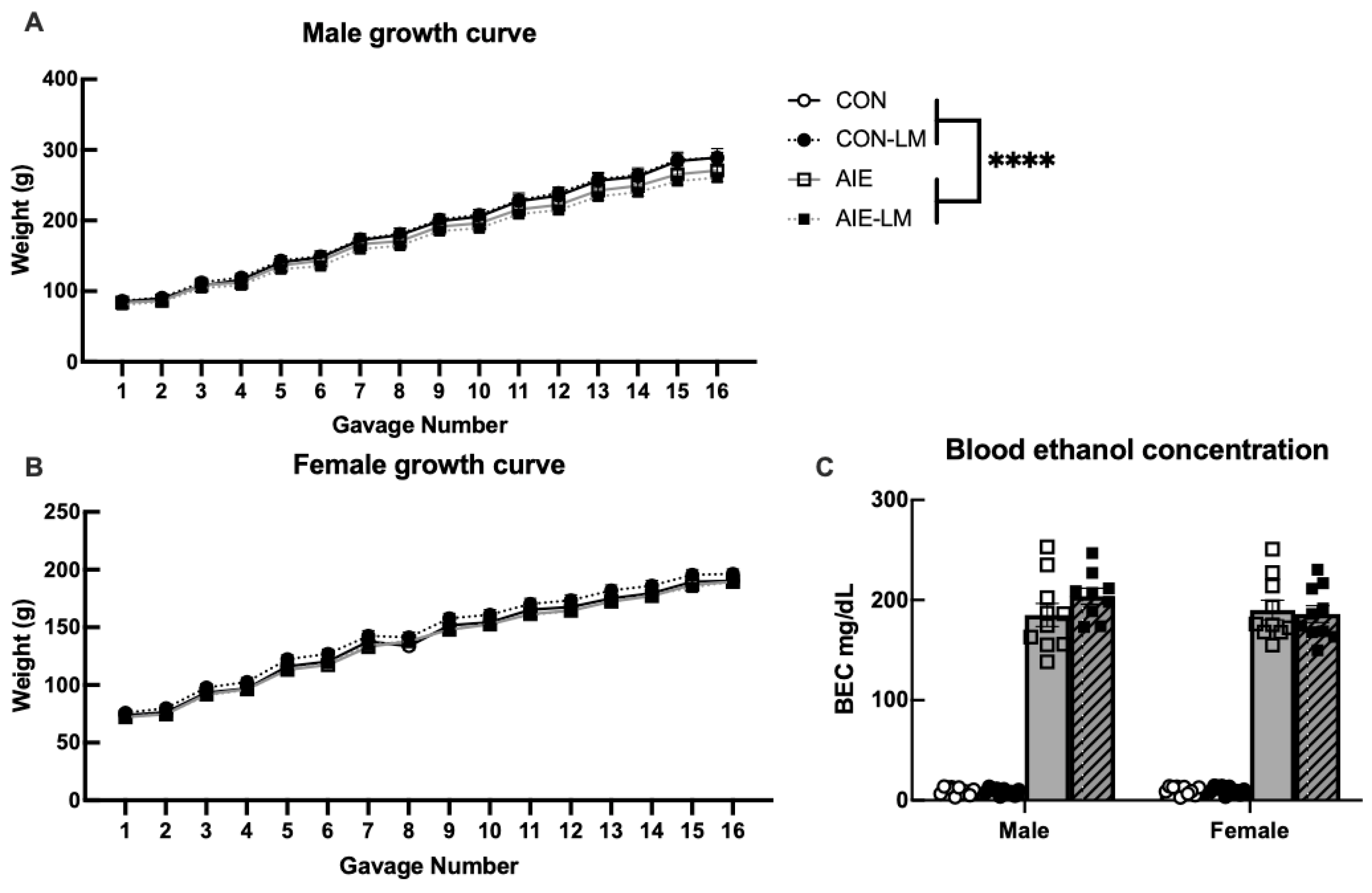
2.2. Treatment Growth Curves and BECs
2.2.1. Male Growth Curve
2.2.2. Female Growth Curve
2.2.3. Blood Ethanol Concentrations
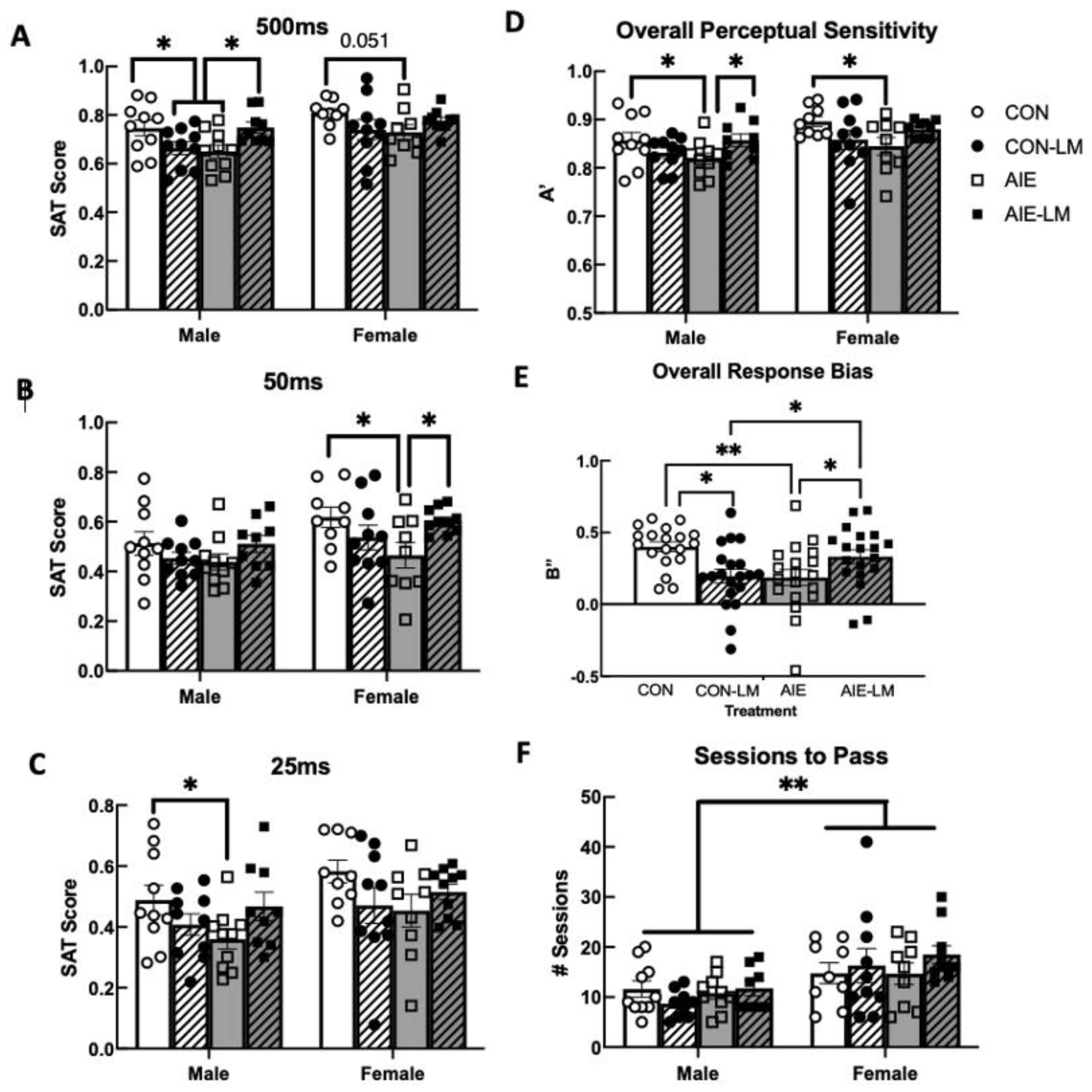
2.3. SAT Pretraining (pSAT)
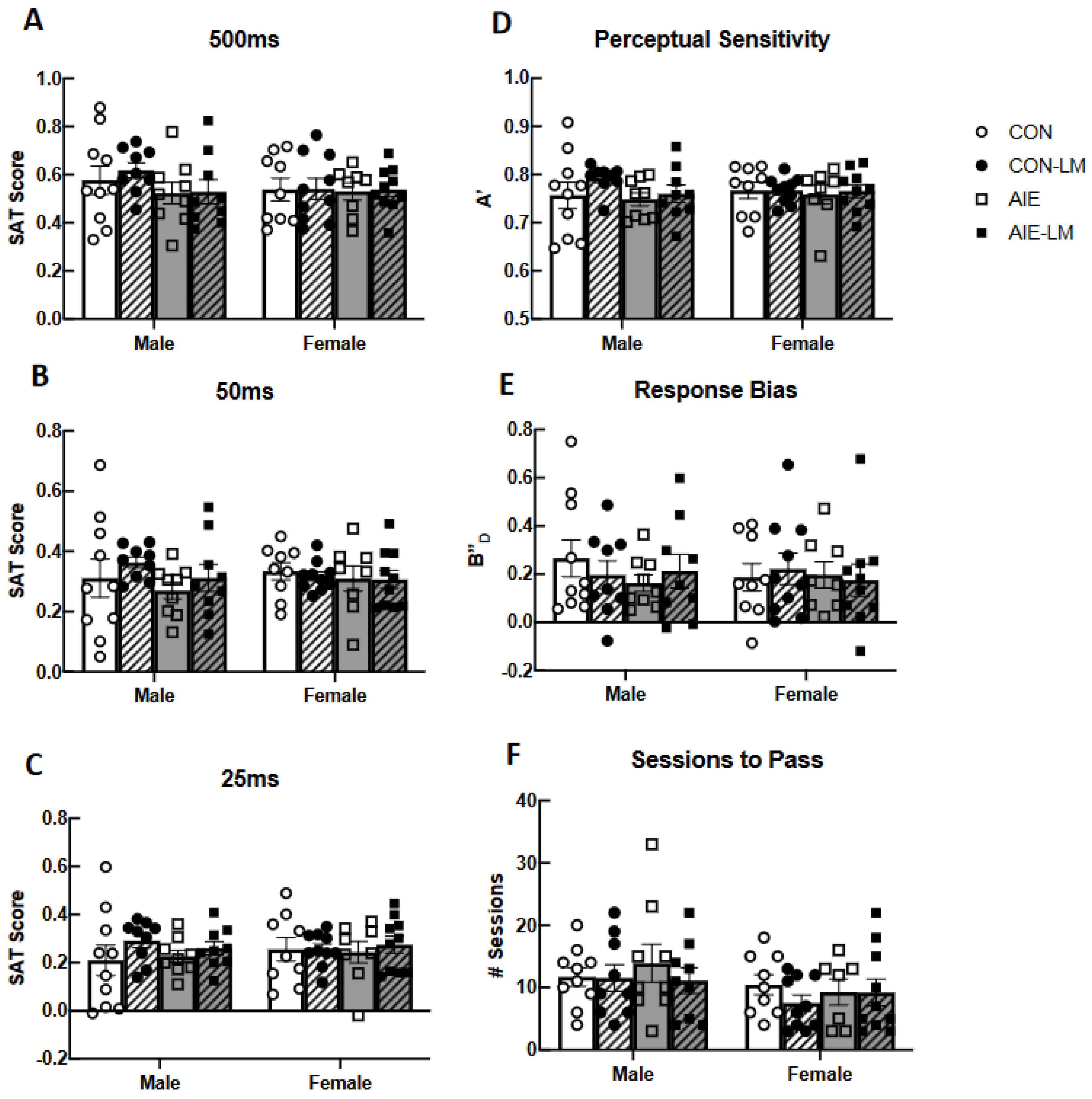
2.4. SAT Task
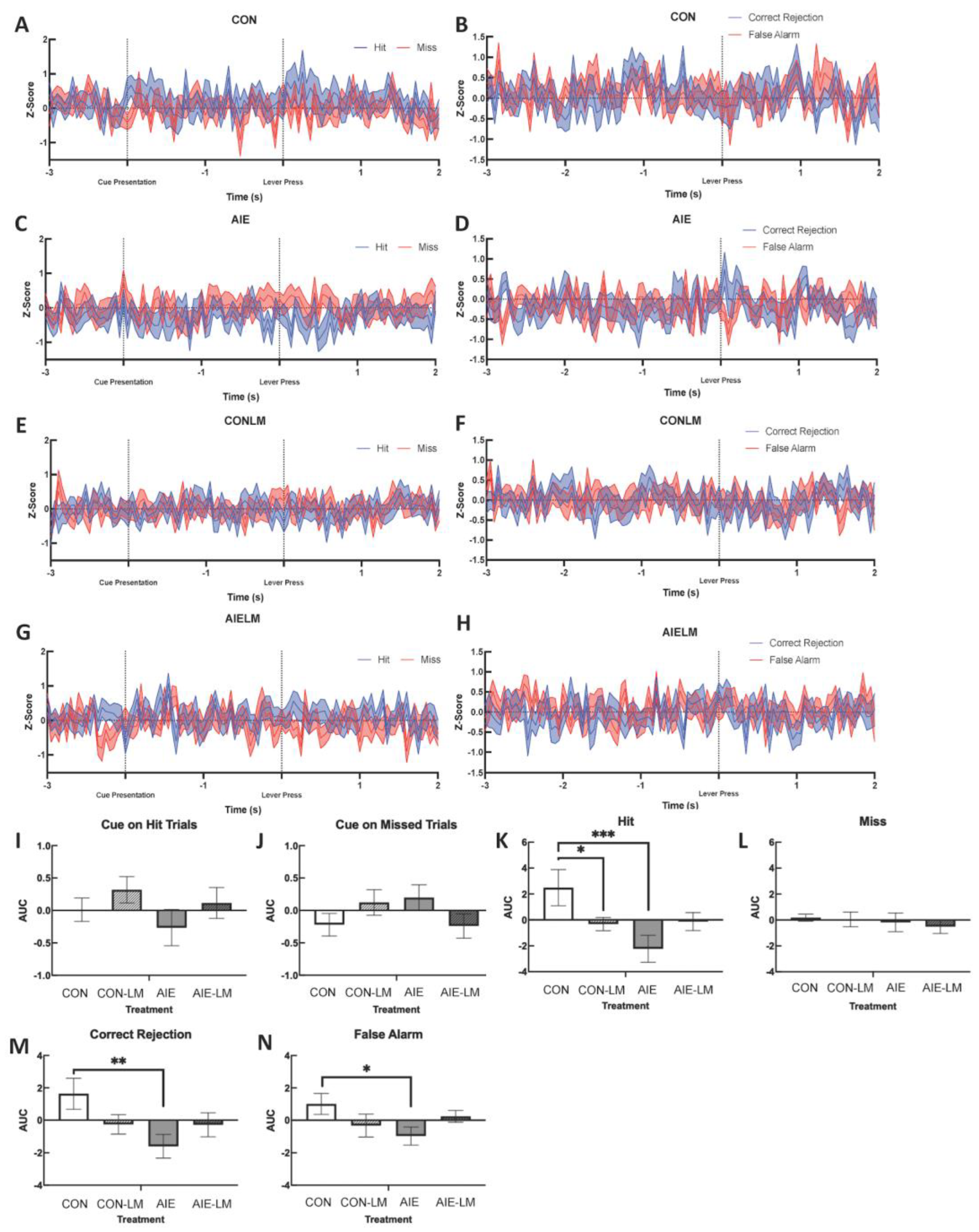
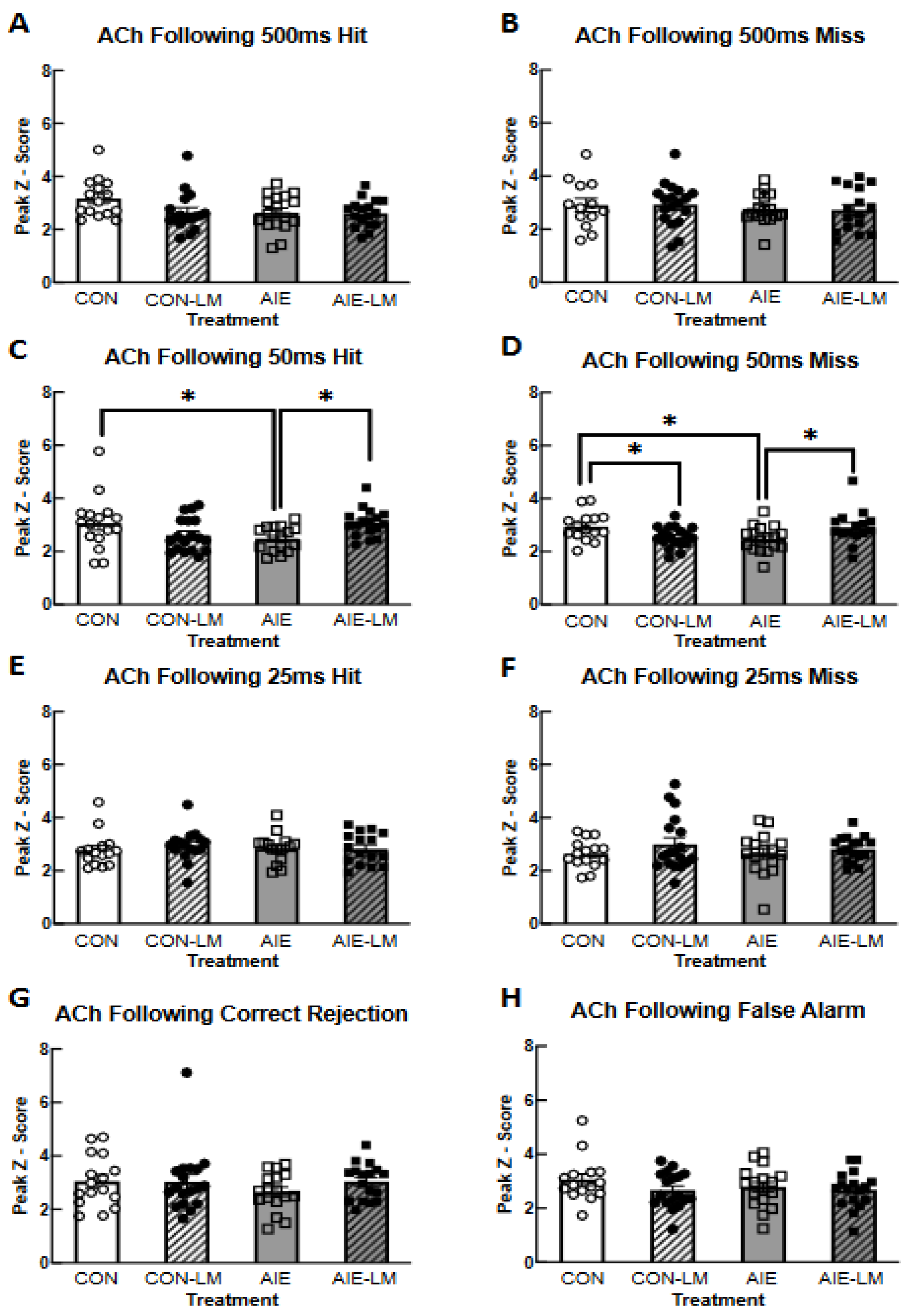
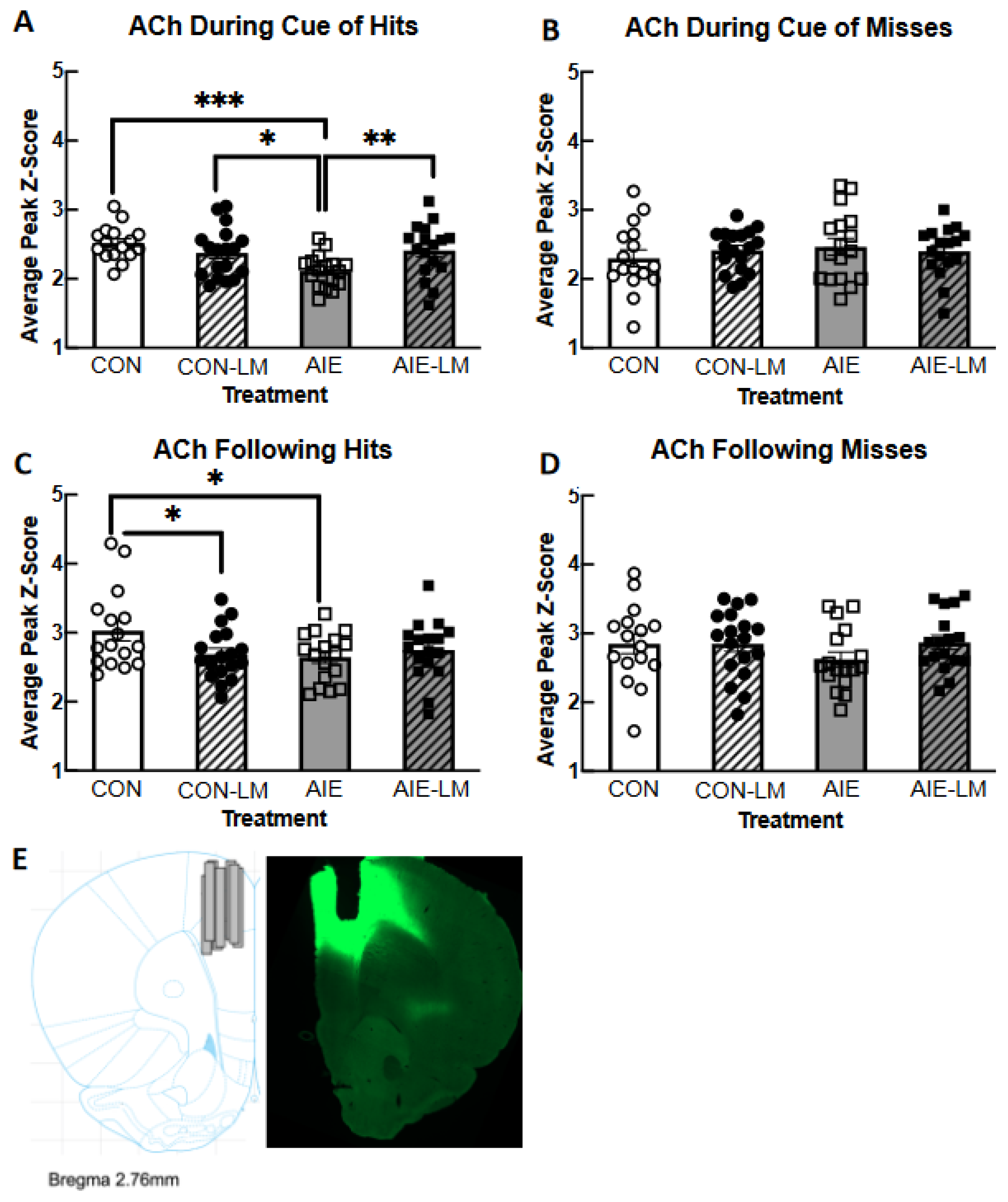
2.5. GRAB ACh 3.0 Activity in the mPFC during SAT
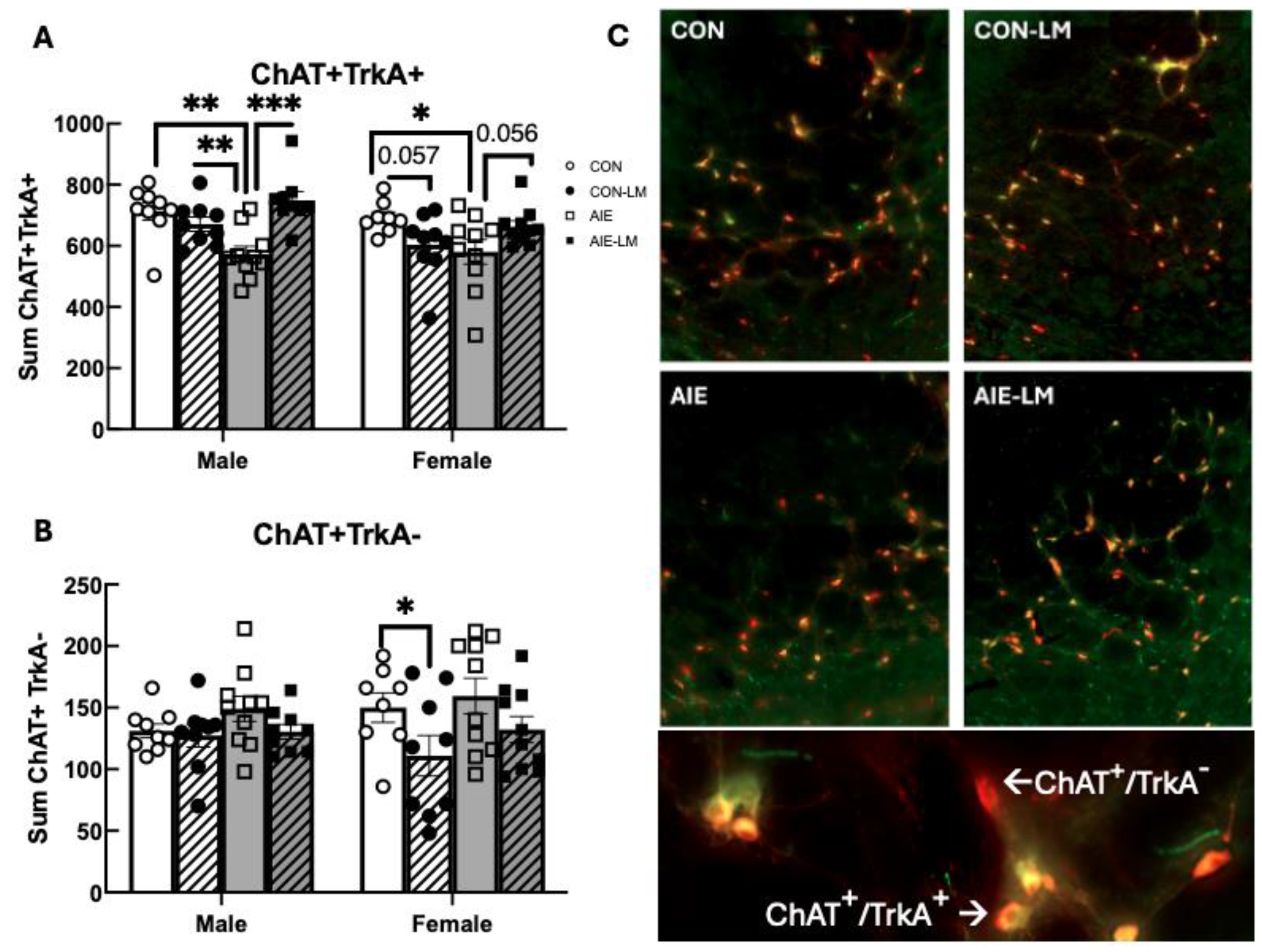
2.6. NbM ChAT+ TrkA+ Cell Counts
3. Materials and Methods
3.1. Subjects
3.2. Treatment
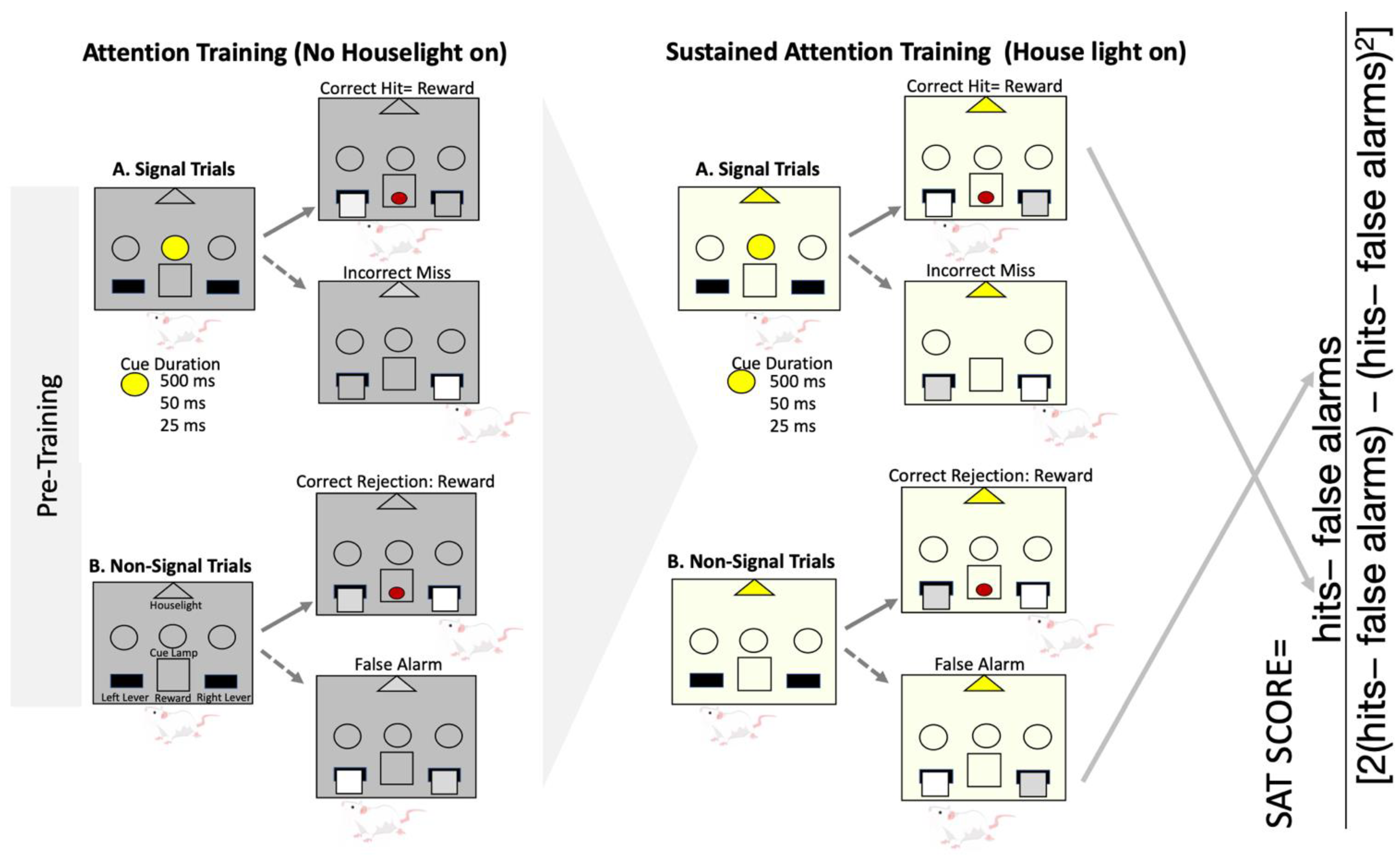
3.3. Operant Sustained Attention Task
3.4. ACh Grab 3.0 Viral Infusion Surgery and ACh 3.0 Recording
3.5. ACh 3.0 Data Analysis
3.6. Tissue Collection
3.7. ChAT and TrkA Immunofluorescence
3.8. Microscopy and ChAT/TrkA Cell Counting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nunes, P.T.; Kipp, B.T.; Reitz, N.L.; Savage, L.M. Aging with alcohol-related brain damage: Critical brain circuits associated with cognitive dysfunction. Int. Rev. Neurobiol. 2019, 148, 101–168. [Google Scholar] [PubMed]
- Crews, F.T.; Robinson, D.L.; Chandler, L.J.; Ehlers, C.L.; Mulholland, P.J.; Pandey, S.C.; Rodd, Z.A.; Spear, L.P.; Swartzwelder, H.S.; Vetreno, R.P. Mechanisms of Persistent Neurobiological Changes Following Adolescent Alcohol Exposure: NADIA Consortium Findings. Alcohol. Clin. Exp. Res. 2019, 43, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- Seemiller, L.R.; Gould, T.J. The effects of adolescent alcohol exposure on learning and related neurobiology in humans and rodents. Neurobiol. Learn. Mem. 2020, 172, 107234. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Sarter, M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Záborszky, L.; Gombkoto, P.; Varsanyi, P.; Gielow, M.R.; Poe, G.; Role, L.W.; Ananth, M.; Rajebhosale, P.; Talmage, D.A.; Hasselmo, M.E.; et al. Specific Basal Forebrain-Cortical Cholinergic Circuits Coordinate Cognitive Operations. J. Neurosci. 2018, 38, 9446–9458. [Google Scholar] [CrossRef] [PubMed]
- Kucinski, A.; Phillips, K.B.; Koshy Cherian, A.; Sarter, M. Rescuing the attentional performance of rats with cholinergic losses by the M1 positive allosteric modulator TAK-071. Psychopharmacology 2020, 237, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Gritton, H.J.; Howe, W.M.; Mallory, C.S.; Hetrick, V.L.; Berke, J.D.; Sarter, M. Cortical cholinergic signaling controls the detection of cues. Proc. Natl. Acad. Sci. USA 2016, 113, E1089–E1097. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Kozak, R.; Martinez, V.; Sarter, M. Prefrontal Acetylcholine Release Controls Cue Detection on Multiple Timescales. Neuron 2007, 56, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, C.; Shichkova, P.; Keller, D.; Markram, H.; Ramaswamy, S. Cellular, Synaptic and Network Effects of Acetylcholine in the Neocortex. Front. Neural Circuits 2019, 13, 24. [Google Scholar] [CrossRef]
- Cole, A.E.; Nicoll, R.A. The pharmacology of cholinergic excitatory responses in hippocampal pyramidal cells. Brain Res. 1984, 305, 283–290. [Google Scholar] [CrossRef]
- Eggermann, E.; Feldmeyer, D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proc. Natl. Acad. Sci. USA 2009, 106, 11753–11758. [Google Scholar] [CrossRef] [PubMed]
- Hangya, B.; Ranade, S.P.; Lorenc, M.; Kepecs, A. Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell 2015, 162, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E.; Bower, J.M. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J. Neurophysiol. 1992, 67, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Crawford, J.; Callahan, P.M.; Terry, A.V., Jr.; Constantinidis, C.; Blake, D.T. Intermittent Stimulation of the Nucleus Basalis of Meynert Improves Working Memory in Adult Monkeys. Curr. Biol. 2017, 27, 2640–2646.e4. [Google Scholar] [CrossRef] [PubMed]
- Monosov, I.E.; Leopold, D.A.; Hikosaka, O. Neurons in the Primate Medial Basal Forebrain Signal Combined Information about Reward Uncertainty, Value, and Punishment Anticipation. J. Neurosci. 2015, 35, 7443–7459. [Google Scholar] [CrossRef] [PubMed]
- Gombkoto, P.; Gielow, M.; Varsanyi, P.; Chavez, C.; Zaborszky, L. Contribution of the basal forebrain to corticocortical network interactions. Brain Struct. Funct. 2021, 226, 1803–1821. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.C.; Pinto, L.; Brock, J.R.; Dan, Y. Calcium Imaging of Basal Forebrain Activity during Innate and Learned Behaviors. Front. Neural Circuits 2016, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.T.; Nunes, P.T.; Galaj, E.; Hitchcock, B.; Nasra, T.; Poynor, K.R.; Heide, S.K.; Reitz, N.L.; Savage, L.M. Adolescent Ethanol Exposure Alters Cholinergic Function and Apical Dendritic Branching within the Orbital Frontal Cortex. Neuroscience 2021, 473, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Fisher, R.; Deason, C.; Vetreno, R.P. Loss of Basal Forebrain Cholinergic Neurons Following Adolescent Binge Ethanol Exposure: Recovery with the Cholinesterase Inhibitor Galantamine. Front. Behav. Neurosci. 2021, 15, 652494. [Google Scholar] [CrossRef]
- Fernandez, G.M.; Savage, L.M. Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience 2017, 361, 129–143. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Broadwater, M.; Liu, W.; Spear, L.P.; Crews, F.T. Adolescent, but Not Adult, Binge Ethanol Exposure Leads to Persistent Global Reductions of Choline Acetyltransferase Expressing Neurons in Brain. PLoS ONE 2014, 9, e113421. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Crews, F.T. Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin. PLoS ONE 2018, 13, e0204500. [Google Scholar] [CrossRef]
- Galaj, E.; Kipp, B.T.; Floresco, S.B.; Savage, L.M. Persistent Alterations of Accumbal Cholinergic Interneurons and Cognitive Dysfunction after Adolescent Intermittent Ethanol Exposure. Neuroscience 2019, 404, 153–164. [Google Scholar] [CrossRef]
- Fernandez, G.M.; Lew, B.J.; Vedder, L.C.; Savage, L.M. Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience 2017, 348, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Li, Y.; Zeng, J.; Huang, P.; Skirzewski, M.; Kljakic, O.; Peng, W.; Qian, T.; Tan, K.; Zou, J.; et al. An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat. Methods 2020, 17, 1139–1146. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.; Varga, V.; Jing, M.; Karadas, M.; Li, Y.; Buzsáki, G. Cholinergic suppression of hippocampal sharp-wave ripples impairs working memory. Proc. Natl. Acad. Sci. USA 2021, 118, e2016432118. [Google Scholar] [CrossRef]
- Crouse, R.B.; Kim, K.; Batchelor, H.M.; Girardi, E.M.; Kamaletdinova, R.; Chan, J.; Rajebhosale, P.; Pittenger, S.T.; Role, L.W.; Talmage, D.A.; et al. Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife 2020, 9, e57335. [Google Scholar] [CrossRef] [PubMed]
- Clewes, O.; Fahey, M.S.; Tyler, S.J.; Watson, J.J.; Seok, H.; Catania, C.; Cho, K.; Dawbarn, D.; Allen, S.J. Human ProNGF: Biological effects and binding profiles at TrkA, P75NTR and sortilin. J. Neurochem. 2008, 107, 1124–1135. [Google Scholar] [CrossRef]
- Bandtlow, C.; Dechant, G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci. STKE 2004, 2004, pe24. [Google Scholar] [CrossRef]
- Barker, P.A. p75NTR is positively promiscuous: Novel partners and new insights. Neuron 2004, 42, 529–533. [Google Scholar] [CrossRef]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.K. ProBDNF Induces Neuronal Apoptosis via Activation of a Receptor Complex of p75NTR and Sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef]
- Teng, K.K.; Felice, S.; Kim, T.; Hempstead, B.L. Understanding proneurotrophin actions: Recent advances and challenges. Dev. Neurobiol. 2010, 70, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.K.; Hempstead, B.L. Neurotrophins and their receptors: Signaling trios in complex biological systems. Cell. Mol. Life Sci. 2004, 61, 35–48. [Google Scholar] [CrossRef]
- Tomellini, E.; Lagadec, C.; Polakowska, R.; Le Bourhis, X. Role of p75 neurotrophin receptor in stem cell biology: More than just a marker. Cell. Mol. Life Sci. 2014, 71, 2467–2481. [Google Scholar] [CrossRef] [PubMed]
- Fortress, A.M.; Buhusi, M.; Helke, K.L.; Granholm, A.C. Cholinergic Degeneration and Alterations in the TrkA and p75NTR Balance as a Result of Pro-NGF Injection into Aged Rats. J. Aging Res. 2011, 2011, 460543. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ménard, M.; Atkinson, T.; Brown, L.; Whitfield, J.; Chakravarthy, B. Relative expression of the p75 neurotrophin receptor, tyrosine receptor kinase A, and insulin receptor in SH-SY5Y neuroblastoma cells and hippocampi from Alzheimer’s disease patients. Neurochem. Int. 2016, 101, 22–29. [Google Scholar] [CrossRef]
- Darcq, E.; Morisot, N.; Phamluong, K.; Warnault, V.; Jeanblanc, J.; Longo, F.M.; Massa, S.M.; Ron, D. The Neurotrophic Factor Receptor p75 in the Rat Dorsolateral Striatum Drives Excessive Alcohol Drinking. J. Neurosci. 2016, 36, 10116–10127. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.M.; Xie, Y.; Yang, T.; Harrington, A.W.; Kim, M.L.; Yoon, S.O.; Kraemer, R.; Moore, L.A.; Hempstead, B.L.; Longo, F.M. Small, Nonpeptide p75NTR Ligands Induce Survival Signaling and Inhibit proNGF-Induced Death. J. Neurosci. 2006, 26, 5288–5300. [Google Scholar] [CrossRef]
- Xie, Y.; Seawell, J.; Boesch, E.; Allen, L.; Suchy, A.; Longo, F.M.; Meeker, R.B. Small molecule modulation of the p75 neurotrophin receptor suppresses age- and genotype-associated neurodegeneration in HIV gp120 transgenic mice. Exp. Neurol. 2021, 335, 113489. [Google Scholar] [CrossRef]
- Xie, Y.; Meeker, R.B.; Massa, S.M.; Longo, F.M. Modulation of the p75 neurotrophin receptor suppresses age-related basal forebrain cholinergic neuron degeneration. Sci. Rep. 2019, 9, 5273. [Google Scholar] [CrossRef]
- Yang, T.; Knowles, J.K.; Lu, Q.; Zhang, H.; Arancio, O.; Moore, L.A.; Chang, T.; Wang, Q.; Andreasson, K.; Rajadas, J.; et al. Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS ONE 2008, 3, e3604. [Google Scholar] [CrossRef]
- Haefeli, J.; Ferguson, A.R.; Bingham, D.; Orr, A.; Won, S.J.; Lam, T.I.; Shi, J.; Hawley, S.; Liu, J.; Swanson, R.A.; et al. A data-driven approach for evaluating multi-modal therapy in traumatic brain injury. Scientific Reports 2017, 7, 42474. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.A.; Knowles, J.K.; Belichenko, N.P.; Banerjee, G.; Finkle, C.; Massa, S.M.; Longo, F.M. A small molecule p75NTR ligand, LM11A-31, reverses cholinergic neurite dystrophy in Alzheimer’s disease mouse models with mid- to late-stage disease progression. PLoS ONE 2014, 9, e102136. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Longo, F.M.; Massa, S.M. A small molecule p75(NTR) ligand protects neurogenesis after traumatic brain injury. Stem Cells 2013, 31, 2561–2574. [Google Scholar] [CrossRef]
- Phillips, K.B.; Sarter, M. Addiction vulnerability and the processing of significant cues: Sign-, but not goal-, tracker perceptual sensitivity relies on cue salience. Behav. Neurosci. 2020, 134, 133–143. [Google Scholar] [CrossRef]
- Green, D.M.; Swets, J.A. Signal detection theory and psychophysics; John Wiley: Oxford, England, 1966; p. xi, 455. [Google Scholar]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef]
- Frey, P.W.; Colliver, J.A. Sensitivity and responsivity measures for discrimination learning. Learn. Motiv. 1973, 4, 327–342. [Google Scholar] [CrossRef]
- Pollack, I.; Norman, D.A. A non-parametric analysis of recognition experiments. Psychon. Sci. 1964, 1, 125–126. [Google Scholar] [CrossRef]
- Grier, J.B. Nonparametric indexes for sensitivity and bias: Computing formulas. Psychol. Bull. 1971, 75, 424–429. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Coleman, L.G., Jr.; Crews, F.T.; Vetreno, R.P. The persistent impact of adolescent binge alcohol on adult brain structural, cellular, and behavioral pathology: A role for the neuroimmune system and epigenetics. Int. Rev. Neurobiol. 2021, 160, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Vetreno, R.P. Cholinergic REST-G9a gene repression through HMGB1-TLR4 neuroimmune signaling regulates basal forebrain cholinergic neuron phenotype. Front. Mol. Neurosci. 2022, 15, 992627. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2021, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Bohnsack, J.P.; Kusumo, H.; Liu, W.; Pandey, S.C.; Crews, F.T. Neuroimmune and epigenetic involvement in adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons: Restoration with voluntary exercise. Addict. Biol. 2020, 25, e12731. [Google Scholar] [CrossRef] [PubMed]
- Blatter, K.; Graw, P.; Münch, M.; Knoblauch, V.; Wirz-Justice, A.; Cajochen, C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav. Brain Res. 2006, 168, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.C. A further study on the sustained attention response to task (SART): The effect of age, gender and education. Brain Inj. 2001, 15, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Conners, C.K.; Epstein, J.N.; Angold, A.; Klaric, J. Continuous performance test performance in a normative epidemiological sample. J. Abnorm. Child Psychol. 2003, 31, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Giambra, L.M.; Quilter, R.E. Sex differences in sustained attention across the adult life span. J. Appl. Psychol. 1989, 74, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, J.D.; Taylor, J.R. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav. Neurosci. 2003, 117, 76–83. [Google Scholar] [CrossRef]
- Riley, E.; Okabe, H.; Germine, L.; Wilmer, J.; Esterman, M.; DeGutis, J. Gender Differences in Sustained Attentional Control Relate to Gender Inequality across Countries. PLoS ONE 2016, 11, e0165100. [Google Scholar] [CrossRef]
- Yuan, J.; He, Y.; Qinglin, Z.; Chen, A.; Li, H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology 2008, 45, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Paolone, G.; Mallory, C.S.; Cherian, A.K.; Miller, T.R.; Blakely, R.D.; Sarter, M. Monitoring cholinergic activity during attentional performance in mice heterozygous for the choline transporter: A model of cholinergic capacity limits. Neuropharmacology 2013, 75, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Holley, L.A.; Wiley, R.G.; Lappi, D.A.; Sarter, M. Cortical cholinergic deafferentation following the intracortical infusion of 192 IgG-saporin: A quantitative histochemical study. Brain Res. 1994, 663, 277–286. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Spadoni, A.D.; Infante, M.A.; Myers, M.G.; Tapert, S.F. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 2009, 23, 715–722. [Google Scholar] [CrossRef]
- Tapert, S.F.; Brown, S.A. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. J. Int. Neuropsychol. Soc. 1999, 5, 481–493. [Google Scholar] [CrossRef]
- Tarter, R.E.; Mezzich, A.C.; Hsieh, Y.-C.; Parks, S.M. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Boutros, N.; Der-Avakian, A.; Markou, A.; Semenova, S. Effects of early life stress and adolescent ethanol exposure on adult cognitive performance in the 5-choice serial reaction time task in Wistar male rats. Psychopharmacology 2017, 234, 1549–1556. [Google Scholar] [CrossRef]
- Sanchez-Roige, S.; Baro, V.; Trick, L.; Peña-Oliver, Y.; Stephens, D.N.; Duka, T. Exaggerated Waiting Impulsivity Associated with Human Binge Drinking, and High Alcohol Consumption in Mice. Neuropsychopharmacology 2014, 39, 2919–2927. [Google Scholar] [CrossRef]
- Semenova, S. Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: The effects of task and ethanol challenges. Psychopharmacology 2012, 219, 433–442. [Google Scholar] [CrossRef]
- Sey, N.Y.A.; Gómez, A.A.; Madayag, A.C.; Boettiger, C.A.; Robinson, D.L. Adolescent intermittent ethanol impairs behavioral flexibility in a rat foraging task in adulthood. Behav. Brain Res. 2019, 373, 112085. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Hosová, D.; Towner, T.; Werner, D.F.; Spear, L.P. Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behav. Brain Res. 2020, 378, 112292. [Google Scholar] [CrossRef]
- Madayag, A.C.; Stringfield, S.J.; Reissner, K.J.; Boettiger, C.A.; Robinson, D.L. Sex and Adolescent Ethanol Exposure Influence Pavlovian Conditioned Approach. Alcohol. Clin. Exp. Res. 2017, 41, 846–856. [Google Scholar] [CrossRef] [PubMed]
- McClory, A.J.; Spear, L.P. Effects of ethanol exposure during adolescence or in adulthood on Pavlovian conditioned approach in Sprague-Dawley rats. Alcohol 2014, 48, 755–763. [Google Scholar] [CrossRef]
- Enkel, T.; Bartsch, D.; Bähner, F. Sign- and goal-tracking rats show differences in various executive functions. Behav. Brain Res. 2019, 371, 111979. [Google Scholar] [CrossRef]
- Maillet, D.; Mandin, V.; Huppé-Gourgues, F. Speed of task acquisition in sign-tracker and goal-tracker rats using 5-CSRTT. Psychol. Neurosci. 2022, 15, 281–291. [Google Scholar] [CrossRef]
- Sarter, M.; Hasselmo, M.E.; Bruno, J.P.; Givens, B. Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Rev. 2005, 48, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Sobreviela, T.; Clary, D.O.; Reichardt, L.F.; Brandabur, M.M.; Kordower, J.H.; Mufson, E.J. TrkA-immunoreactive profiles in the central nervous system: Colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J. Comp. Neurol. 1994, 350, 587–611. [Google Scholar] [CrossRef]
- Batchelor, P.E.; Armstrong, D.M.; Blaker, S.N.; Gage, F.H. Nerve growth factor receptor and choline acetyltransferase colocalization in neurons within the rat forebrain: Response to fimbria-fornix transection. J. Comp. Neurol. 1989, 284, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S.; Fahnestock, M. ProNGF, but Not NGF, Switches from Neurotrophic to Apoptotic Activity in Response to Reductions in TrkA Receptor Levels. Int. J. Mol. Sci. 2017, 18, 599. [Google Scholar] [CrossRef]
- Allard, S.; Leon, W.C.; Pakavathkumar, P.; Bruno, M.A.; Ribeiro-da-Silva, A.; Cuello, A.C. Impact of the NGF Maturation and Degradation Pathway on the Cortical Cholinergic System Phenotype. J. Neurosci. 2012, 32, 2002–2012. [Google Scholar] [CrossRef]
- Venero, J.L.; Knüsel, B.; Beck, K.D.; Hefti, F. Expression of neurotrophin and trk receptor genes in adult rats with fimbria transections: Effect of intraventricular nerve growth factor and brain-derived neurotrophic factor administration. Neuroscience 1994, 59, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Nelson, M.; Granholm, A.C.; Coultrap, S.; Browning, M.; Curtis, M. Altered NGF response but not release in the aged septo-hippocampal cholinergic system. Exp. Neurol. 2005, 196, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.K.; Simmons, D.A.; Nguyen, T.V.; Vander Griend, L.; Xie, Y.; Zhang, H.; Yang, T.; Pollak, J.; Chang, T.; Arancio, O.; et al. Small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol. Aging 2013, 34, 2052–2063. [Google Scholar] [CrossRef]
- Reitz, N.L.; Nunes, P.T.; Savage, L.M. Adolescent Binge-Type Ethanol Exposure in Rats Mirrors Age-Related Cognitive Decline by Suppressing Cholinergic Tone and Hippocampal Neurogenesis. Front. Behav. Neurosci. 2021, 22, 772857. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kipp, B.T.; Savage, L.M. Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction. Int. J. Mol. Sci. 2024, 25, 5792. https://doi.org/10.3390/ijms25115792
Kipp BT, Savage LM. Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction. International Journal of Molecular Sciences. 2024; 25(11):5792. https://doi.org/10.3390/ijms25115792
Chicago/Turabian StyleKipp, Brian T., and Lisa M. Savage. 2024. "Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction" International Journal of Molecular Sciences 25, no. 11: 5792. https://doi.org/10.3390/ijms25115792
APA StyleKipp, B. T., & Savage, L. M. (2024). Modulation of the p75NTR during Adolescent Alcohol Exposure Prevents Cholinergic Neuronal Atrophy and Associated Acetylcholine Activity and Behavioral Dysfunction. International Journal of Molecular Sciences, 25(11), 5792. https://doi.org/10.3390/ijms25115792



