Insight into the Mechanism of Lysogeny Control of phiCDKH01 Bacteriophage Infecting Clinical Isolate of Clostridioides difficile
Abstract
1. Introduction
2. Results
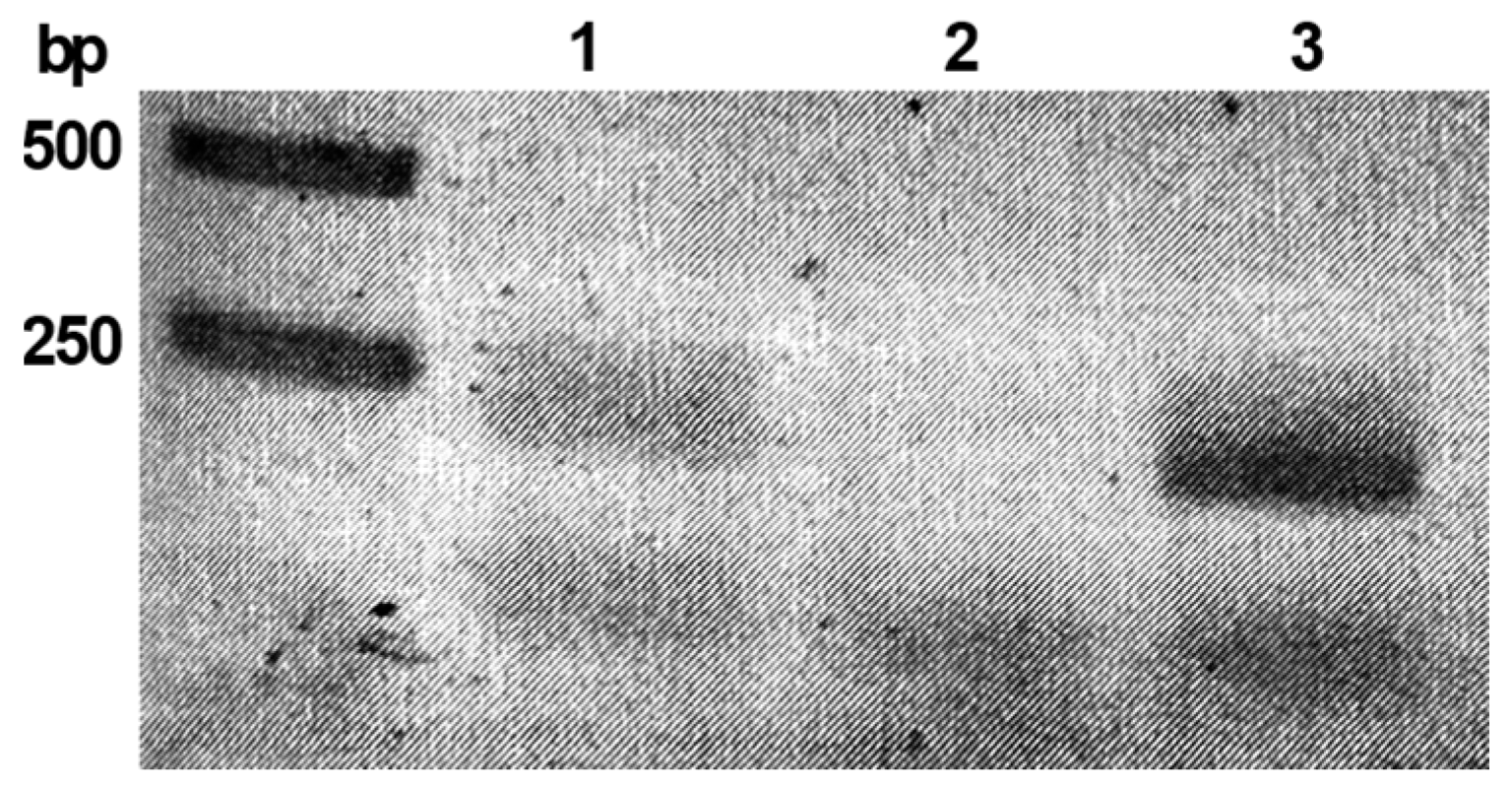
2.1. The phiCDKH01_17 Is Expressed in Lysogenic Strain C. difficile CD34/Sr
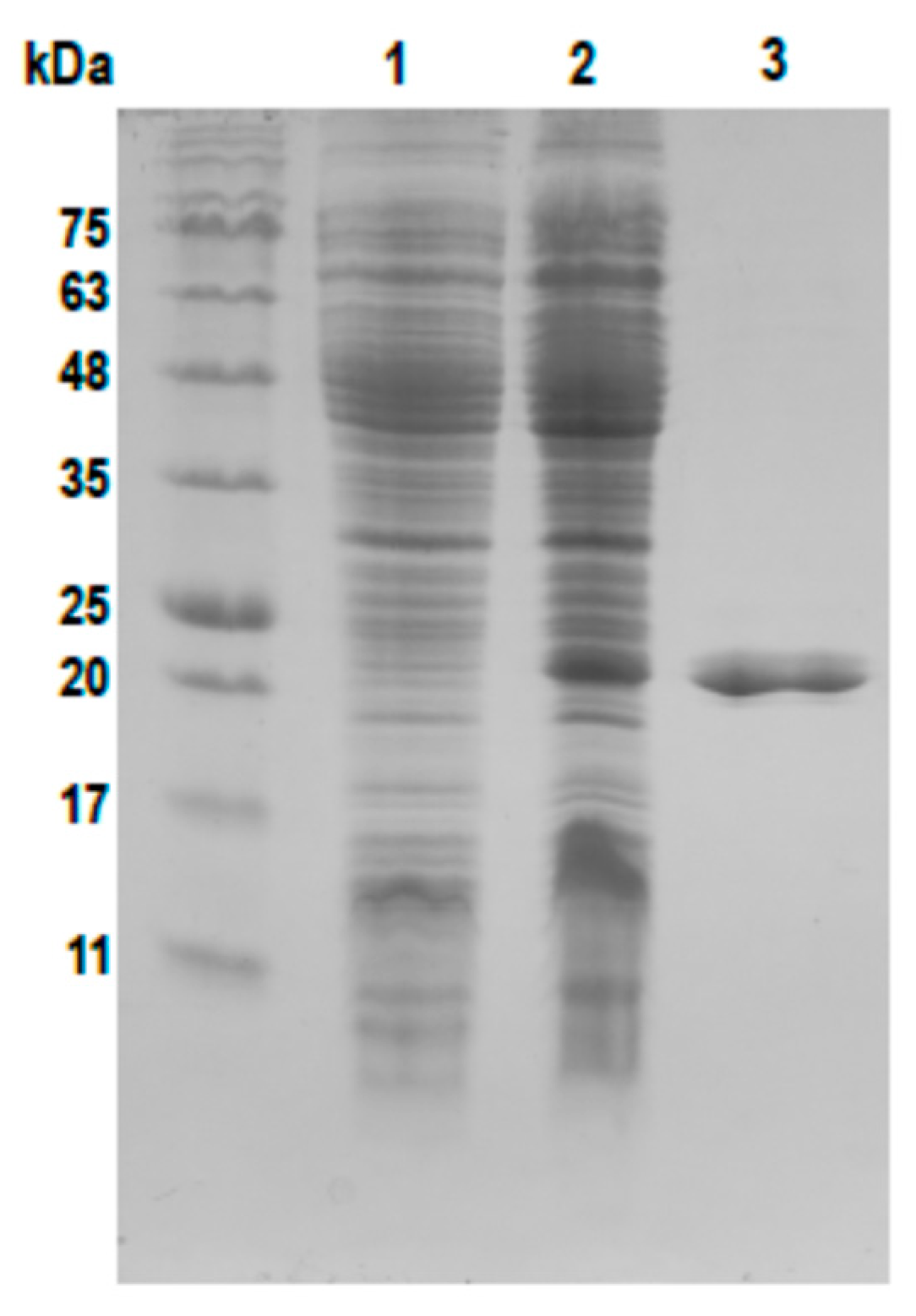
2.2. Cloning and Purification of the phiCDKH01 XRE Protein
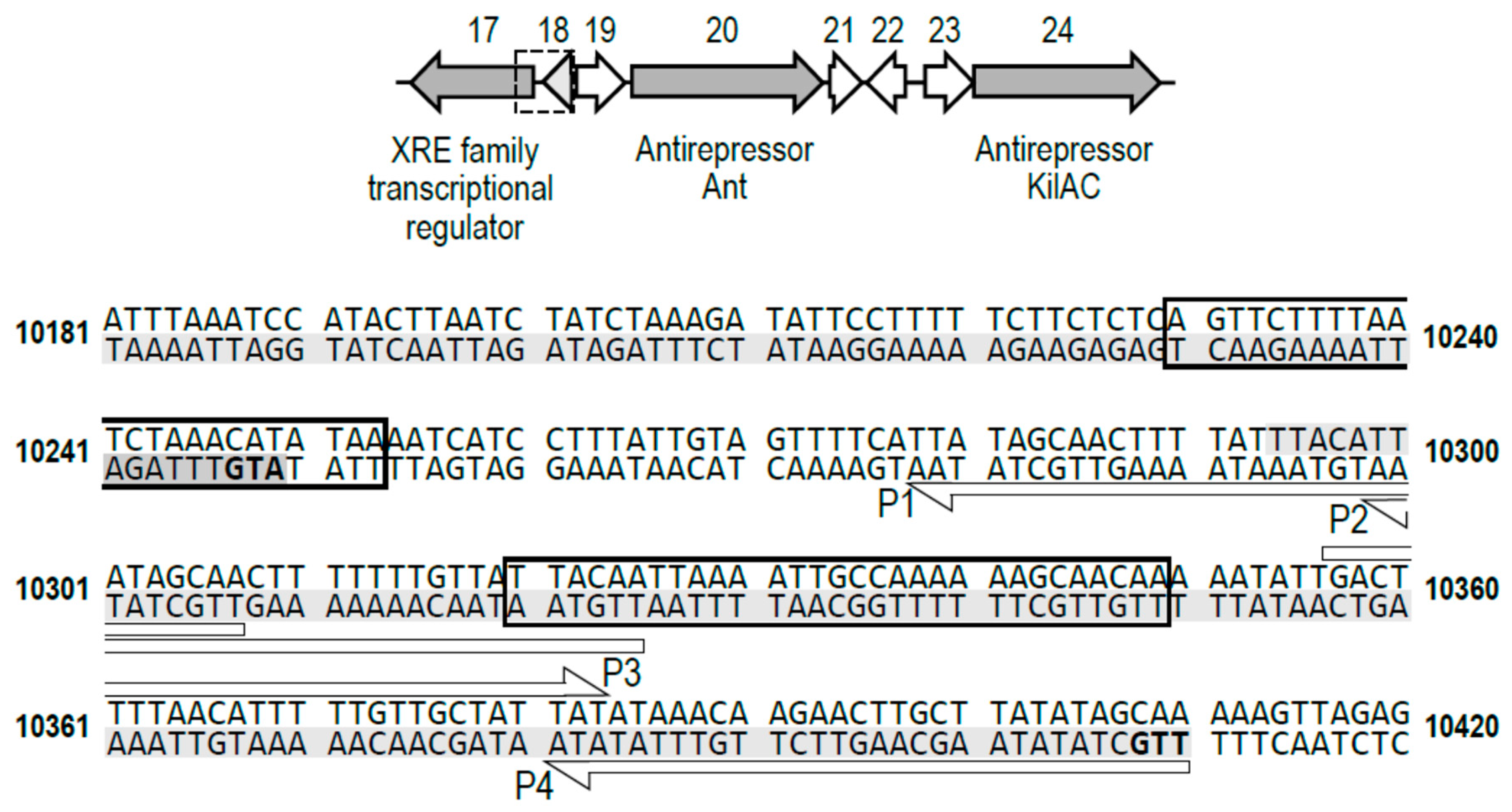
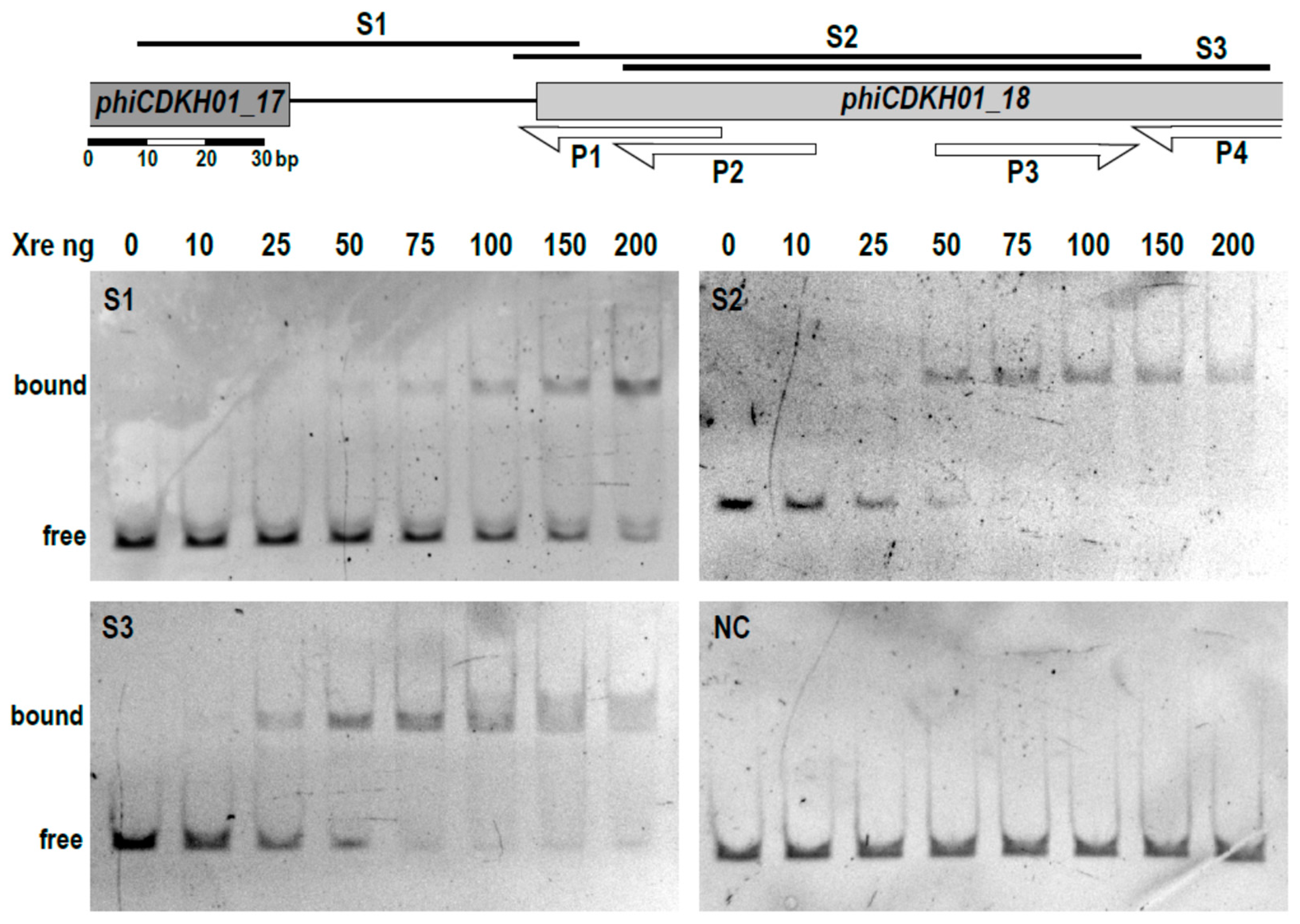
2.3. The XRE Protein Binds to the Putative Lysis/Lysogeny Regulatory Region within the phiCDKH01 Genome
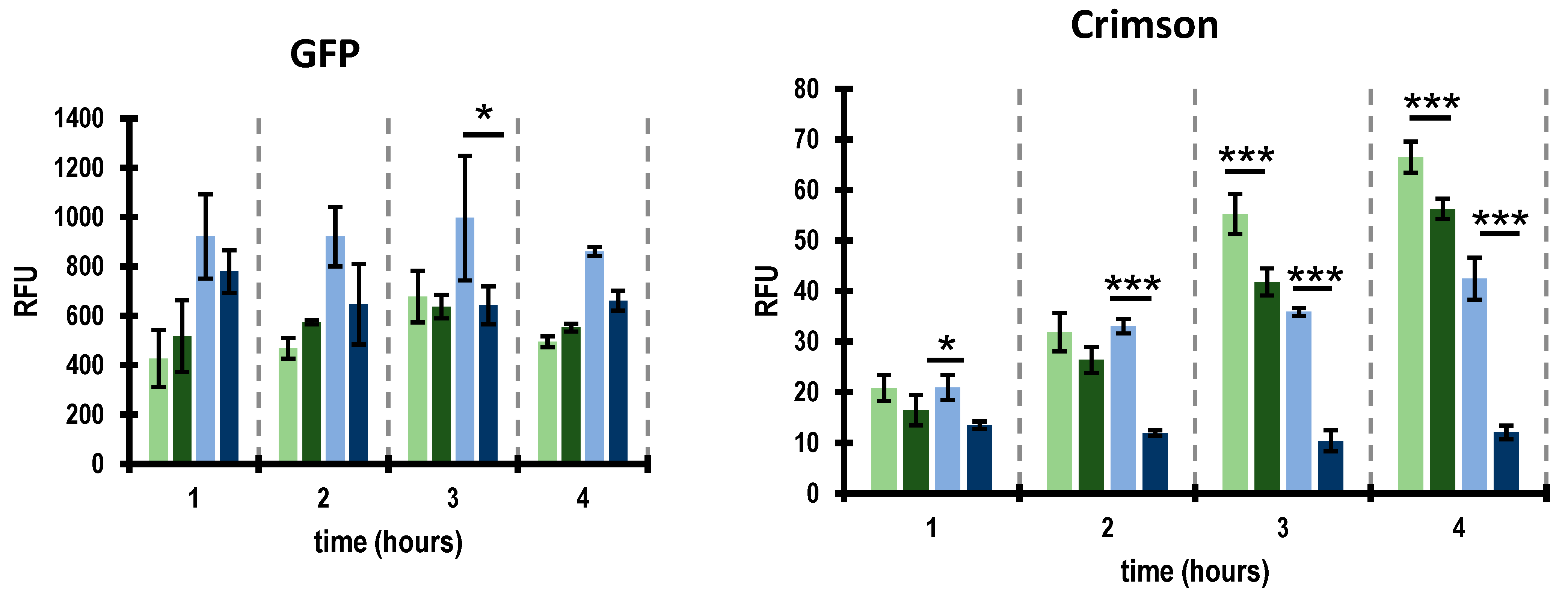
2.4. Activity of Promoters Located within the Putative Lysis/Lysogeny Regulatory Region Are Controlled by the XRE Protein
3. Discussion
4. Materials and Methods
4.1. The phiCDKH01_17 Encodes a Putative XRE-Family Transcription Factor
4.2. Bacterial Strains and Culture Conditions
4.3. RNA Isolation from C. difficile, Reverse Transcription and Analysis of the phiCDKH01_17 Gene Transcription
4.4. Phage Induction, Purification and DNA Extraction
4.5. Plasmid Construction
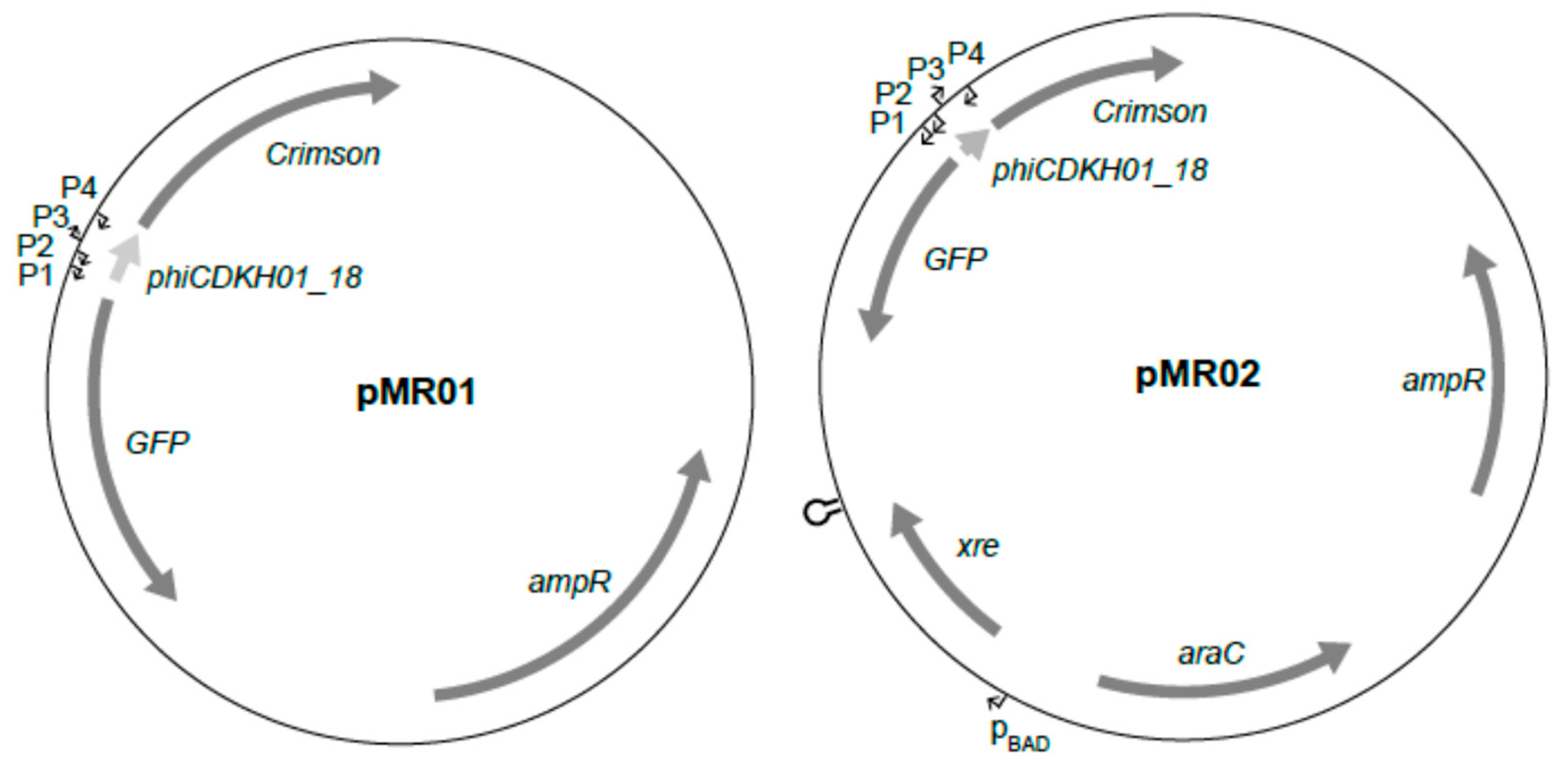
4.5.1. phiCDKH01
4.5.2. pMR01
4.5.3. pMR02
4.6. Overexpression and Purification of phiCDKH01 XRE Protein
4.7. Gel Retardation Electrophoresis
4.8. Monitoring Putative Phage Promoters Activity
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freeman, J.; Bauer, M.P.; Baines, S.D.; Corver, J.; Fawley, W.N.; Goorhuis, B.; Kuijper, E.J.; Wilcox, M.H. The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 2010, 23, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Arthur, A.K.; Anibueze, C.I.; Singh, S.; Cavallazzi, R.; Loke, Y.K. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: Meta-analysis. Am. J. Gastroenterol. 2012, 107, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Slimings, C.; Riley, T.V. Antibiotics and hospital-acquired Clostridium difficile infection: Update of systematic review and meta-analysis. J. Antimicrob. Chemother. 2014, 69, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Shen, A. Clostridium difficile toxins: Mediators of inflammation. J. Innate Immun. 2012, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Heuler, J.; Fortier, L.-C.; Sun, X. Clostridioides difficile phage biology and application. FEMS Microbiol. Rev. 2021, 45, fuab012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shang, J.; Peng, C.; Sun, Y. Phage family classification under Caudoviricetes: A review of current tools using the latest ICTV classification framework. Front. Microbiol. 2022, 13, 1032186. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.; Ong, P.F.; Song, K.P.; Riley, T.V.; Chang, B.J. The complete genome sequence of Clostridium difficile phage ϕC2 and comparisons to ϕCD119 and inducible prophages of CD630. Microbiology 2007, 153, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Govind, R.; Fralick, J.A.; Rolfe, R.D. Genomic organization and molecular characterization of Clostridium difficile bacteriophage ΦCD119. J. Bacteriol. 2006, 188, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Sekulovic, O.; Meessen-Pinard, M.; Fortier, L.-C. Prophage-stimulated toxin production in Clostridium difficile NAP1/027 Lysogens. J. Bacteriol. 2011, 193, 2726–2734. [Google Scholar] [CrossRef]
- Boudry, P.; Semenova, E.; Monot, M.; Datsenko, K.A.; Lopatina, A.; Sekulovic, O.; Ospina-Bedoya, M.; Fortier, L.-C.; Severinov, K.; Dupuy, B.; et al. Function of the CRISPR-Cas System of the Human Pathogen Clostridium difficile. mBio 2015, 6, e01112-15. [Google Scholar] [CrossRef]
- Wittmann, J.; Riedel, T.; Bunk, B.; Spröer, C.; Gronow, S.; Overmann, J. Complete Genome Sequence of the Novel Temperate Clostridium difficile Phage phiCDIF1296T. Genome Announc. 2015, 3, e00839-15. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.; Wittmann, J.; Bunk, B.; Schober, I.; Spröer, C.; Gronow, S.; Overmann, J. A Clostridioides difficile bacteriophage genome encodes functional binary toxin-associated genes. J. Biotechnol. 2017, 250, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mahony, D.E.; Bell, P.D.; Easterbrook, K.B. Two bacteriophages of Clostridium difficile. J. Clin. Microbiol. 1985, 21, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Meessen-Pinard, M.; Sekulovic, O.; Fortier, L.-C. Evidence of in vivo prophage induction during Clostridium difficile infection. Appl. Environ. Microbiol. 2012, 78, 7662–7670. [Google Scholar] [CrossRef] [PubMed]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Sekulovic, O.; Garneau, J.R.; Néron, A.; Fortier, L.-C. Characterization of temperate phages infecting Clostridium difficile isolates of human and animal origins. Appl. Environ. Microbiol. 2014, 80, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.-C.; Moineau, S. Morphological and genetic diversity of temperate phages in Clostridium difficile. Appl. Environ. Microbiol. 2007, 73, 7358–7366. [Google Scholar] [CrossRef] [PubMed]
- Nale, J.Y.; Shan, J.; Hickenbotham, P.T.; Fawley, W.N.; Wilcox, M.H.; Clokie, M.R.J. Diverse temperate bacteriophage carriage in Clostridium difficile 027 strains. PLoS ONE 2012, 7, e37263. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lewis, D.E.A.; Adhya, S. The Developmental Switch in Bacteriophage λ: A Critical Role of the Cro Protein. J. Mol. Biol. 2017, 430, 58–68. [Google Scholar] [CrossRef]
- Mayer, M.J.; Narbad, A.; Gasson, M.J. Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J. Bacteriol. 2008, 190, 6734–6740. [Google Scholar] [CrossRef]
- Hinc, K.; Kabała, M.; Iwanicki, A.; Martirosian, G.; Negri, A.; Obuchowski, M. Complete genome sequence of the newly discovered temperate Clostridioides difficile bacteriophage phiCDKH01 of the family Siphoviridae. Arch. Virol. 2021, 166, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Tavares, F.; Thioulouse, J.; Normand, P. A phylogenomic analysis of bacterial helix–turn–helix transcription factors. FEMS Microbiol. Rev. 2009, 33, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.S.; Alber, B.E. Transcriptional Regulation by the Short-Chain Fatty Acyl Coenzyme A Regulator (ScfR) PccR Controls Propionyl Coenzyme a Assimilation by Rhodobacter sphaeroides. J. Bacteriol. 2015, 197, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Gerstmeir, R.; Cramer, A.; Dangel, P.; Schaffer, S.; Eikmanns, B.J. RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 2004, 186, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, Q.; Wei, R.; Li, R.; Zhao, D.; Ge, M.; Yao, Q.; Yu, X. The XRE Family Transcriptional Regulator SrtR in Streptococcus suis Is Involved in Oxidant Tolerance and Virulence. Front. Cell. Infect. Microbiol. 2019, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Kearns, D.B.; Branda, S.S.; Kolter, R.; Losick, R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2006, 59, 1216–1228. [Google Scholar] [CrossRef]
- Kearns, D.B.; Chu, F.; Branda, S.S.; Kolter, R.; Losick, R. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 2005, 55, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Ptashne, M. A Genetic Switch: Phage Lambda Revisited, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2004. [Google Scholar]
- Wood, H.E.; Devine, K.M.; McConnell, D.J. Characterisation of a repressor gene (xre) and a temperature-sensitive allele from the Bacillus subtilis prophage, PBSX. Gene 1990, 96, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sekulovic, O.; Fortier, L.-C. Global transcriptional response of Clostridium difficile carrying the ϕCD38-2 prophage. Appl. Environ. Microbiol. 2015, 81, 1364–1374. [Google Scholar] [CrossRef]
- Williams, R.; Meader, E.; Mayer, M.; Narbad, A.; Roberts, A.P.; Mullany, P. Determination of the attP and attB sites of phage ϕCD27 from Clostridium difficile NCTC 12727. J. Med. Microbiol. 2013, 62, 1439–1443. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, M.; Rocha, M.; Oliveira, H.; Dias, O. Predicting promoters in phage genomes using PhagePromoter. Bioinformatics 2019, 35, 5301–5302. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in US Burden of Clostridioides difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.; Velleman, M.; Schuster, H. The tripartite immunity system of phages P1 and P7. FEMS Microbiol. Rev. 1995, 17, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Koonin, E.V.; Aravind, L. Extensive domain shuffling in transcription regulators of DNA viruses and implications for the origin of fungal APSES transcription factors. Genome Biol. 2002, 3, research0012. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, A.B.; Kobiler, O.; Stavans, J.; Court, D.L.; Adhya, S. Switches in bacteriophage lambda development. Annu. Rev. Genet. 2005, 39, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Canchaya, C.; Pridmore, R.; Brüssow, H. The prophages of Lactobacillus johnsonii NCC 533: Comparative genomics and transcription analysis. Virology 2004, 320, 229–242. [Google Scholar] [CrossRef]
- Ptashne, M.; Jeffrey, A.; Johnson, A.; Maurer, R.; Meyer, B.; Pabo, C.; Roberts, T.; Sauer, R. How the λ repressor and cro work. Cell 1980, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.E.; McConnell, D.J. Overproduction, isolation, and DNA-binding characteristics of Xre, the repressor protein from the Bacillus subtilis defective prophage PBSX. J. Bacteriol. 1994, 176, 5831–5834. [Google Scholar] [CrossRef]
- McLaughlin, M.; Fiebig, A.; Crosson, S. XRE transcription factors conserved in Caulobacter and φCbK modulate adhesin development and phage production. PLoS Genet. 2023, 19, e1011048. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, D.; Crowther, P.; Doherty, J.; Jefferson, S.; DeCruz, E.; Noyer-Weidner, M.; Smith, S.; Michael, M.; Graham, M. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989, 17, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Phothichaisri, W.; Ounjai, P.; Phetruen, T.; Janvilisri, T.; Khunrae, P.; Singhakaew, S.; Wangroongsarb, P.; Chankhamhaengdecha, S. Characterization of Bacteriophages Infecting Clinical Isolates of Clostridium difficile. Front. Microbiol. 2018, 9, 1701. [Google Scholar] [CrossRef] [PubMed]
- Sekulović, O.; Fortier, L.-C. Clostridium Difficile: Methods and Protocols, 2nd ed.; Roberts, A.P., Mullany, P., Eds.; Humana: New York, NY, USA, 2016. [Google Scholar]

| Name | Relevant Genotype | Source of Reference |
|---|---|---|
| Escherichia coli | ||
| DH5α | fhuA2 lac(del)U169 phoA glnV44 W809 lacZ(del)M15 gyrA96 recA1 relA1endA1 thi-1 hsdR17 | [43] |
| EMB2401 | DH5α pBAD-XRE | This study |
| EMR01 | DH5α pMR01 | This study |
| EMR02 | DH5α pMR02 | This study |
| Clostridioides difficile | ||
| CD34-Sr | [21] | |
| phiCDKH01 phage | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanicki, A.; Roskwitalska, M.; Frankowska, N.; Wultańska, D.; Kabała, M.; Pituch, H.; Obuchowski, M.; Hinc, K. Insight into the Mechanism of Lysogeny Control of phiCDKH01 Bacteriophage Infecting Clinical Isolate of Clostridioides difficile. Int. J. Mol. Sci. 2024, 25, 5662. https://doi.org/10.3390/ijms25115662
Iwanicki A, Roskwitalska M, Frankowska N, Wultańska D, Kabała M, Pituch H, Obuchowski M, Hinc K. Insight into the Mechanism of Lysogeny Control of phiCDKH01 Bacteriophage Infecting Clinical Isolate of Clostridioides difficile. International Journal of Molecular Sciences. 2024; 25(11):5662. https://doi.org/10.3390/ijms25115662
Chicago/Turabian StyleIwanicki, Adam, Małgorzata Roskwitalska, Natalia Frankowska, Dorota Wultańska, Monika Kabała, Hanna Pituch, Michał Obuchowski, and Krzysztof Hinc. 2024. "Insight into the Mechanism of Lysogeny Control of phiCDKH01 Bacteriophage Infecting Clinical Isolate of Clostridioides difficile" International Journal of Molecular Sciences 25, no. 11: 5662. https://doi.org/10.3390/ijms25115662
APA StyleIwanicki, A., Roskwitalska, M., Frankowska, N., Wultańska, D., Kabała, M., Pituch, H., Obuchowski, M., & Hinc, K. (2024). Insight into the Mechanism of Lysogeny Control of phiCDKH01 Bacteriophage Infecting Clinical Isolate of Clostridioides difficile. International Journal of Molecular Sciences, 25(11), 5662. https://doi.org/10.3390/ijms25115662






