Downregulation of hsa-miR-100-5p May Be a Protective Factor in the Early Stages of Nephropathy in Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Sample Characterization
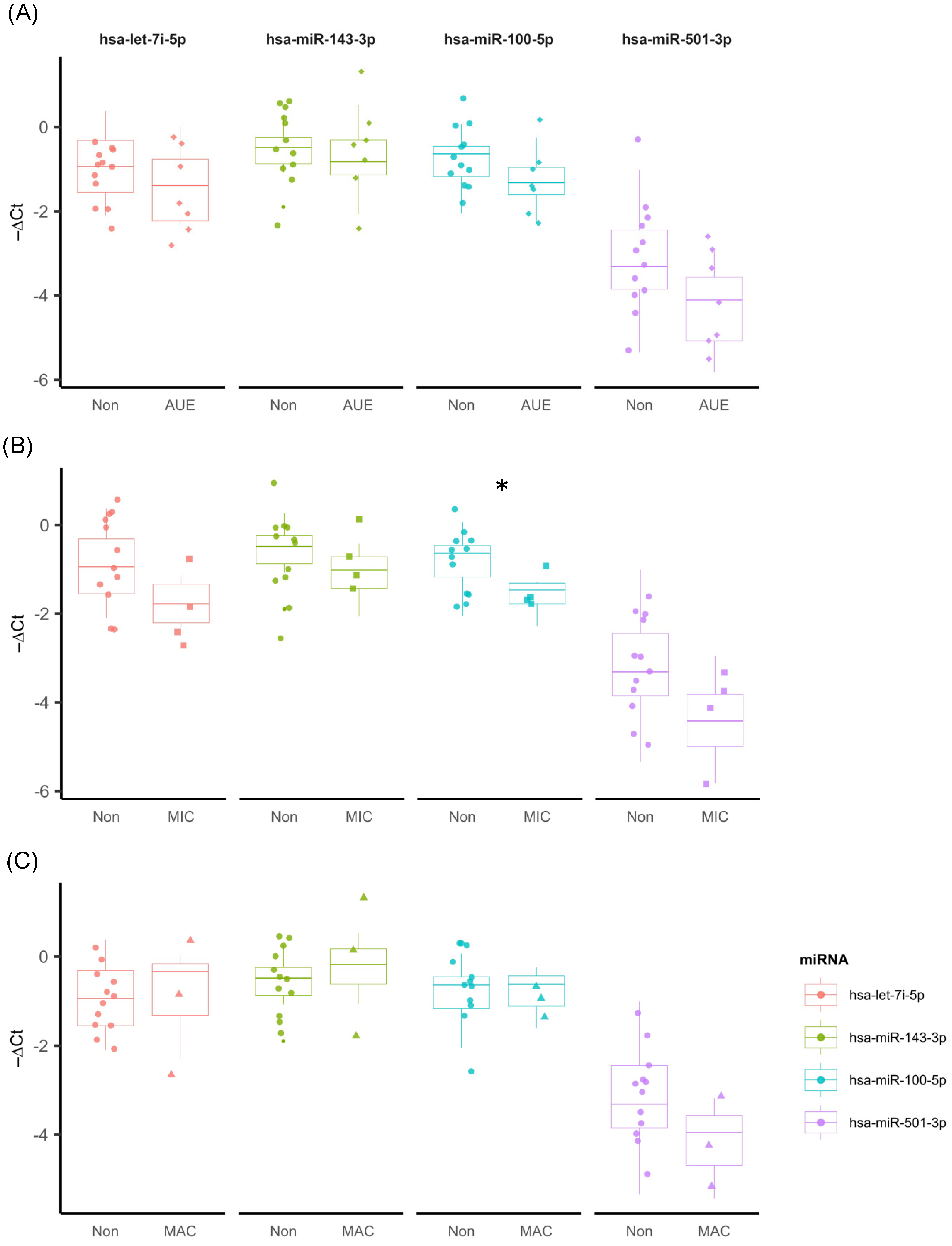
2.2. Characterization of miRNAs
3. Discussion
3.1. hsa-let-7i-5p
3.2. hsa-miR-143-3p
3.3. hsa-miR-501-3p
3.4. hsa-miR-100-5p
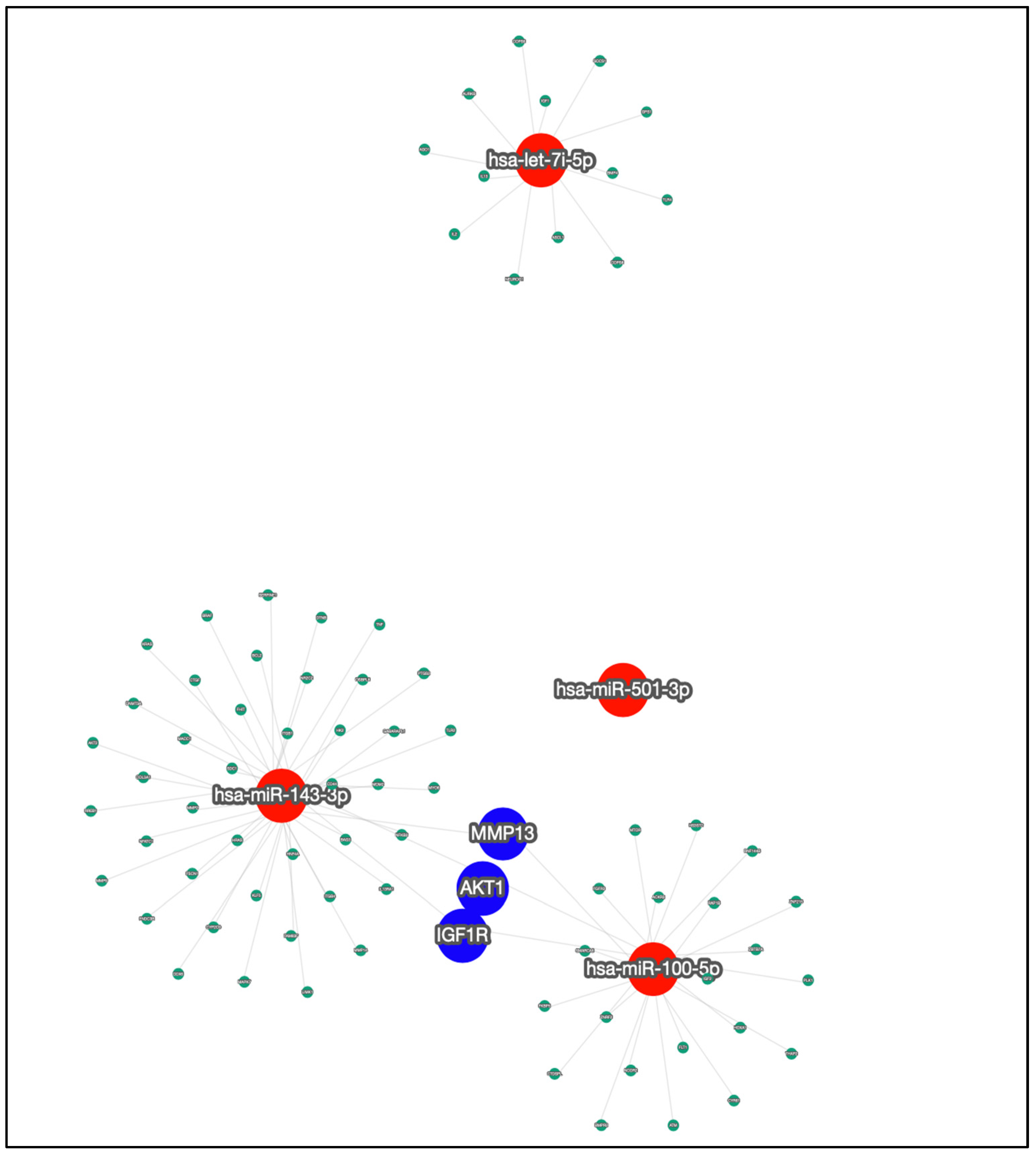
3.5. Target Genes
4. Materials and Methods
4.1. Sampling and Ethical Aspects
4.2. Selection of miRNAs
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akil, A.A.-S.; Yassin, E.; Al-Maraghi, A.; Aliyev, E.; Al-Malki, K.; Fakhro, K.A. Diagnosis and Treatment of Type 1 Diabetes at the Dawn of the Personalized Medicine Era. J. Transl. Med. 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Ciężki, S.; Kurpiewska, E.; Bossowski, A.; Głowińska-Olszewska, B. Multi-Faceted Influence of Obesity on Type 1 Diabetes in Children—From Disease Pathogenesis to Complications. Front. Endocrinol. 2022, 13, 890833. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, X.; Cai, J.; Chen, J.; Huang, L.; Wu, W.; Pugliese, A.; Li, S.; Ricordi, C.; Tan, J. Prevention of Chronic Diabetic Complications in Type 1 Diabetes by Co-Transplantation of Umbilical Cord Mesenchymal Stromal Cells and Autologous Bone Marrow: A Pilot Randomized Controlled Open-Label Clinical Study with 8-Year Follow-Up. Cytotherapy 2022, 24, 421–427. [Google Scholar] [CrossRef] [PubMed]
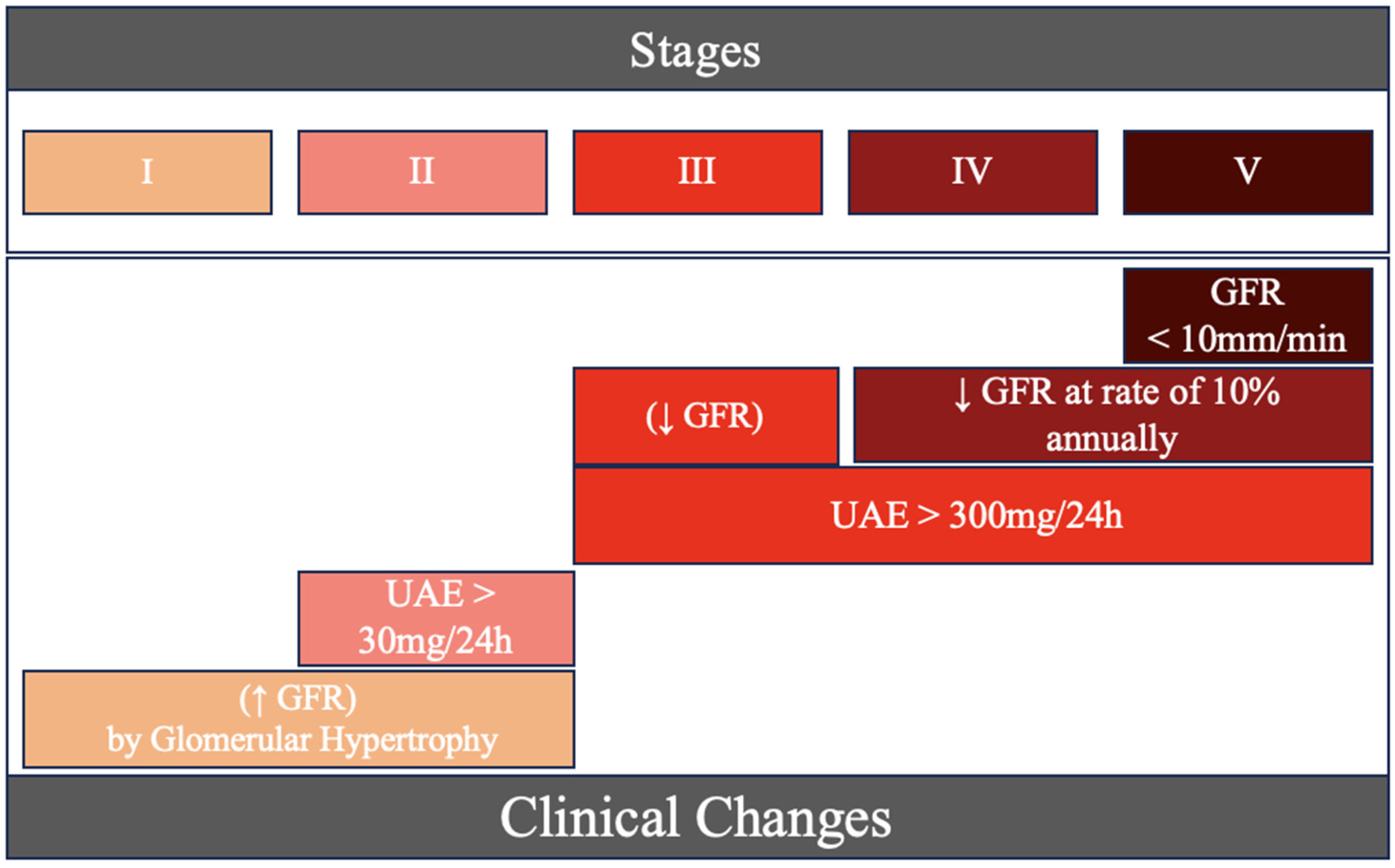
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Sociedade Brasileira de Diabetes. Diretrizes Da Sociedade Brasileira de Diabetes; Editora Clannad: São Paulo, Brazil, 2017. [Google Scholar]
- Mahtal, N.; Lenoir, O.; Tinel, C.; Anglicheau, D.; Tharaux, P.-L. MicroRNAs in Kidney Injury and Disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef]
- Papadopoulou-Marketou, N.; Paschou, S.A.; Marketos, N.; Adamidi, S.; Adamidis, S.; Kanaka-Gantenbein, C. Diabetic Nephropathy in Type 1 Diabetes. Minerva Med. 2018, 109, 218–228. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S175–S184. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.R.D.; Domingueti, C.P. MicroRNAs: New Biomarkers and Promising Therapeutic Targets for Diabetic Kidney Disease. J. Bras. Nefrol. 2019, 41, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Porto, J.R.; Gomes, K.B.; Fernandes, A.P.; Domingueti, C.P. Evaluation of Renal Function in Chronic Kidney Disease. Rev. Bras. Anál. Clín. 2017, 49, 26–35. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Bhatt, K.; Kato, M.; Natarajan, R. Mini-Review: Emerging Roles of microRNAs in the Pathophysiology of Renal Diseases. Am. J. Physiol.-Ren. Physiol. 2016, 310, F109–F118. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yao, D.; Yan, H.; Chen, X.; Wang, L.; Zhan, H. The Role of MicroRNAs in the Pathogenesis of Diabetic Nephropathy. Int. J. Endocrinol. 2019, 2019, 8719060. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.M.D.; Resende, S.S.; Sousa, T.N.D.; Brito, C.F.A.D. A Systematic Scoping Review of the Genetic Ancestry of the Brazilian Population. Genet. Mol. Biol. 2019, 42, 495–508. [Google Scholar] [CrossRef]
- Ferraz, R.S.; Santos, L.C.B.; da-Silva-Cruz, R.L.; Braga-da-Silva, C.H.; Magalhães, L.; Ribeiro-dos-Santos, A.; Vidal, A.; Vinasco-Sandoval, T.; Reis-das-Mercês, L.; Sena-dos-Santos, C.; et al. Global miRNA Expression Reveals Novel Nuclear and Mitochondrial Interactions in Type 1 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 1033809. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.; Rome, S.; Sathishkumar, C.; Gastebois, C.; Meugnier, E.; Mohan, V.; Balasubramanyam, M. MicroRNAs from Urinary Extracellular Vesicles Are Non-Invasive Early Biomarkers of Diabetic Nephropathy in Type 2 Diabetes Patients with the ‘Asian Indian Phenotype’. Diabetes Metab. 2019, 45, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Riffo-Campos, A.L.; Ortega, A.; Martinez-Arroyo, O.; Perez-Gil, D.; Olivares, D.; Solaz, E.; Martinez, F.; Martínez-Hervás, S.; Chaves, F.J.; et al. Urinary- and Plasma-Derived Exosomes Reveal a Distinct MicroRNA Signature Associated With Albuminuria in Hypertension. Hypertension 2021, 77, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Bai, Y.; Zhang, H.; Zhang, S.; He, L.; Zhou, W.; Zhang, D.; Xu, J. Identification of a Genome-Wide Serum microRNA Expression Profile as Potential Noninvasive Biomarkers for Chronic Kidney Disease Using next-Generation Sequencing. J. Int. Med. Res. 2020, 48, 030006052096948. [Google Scholar] [CrossRef] [PubMed]
- Assmann, T.S.; Recamonde-Mendoza, M.; De Souza, B.M.; Crispim, D. MicroRNA Expression Profiles and Type 1 Diabetes Mellitus: Systematic Review and Bioinformatic Analysis. Endocr. Connect. 2017, 6, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Berdine, G. The Receiver Operating Characteristic (ROC) Curve. SW Resp. Crit. Care Chron. 2017, 5, 34. [Google Scholar] [CrossRef]
- Polo, T.C.F.; Miot, H.A. Aplicações Da Curva ROC Em Estudos Clínicos e Experimentais. J. Vasc. Bras. 2020, 19, e20200186. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y. Wnt/β-Catenin Signalling and Podocyte Dysfunction in Proteinuric Kidney Disease. Nat. Rev. Nephrol. 2015, 11, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Goru, S.K.; Kadakol, A.; Gaikwad, A.B. Hidden Targets of Ubiquitin Proteasome System: To Prevent Diabetic Nephropathy. Pharmacol. Res. 2017, 120, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Garud, M.; Kulkarni, Y. Hyperglycemia to Nephropathy via Transforming Growth Factor Beta. Curr. Diabetes Rev. 2014, 10, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Müller-Deile, J.; Gellrich, F.; Schenk, H.; Schroder, P.; Nyström, J.; Lorenzen, J.; Haller, H.; Schiffer, M. Overexpression of TGF-β Inducible microRNA-143 in Zebrafish Leads to Impairment of the Glomerular Filtration Barrier by Targeting Proteoglycans. Cell Physiol. Biochem. 2016, 40, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Tabei, A.; Sakairi, T.; Hamatani, H.; Ohishi, Y.; Watanabe, M.; Nakasatomi, M.; Ikeuchi, H.; Kaneko, Y.; Kopp, J.B.; Hiromura, K. The miR-143/145 Cluster Induced by TGF-Β1 Suppresses Wilms’ Tumor 1 Expression in Cultured Human Podocytes. Am. J. Physiol.-Ren. Physiol. 2023, 325, F121–F133. [Google Scholar] [CrossRef] [PubMed]
- Czarnowski, D. Syndecans in Cancer: A Review of Function, Expression, Prognostic Value, and Therapeutic Significance. Cancer Treat. Res. Commun. 2021, 27, 100312. [Google Scholar] [CrossRef]
- Deng, L.; Blanco, F.J.; Stevens, H.; Lu, R.; Caudrillier, A.; McBride, M.; McClure, J.D.; Grant, J.; Thomas, M.; Frid, M.; et al. MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ. Res. 2015, 117, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Arif, E.; Nihalani, D. Glomerular Filtration Barrier Assembly: An Insight. Postdoc J. 2013, 1, 33–45. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, S.; Ying, Y.; Xie, H.; Li, J.; Ma, X.; Wang, W.; Shen, H.; Wang, X.; Zheng, X.; et al. MicroRNA-501-3p Inhibits the Proliferation of Kidney Cancer Cells by Targeting WTAP. Cancer Med. 2021, 10, 7222–7232. [Google Scholar] [CrossRef]
- Hara, N.; Kikuchi, M.; Miyashita, A.; Hatsuta, H.; Saito, Y.; Kasuga, K.; Murayama, S.; Ikeuchi, T.; Kuwano, R. Serum microRNA miR-501-3p as a Potential Biomarker Related to the Progression of Alzheimer’s Disease. Acta Neuropathol. Commun. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ding, Y.; Wang, Y.; Wang, C.; Sun, K.; Wang, L. Identification of Serum miR-501-3p and miR-338-3p as Novel Diagnostic Biomarkers for Breast Cancer and Their Target Genes Associated with Immune Infiltration. Int. J. Gen. Med. 2023, 16, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, P.; Liu, K.; Xu, W.; Wang, J.; Li, Q.; Chen, B.; Deng, Y.; Han, C.; Sun, T.; et al. MiRNA-100 Ameliorates Diabetes Mellitus-induced Erectile Dysfunction by Modulating Autophagy, Anti-inflammatory, and Antifibrotic Effects. Andrology 2024. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Chen, T.; Feng, G. MiR-100-5p Transfected MSCs-Derived Exosomes Can Suppress NSCLC Progression via PI3K-AKT-mTOR. Oncologie 2023, 25, 705–715. [Google Scholar] [CrossRef]
- Dunlop, E.A.; Tee, A.R. mTOR and Autophagy: A Dynamic Relationship Governed by Nutrients and Energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Miloudi, K.; Oubaha, M.; Ménard, C.; Dejda, A.; Guber, V.; Cagnone, G.; Wilson, A.M.; Tétreault, N.; Mawambo, G.; Binet, F.; et al. NOTCH1 Signaling Induces Pathological Vascular Permeability in Diabetic Retinopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 4538–4547. [Google Scholar] [CrossRef] [PubMed]
- Kern, F.; Aparicio-Puerta, E.; Li, Y.; Fehlmann, T.; Kehl, T.; Wagner, V.; Ray, K.; Ludwig, N.; Lenhof, H.-P.; Meese, E.; et al. miRTargetLink 2.0—Interactive miRNA Target Gene and Target Pathway Networks. Nucleic Acids Res. 2021, 49, W409–W416. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yu, J.; Yu, F.; Yang, S.; Qian, Q.; Zha, Y. IGF-1/IGF-1R Blockade Ameliorates Diabetic Kidney Disease through Normalizing Snail1 Expression in a Mouse Model. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E686–E698. [Google Scholar] [CrossRef] [PubMed]
- Asmy, V.K.S.S.; Natarajan, J. Comparative Co-Expression Analysis of RNA-Seq Transcriptome Revealing Key Genes, miRNA and Transcription Factor in Distinct Metabolic Pathways in Diabetic Nerve, Eye, and Kidney Disease. Genom. Inform. 2022, 20, e26. [Google Scholar] [CrossRef]
- Li, J.; Dong, R.; Yu, J.; Yi, S.; Da, J.; Yu, F.; Zha, Y. Inhibitor of IGF1 Receptor Alleviates the Inflammation Process in the Diabetic Kidney Mouse Model without Activating SOCS2. Drug Des. Dev. Ther. 2018, 12, 2887–2896. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2015 Abridged for Primary Care Providers. Am. Diabetes Assoc. 2015, 33, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Variable | Non (n = 12) | MIC (n = 4) | MAC (n = 3) |
|---|---|---|---|
| BMI | 23.1 ± 3.9 | 22.7 ± 2.2 | 29.8 ± 6.6 |
| FBG | 203.2 ± 101.3 | 76.3 ± 27.5 | 136.7 ± 23.4 |
| GFR | 121.8 ± 12.1 | 117.1 ± 26.5 | 83.7 ± 33.4 |
| Creatinine | 0.7 ± 0.2 | 0.8 ± 0.3 | 1.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, A.H.G.; Pereira, B.d.O.; Silva, L.S.D.; Melo, F.T.C.d.; Souza, A.C.C.B.d.; Leal, V.S.G.; Figueiredo, P.B.B.d.; Neto, J.F.A.; Santos, M.C.d.; Queiroz, N.N.M.d.; et al. Downregulation of hsa-miR-100-5p May Be a Protective Factor in the Early Stages of Nephropathy in Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 5663. https://doi.org/10.3390/ijms25115663
Pinheiro AHG, Pereira BdO, Silva LSD, Melo FTCd, Souza ACCBd, Leal VSG, Figueiredo PBBd, Neto JFA, Santos MCd, Queiroz NNMd, et al. Downregulation of hsa-miR-100-5p May Be a Protective Factor in the Early Stages of Nephropathy in Type 1 Diabetes Mellitus. International Journal of Molecular Sciences. 2024; 25(11):5663. https://doi.org/10.3390/ijms25115663
Chicago/Turabian StylePinheiro, Andrey Henrique Gama, Beatriz de Oliveira Pereira, Lilian Souza D’Albuquerque Silva, Franciane T. Cunha de Melo, Ana Carolina C. Braga de Souza, Valéria S. Galvão Leal, Priscila B. Barbosa de Figueiredo, João F. Abrahão Neto, Marcia Costa dos Santos, Natércia Neves Marques de Queiroz, and et al. 2024. "Downregulation of hsa-miR-100-5p May Be a Protective Factor in the Early Stages of Nephropathy in Type 1 Diabetes Mellitus" International Journal of Molecular Sciences 25, no. 11: 5663. https://doi.org/10.3390/ijms25115663
APA StylePinheiro, A. H. G., Pereira, B. d. O., Silva, L. S. D., Melo, F. T. C. d., Souza, A. C. C. B. d., Leal, V. S. G., Figueiredo, P. B. B. d., Neto, J. F. A., Santos, M. C. d., Queiroz, N. N. M. d., Felício, K. M., Ribeiro-dos-Santos, Â., Felício, J. S., & Cavalcante, G. C. (2024). Downregulation of hsa-miR-100-5p May Be a Protective Factor in the Early Stages of Nephropathy in Type 1 Diabetes Mellitus. International Journal of Molecular Sciences, 25(11), 5663. https://doi.org/10.3390/ijms25115663





