Does Salmonella diarizonae 58:r:z53 Isolated from a Mallard Duck Pose a Threat to Human Health?
Abstract
1. Introduction
2. Results
2.1. Source Animal and Antimicrobial Resistance of the Isolate
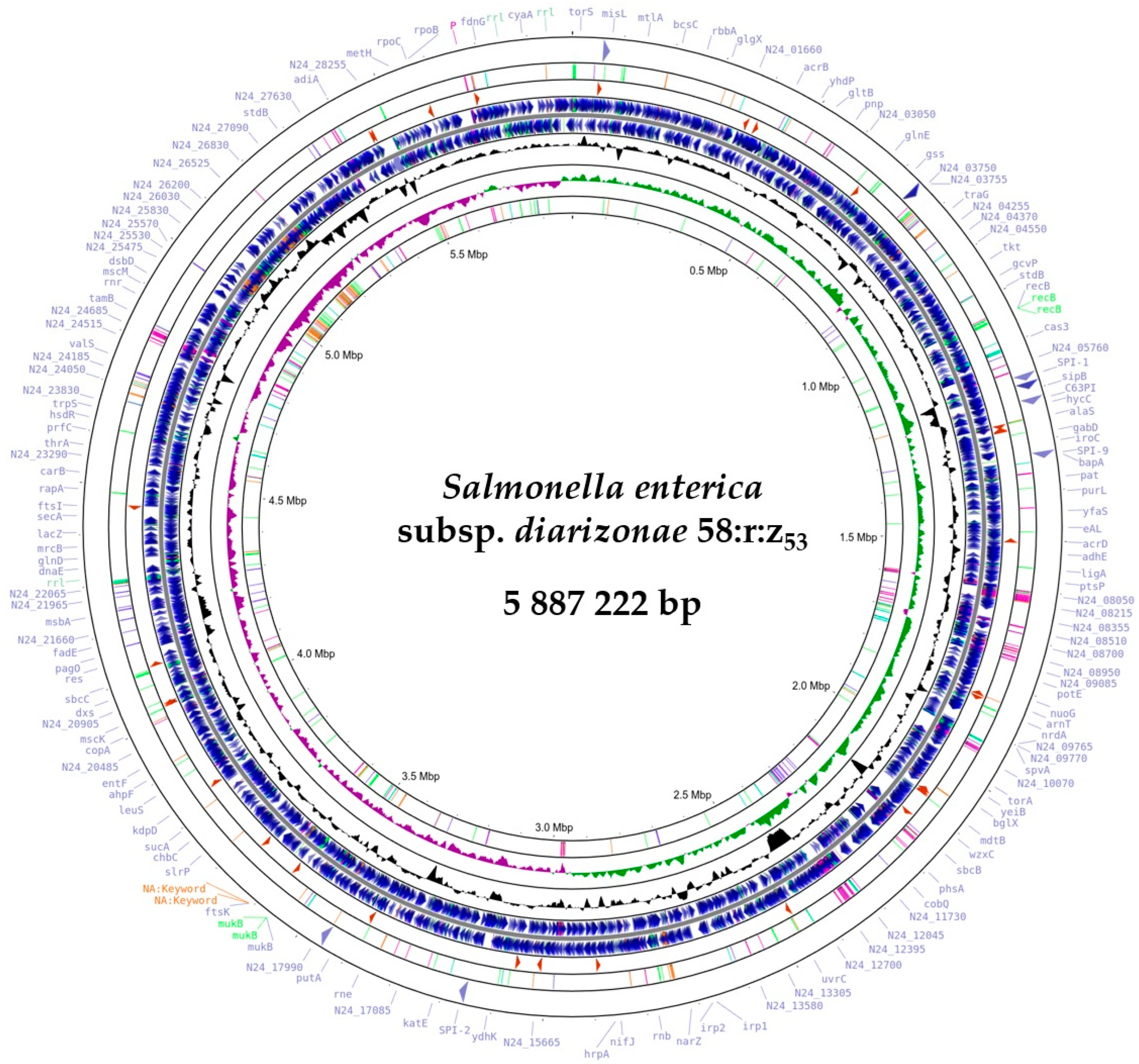
2.2. Phylogenetic Analysis of Salmonella enterica subsp. diarizonae IIIb 58:r:z53
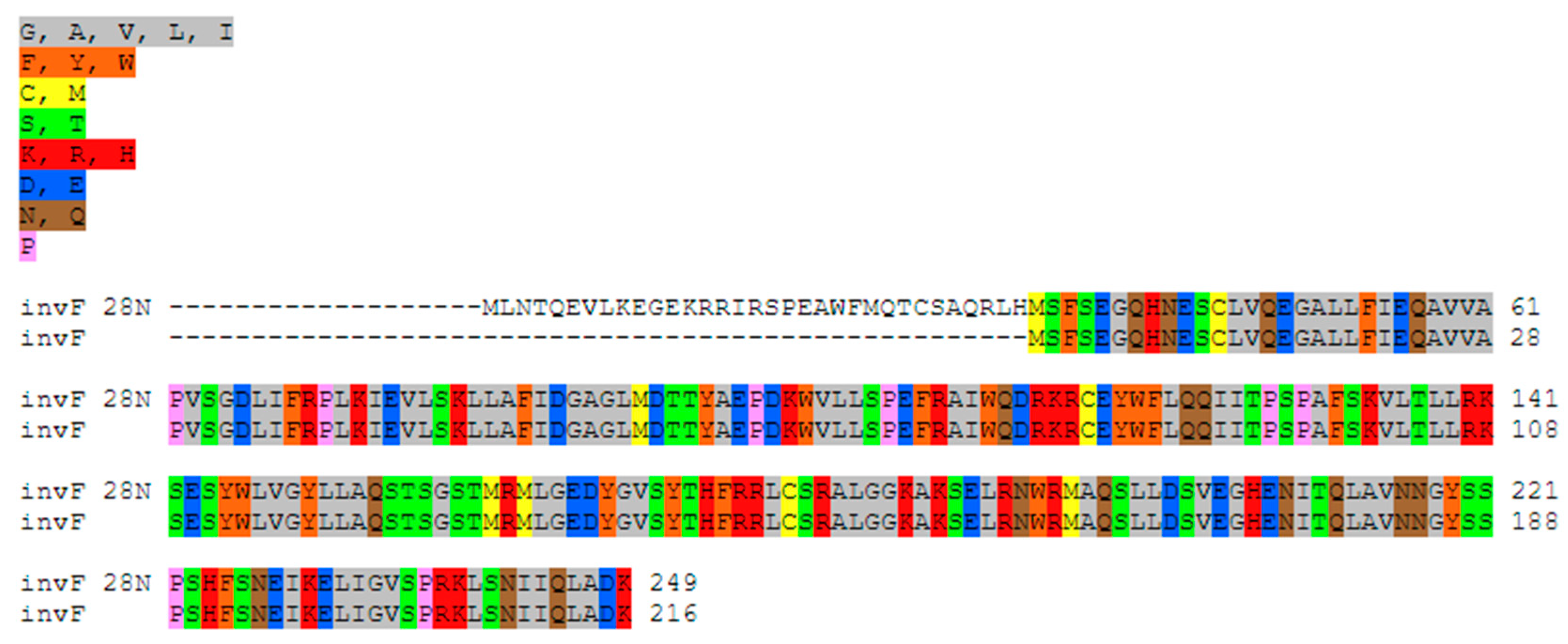
2.3. Genotypic Serotyping
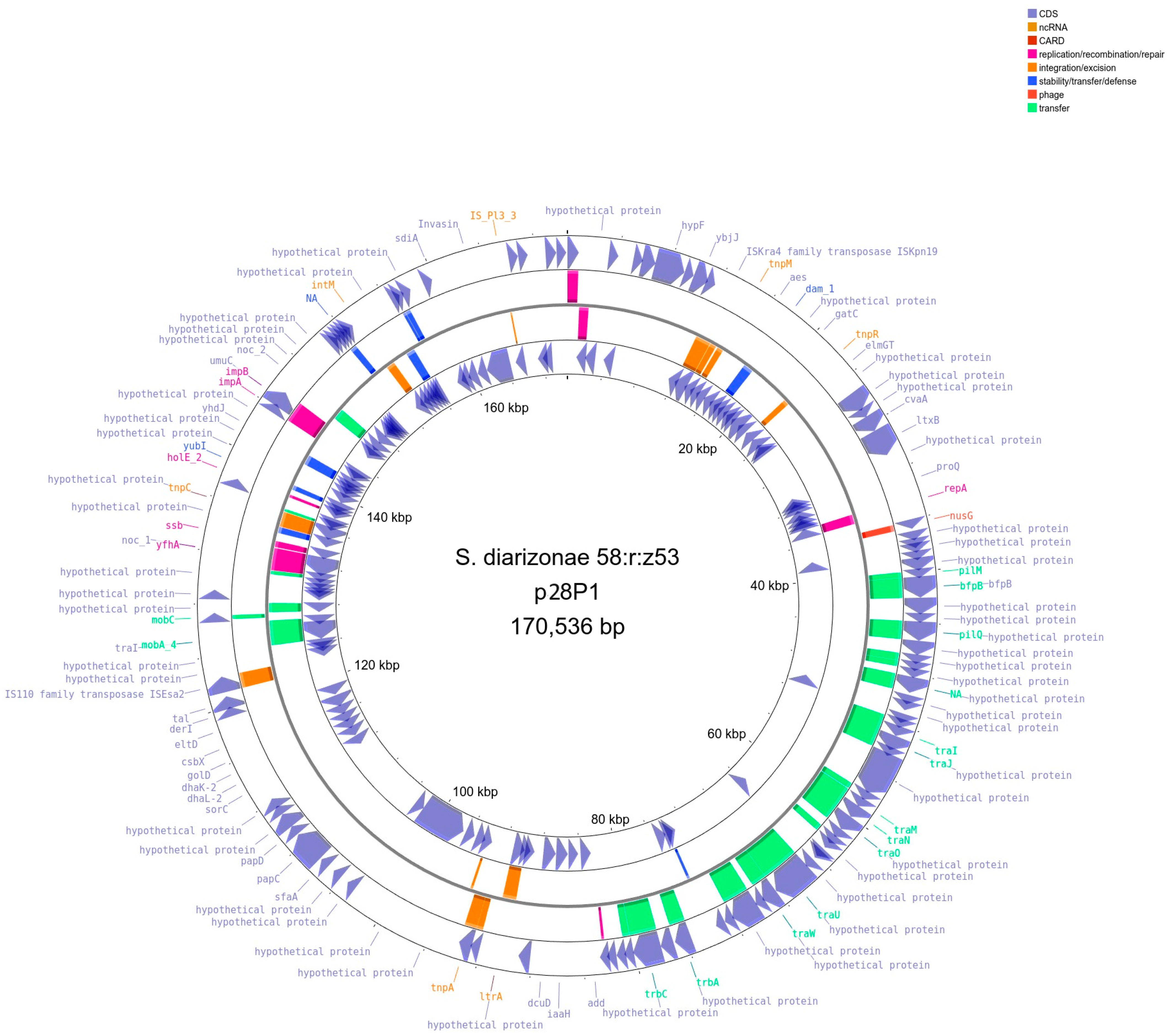
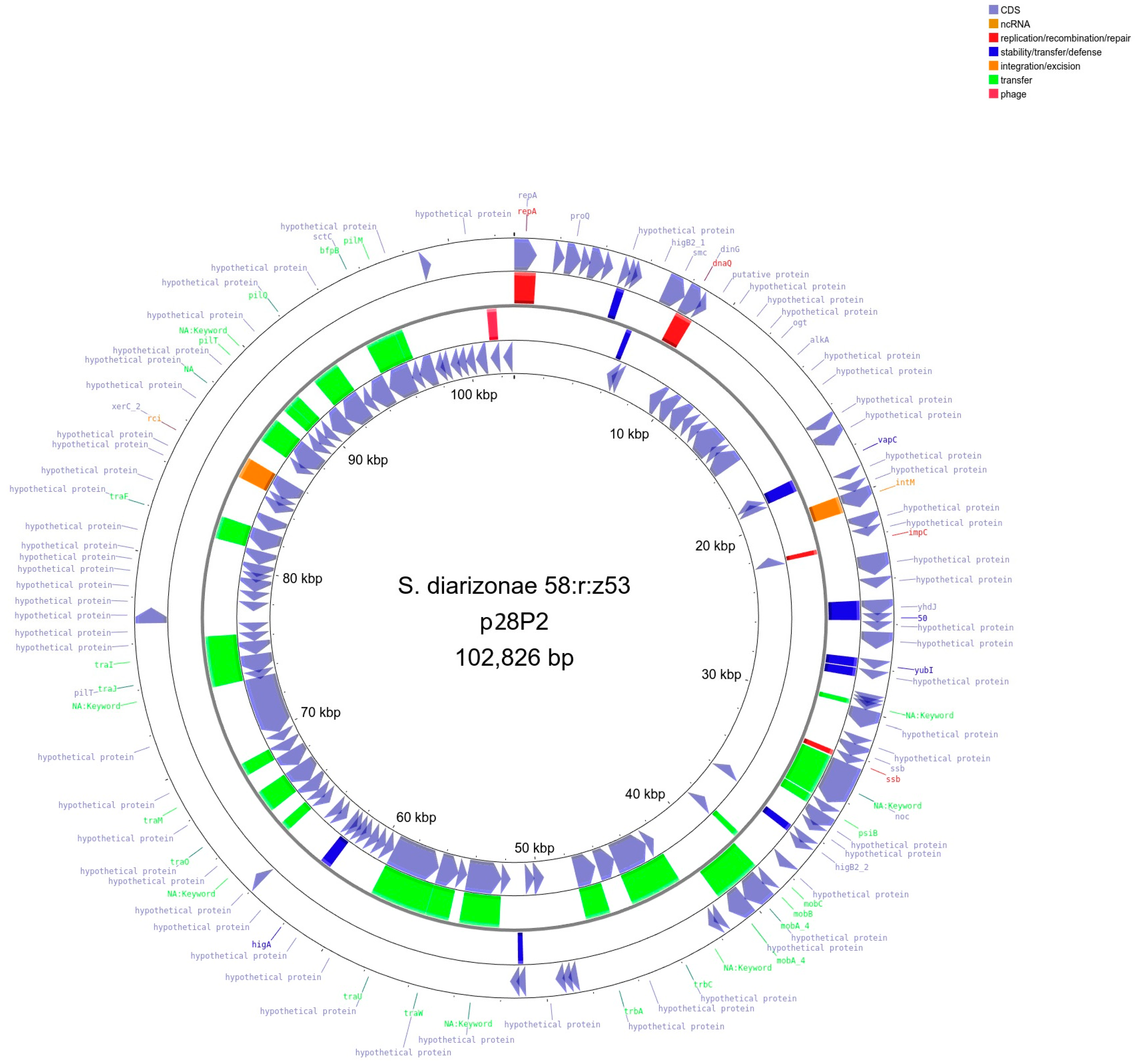
2.4. Detection and Initial Characterization of S. diarizonae 58:r:z53 Plasmids
2.5. Salmonella diarizonae 58:r:z53 Pathogenicity Islands
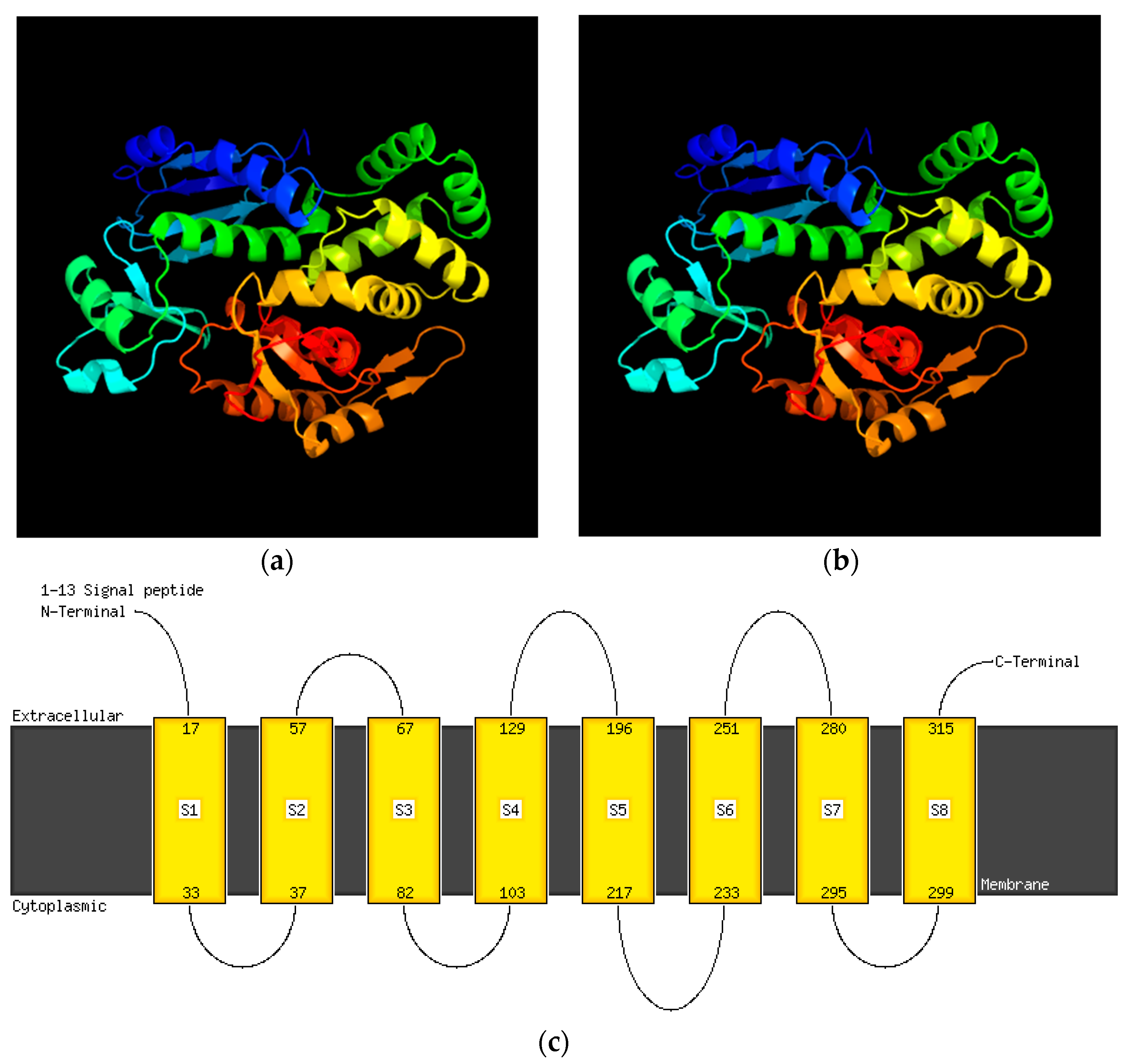
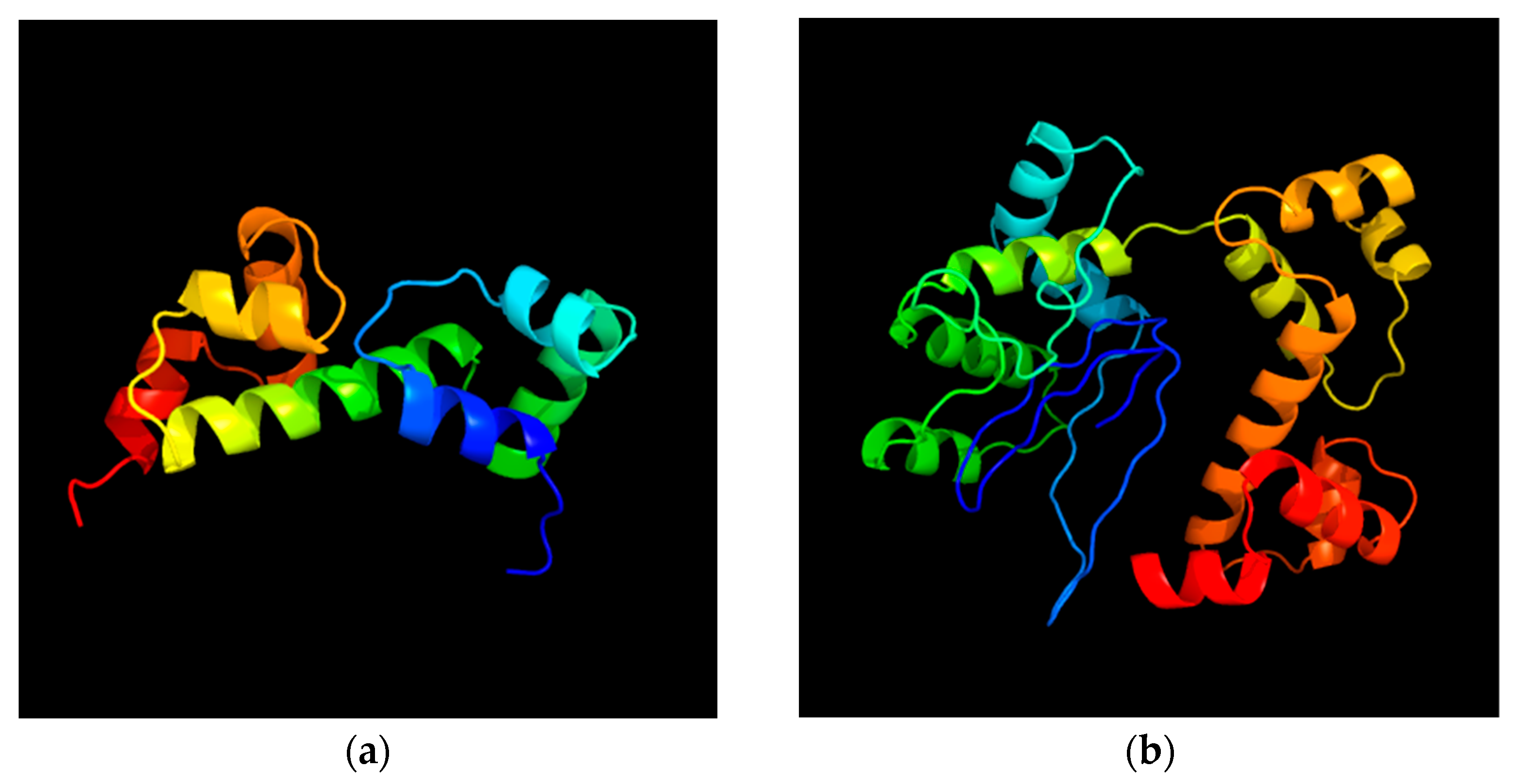
2.6. Virulence and Pathogenic Genes
2.7. Comparative Analysis of Virulence Determinants in Salmonella enterica subsp. diarizonae Strains
2.8. Antimicrobial Resistance of Salmonella IIIb 58:r:z53
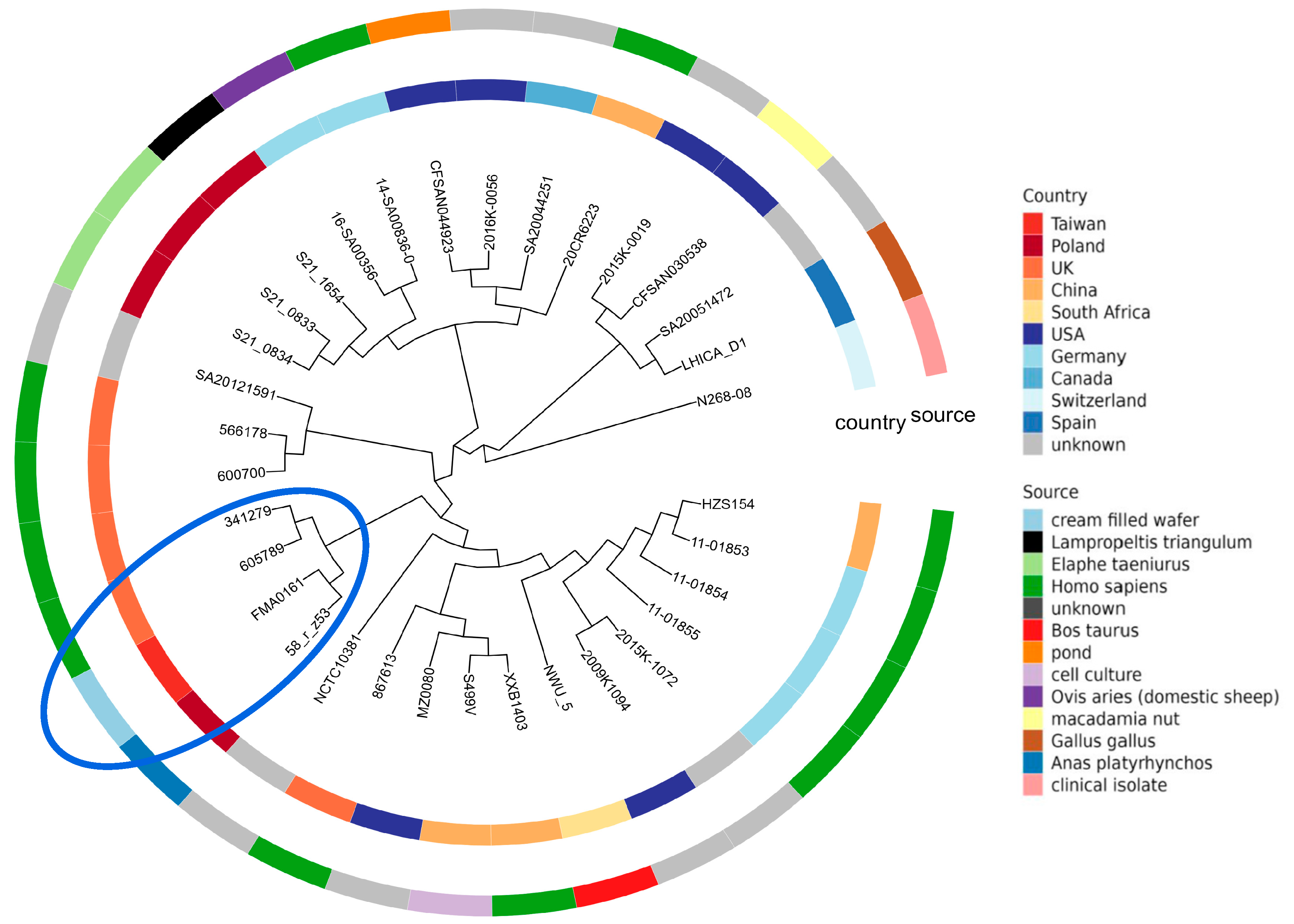
2.9. Phylogenetic Analysis of Salmonella IIIb 58:r:z53
3. Discussion
4. Materials and Methods
4.1. Sampled Animal
4.2. Salmonella spp. Isolation and Identification
4.3. Antimicrobial Sensitivity Testing
4.4. Whole-Genome Sequencing
4.5. Whole-Genome Analysis of Salmonella enterica spp. diarizonae 58:r:z53
4.6. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; De Pinna, E.; Nair, S.; Fields, P.I.; Guibourdenche, M.; Weill, F.-X. Supplement 2008–2010 (no. 48) to the White–Kauffmann–Le Minor scheme. Res. Microbiol. 2014, 165, 526–530. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Duma-kocan, P.; Rudy, M.; Stanisławczyk, R. Serowary Salmonella w żywności wg powiadomień systemu RASFF w latach 2000–2017. Med. Weter. 2019, 75, 394–397. [Google Scholar]
- Pires, S.M.; Vieira, A.R.; Hald, T.; Cole, D. Source attribution of human salmonellosis: An overview of methods and estimates. Foodborne Pathog. Dis. 2014, 11, 667–676. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef]
- EFSA. (European Food Safety Authority) Multi-country outbreak of multiple Salmonella enterica serotypes linked to imported sesame-based products. EFSA Support. Publ. 2021, 18, 6922E. [Google Scholar] [CrossRef]
- Toyting, J.; Nuanmuang, N.; Utrarachkij, F.; Supha, N.; Thongpanich, Y. Genomic analysis of Salmonella isolated from canal water in Bangkok, Thailand. Microbiol. Spectr. 2024, 12, e04216-23. [Google Scholar] [CrossRef]
- Schröter, M.; Roggentin, P.; Hofmann, J.; Speicher, A.; Laufs, R.; Mack, D. Pet Snakes as a Reservoir for Salmonella enterica subsp. diarizonae (Serogroup IIIb): A Prospective Study. Appl. Environ. Microbiol. 2004, 70, 613–615. [Google Scholar] [CrossRef]
- Dec, M.; Zając, M.; Puchalski, A.; Szczepaniak, K.; Urban-Chmiel, R. Pet Reptiles in Poland as a Potential Source of Transmission of Salmonella. Pathogens 2022, 11, 1125. [Google Scholar] [CrossRef]
- Molina-Lopez, R.A.; Valverdú, N.; Martin, M.; Mateu, E.; Obon, E.; Cerdà-Cuéllar, M.; Darwich, L. Wild raptors as carriers of antimicrobialresistant Salmonella and Campylobacter strains. Vet. Rec. 2011, 168, 565. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, Q.; Zhang, X.; Mei, L.; Ye, Y.; Fang, C.; Shang, S. Salmonella enterica subsp. diarizonae Harboring ST233, ST1263, and ST1845 in Children. Front. Cell. Infect. Microbiol. 2021, 11, 727811. [Google Scholar] [CrossRef] [PubMed]
- Hervás, J.A.; Rosell, A.; Hervás, D.; Rubio, R.; Dueñas, J.; Mena, A. Reptile Pets–associated Salmonella enterica Subspecies diarizonae Gastroenteritis in a Neonate. Pediatr. Infect. Dis. J. 2012, 31, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Fermaglich, L.J.; Routes, J.M.; Lye, P.S.; Kehl, S.C.; Havens, P.L. Salmonella cervical lymphadenitis in an immunocompetent child exposed to a snake at an educational exhibit: A case report. Infect. Dis. Clin. Pract. 2012, 20, 289–290. [Google Scholar] [CrossRef]
- Chong, Y.; Kwon, O.H.; Lee, S.Y.; Chung, K.S.; Shimada, T. Salmonella enterica subspecies diarizonae bacteremia in an infant with enteritis. A case report. Yonsei Med. J. 1991, 32, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Pławińska-Czarnak, J.; Wódz, K.; Piechowicz, L.; Tokarska-Pietrzak, E.; Bełkot, Z.; Bogdan, J.; Wiśniewski, J.; Kwieciński, P.; Kwieciński, A.; Anusz, K. Wild Duck (Anas platyrhynchos) as a Source of Antibiotic-Resistant Salmonella enterica subsp. diarizonae O58—The First Report in Poland. Antibiotics 2022, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Cumsille, A.; Durán, R.E.; Rodríguez-Delherbe, A.; Saona-Urmeneta, V.; Cámara, B.; Seeger, M.; Araya, M.; Jara, N.; Buil-Aranda, C. GenoVi, an open-source automated circular genome visualizer for bacteria and archaea. PLoS Comput. Biol. 2023, 19, e1010998. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Uelze, L.; Borowiak, M.; Flieger, A.; Simon, S.; Tausch, S.H. Complete Genome Sequence of Salmonella enterica subsp. diarizonae Serovar 61:k:1,5,(7) Strain 14-SA00836-0, Isolated from Human Urine Laura. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, R.G.; Walter, S.; McClelland, M.; Schmidt, C.; Steglich, M.; Prager, R.; Bender, J.K.; Fuchs, S.; Schoerner, C.; Rabsch, W.; et al. Comparative whole genome analysis of three consecutive Salmonella diarizonae isolates. Int. J. Med. Microbiol. 2017, 307, 542–551. [Google Scholar] [CrossRef]
- Dufresne, K.; Saulnier-Bellemare, J.; Daigle, F. Functional analysis of the chaperone-usher fimbrial gene clusters of Salmonella enterica serovar Typhi. Front. Cell. Infect. Microbiol. 2018, 8, 26. [Google Scholar] [CrossRef]
- Seah, X.F.V.; Ngeow, J.H.A.; Thoon, K.C.; Tee, W.S.N.; Maiwald, M.; Chong, C.Y.; Tan, N.W.H. Infant With Invasive Nontyphoidal Salmonellosis and Mastitis. Glob. Pediatr. Health 2015, 2, 2333794X1559156. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Nisbet, J. Building Phylogenetic Trees From Genome Sequences With kSNP4. Mol. Biol. Evol. 2023, 40, msad235. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Kraft, M.; Fostiropoulos, K.; Falkowski, A.; Tarr, P.E. Salmonella enterica subspecies diarizonae maxillary sinusitis in a snake handler: First report. Open Forum Infect. Dis. 2016, 3, ofw066. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Song, X.; Zhang, H.; Ma, S.; Wang, J.; Li, W.; Lv, R.; Liu, X.; Ma, S.; et al. Salmonella Typhimurium reprograms macrophage metabolism via T3SS effector SopE2 to promote intracellular replication and virulence. Nat. Commun. 2021, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Schwan, C.L.; Lomonaco, S.; Bastos, L.M.; Cook, P.W.; Maher, J.; Trinetta, V.; Bhullar, M.; Phebus, R.K.; Gragg, S.; Kastner, J.; et al. Genotypic and Phenotypic Characterization of Antimicrobial Resistance Profiles in Non-typhoidal Salmonella enterica Strains Isolated From Cambodian Informal Markets. Front. Microbiol. 2021, 12, 711472. [Google Scholar] [CrossRef] [PubMed]
- Giner-Lamia, J.; Vinuesa, P.; Betancor, L.; Silva, C.; Bisio, J.; Soleto, L.; Chabalgoity, J.A.; Puente, J.L.; Soncini, F.C.; García-Vescovi, E.; et al. Genome analysis of Salmonella enterica subsp. diarizonae isolates from invasive human infections reveals enrichment of virulence-related functions in lineage ST1256. BMC Genom. 2019, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Jennings, E.; Thurston, T.L.M.; Holden, D.W. Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms And Physiological Consequences. Cell Host Microbe 2017, 22, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, I.E.; Ghori, N.; Falkow, S.; Monack, D. Mig-14 is an inner membrane-associated protein that promotes Salmonella typhimurium resistance to CRAMP, survival within activated macrophages and persistent infection. Mol. Microbiol. 2005, 55, 954–972. [Google Scholar] [CrossRef] [PubMed]
- Gruz, P.; Matsui, K.; Sofuni, T.; Nohmi, T. Roles of the mutagenesis proteins SamA’B and MucA’B in chemically induced frameshift mutagenesis in Salmonella typhimurium hisD3052. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 1998, 398, 33–42. [Google Scholar] [CrossRef]
- Gawin, P. Wild game in Poland. In Hunting Traditions, Law, Game; Gawin, P., Ed.; SBM Sp. z o.o.: Warsaw, Poland, 2015; pp. 129–131. ISBN 9788378458661. [Google Scholar]
- Regulation of the Minister of the Environment of March 11, 2005 on the List of Game Species Dz.U. 2005 nr 45 poz. 433. 2005. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20050450433 (accessed on 3 February 2024).
- PN-EN ISO 6579-1:2017-04; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration, and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. Polish Committee for Standardization: Warsaw, Poland, 2020.
- Grimont, P.A.D.; Weill, F.-X. Antigenic formulae of the Salmonella serovars. In WHO Collaborating Centre for Reference and Research on Salmonella, 9th ed.; WHO: Geneva, Switzerland, 2007; pp. 1–167. [Google Scholar]
- MacLowry, J.D.; Marsh, H.H. Semi-automatic microtechnique for serial dilution antibiotic sensitivity testing in the clinical laboratory. J. Lab. Clin. Med. 1968, 72, 685–687. [Google Scholar]
- Gerlach, E. Microdilution 1: A Comparative Study. In Current Techniques for Antibiotic Susceptibility Testing; Thomas, C.C., Ed.; Charles C. Thomas: Springfield, IL, USA, 1974; pp. 63–76. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume 8, ISBN 156238838X. [Google Scholar]
- Pławińska-Czarnak, J.; Wódz, K.; Kizerwetter-Świda, M.; Bogdan, J.; Kwieciński, P.; Nowak, T.; Strzałkowska, Z.; Anusz, K. Multi-Drug Resistance to Salmonella spp. When Isolated from Raw Meat Products. Antibiotics 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Holt, K.E. Polypolish: Short-read polishing of long-read bacterial genome assemblies. PLoS Comput. Biol. 2022, 18, e1009802. [Google Scholar] [CrossRef] [PubMed]
- Zimin, A.V.; Puiu, D.; Luo, M.C.; Zhu, T.; Koren, S.; Marçais, G.; Yorke, J.A.; Dvořák, J.; Salzberg, S.L. Hybrid assembly of the large and highly repetitive genome of Aegilops tauschii, a progenitor of bread wheat, with the MaSuRCA mega-reads algorithm. Genome Res. 2017, 27, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yin, Y.; Jones, M.B.; Zhang, Z.; Kaiser, B.L.D.; Dinsmore, B.A.; Fitzgerald, C.; Fields, P.I.; Deng, X. Salmonella serotype determination utilizing high-throughput genome sequencing data. J. Clin. Microbiol. 2015, 53, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; den Bakker, H.C.; Li, S.; Chen, J.; Dinsmore, B.A.; Lane, C.; Lauer, A.C.; Fields, P.I.; Deng, X. SeqSero2: Rapid and improved salmonella serotype determination using whole-genome sequencing data. Appl. Environ. Microbiol. 2019, 85, e01746-19. [Google Scholar] [CrossRef]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Schwengers, O.; Hain, T.; Chakraborty, T.; Goesmann, A. ReferenceSeeker: Rapid determination of appropriate reference genomes. J. Open Source Softw. 2020, 5, 1994. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]


| Name | Type | Start/Stop | Identity % | Query/Template Length | Genes | Bacterium | Accession No. |
|---|---|---|---|---|---|---|---|
| C63PI | CDS | 1 219 752/1 223 751 | 95.58 | 4000/4000 | sitA, sitB, sitC, sitD | Salmonella-enterica-Typhimurium-SL1344 | AF128999 |
| SPI-1 | CDS | 1 190 777/1 191 191 | 96.14 | 415/415 | invA | Salmonella-enterica-Gallinarum-SGB_8 | AY956825 |
| SPI-1 | CDS | 1 189 639/1 189 897 | 95.37 | 259/257 | invA | Salmonella-enterica-Typhimurium-J4STEHO | JN982040 |
| SPI-1 | CDS | 1 171 725/1 174 414 | 95.32 | 2692/3141 | fhlA/mutS | Salmonella-enterica-Typhimurium-SL1344 | U16303 |
| SPI-13 | CDS | 745 447/745 784 | 98.22 | 338/338 | gacD | Salmonella-enterica-Gallinarum-SGD_3 | AY956832 |
| SPI-13 | CDS | 746 092/746 495 | 98.02 | 404/404 | gtrA | Salmonella-enterica-Gallinarum-SGG_1 | AY956833 |
| SPI-13 | CDS | 747 863/748 203 | 98.53 | 341/341 | gtrA | Salmonella-enterica-Gallinarum-SGA_10 | AY956834 |
| SPI-2 | CDS | 3 159 479/3 164 104 | 95.47 | 4634/4631 | ORF32, ORF48, pykF | Salmonella-enterica-Typhimurium-LT2 | X99945 |
| SPI-3 | CDS | 66 847/67 584 | 96.75 | 738/738 | mgtC | Salmonella-enterica-Typhimurium-14028s | AJ000509 |
| SPI-5 | CDS | 3 455 305/3 456 557 | 99.36 | 1253/1253 | sopB | Salmonella-diarizonae-SARC7 | AF323077 |
| SPI-9 | CDS | 1 323 107/1 335 754 | 95.65 | 12651/15696 | Salmonella-enterica-Typhi-CT18 | NC_003198 |
| AMR Mechanism | Genes | Drug Class |
|---|---|---|
| antibiotic inactivation | AAC(6′)-Iy | aminoglycoside |
| antibiotic efflux | acrD, kdpE | aminoglycoside |
| mdtB, mdtC | aminocoumarin | |
| baeR, cpxA | aminocoumarin, aminoglycoside | |
| msbA | nitroimidazole | |
| yojI | peptide antibiotic | |
| emrA, emrB, emrR | fluoroquinolone | |
| crp | fluoroquinolone, macrolide, penam | |
| msrB | streptogramin, macrolide | |
| h-ns | macrolide, penam, cephamycin, tetracycline, cephalosporin, fluoroquinolone | |
| emrD | phenicol, disinfecting agent, antiseptic | |
| sdiA, E. coli acrA | cephalosporin, glycylcycline, penam, tetracycline, fluoroquinolone, rifamycin, phenicol, triclosan | |
| acrB | tetracycline, rifamycin, glycylcycline, phenicol, penam, cephalosporin, fluoroquinolone, disinfecting agent, antiseptic | |
| tolC | peptide antibiotic, aminoglycoside, tetracycline, glycylcycline, macrolide, fluoroquinolone, penam, carbapenem, penem, aminocoumarin, phenicol, cephalosporin, rifamycin, cephamycin, disinfecting agent, antiseptic | |
| antibiotic target alteration | bacA | peptide antibiotic |
| glpT, uhpT | fosfomycin | |
| reduced permeability to antibiotics, antibiotic efflux | marA | glycylcycline, cephalosporin, penam, cephamycin, monobactam, penem, tetracycline, fluoroquinolone, rifamycin, phenicol, carbapenem, triclosan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wódz, K.; Piechowicz, L.; Tokarska-Pietrzak, E.; Gawor, J.; Gromadka, R.; Bełkot, Z.; Strzałkowska, Z.; Wiśniewski, J.; Nowak, T.; Bogdan, J.; et al. Does Salmonella diarizonae 58:r:z53 Isolated from a Mallard Duck Pose a Threat to Human Health? Int. J. Mol. Sci. 2024, 25, 5664. https://doi.org/10.3390/ijms25115664
Wódz K, Piechowicz L, Tokarska-Pietrzak E, Gawor J, Gromadka R, Bełkot Z, Strzałkowska Z, Wiśniewski J, Nowak T, Bogdan J, et al. Does Salmonella diarizonae 58:r:z53 Isolated from a Mallard Duck Pose a Threat to Human Health? International Journal of Molecular Sciences. 2024; 25(11):5664. https://doi.org/10.3390/ijms25115664
Chicago/Turabian StyleWódz, Karolina, Lidia Piechowicz, Ewa Tokarska-Pietrzak, Jan Gawor, Robert Gromadka, Zbigniew Bełkot, Zuzanna Strzałkowska, Jan Wiśniewski, Tomasz Nowak, Janusz Bogdan, and et al. 2024. "Does Salmonella diarizonae 58:r:z53 Isolated from a Mallard Duck Pose a Threat to Human Health?" International Journal of Molecular Sciences 25, no. 11: 5664. https://doi.org/10.3390/ijms25115664
APA StyleWódz, K., Piechowicz, L., Tokarska-Pietrzak, E., Gawor, J., Gromadka, R., Bełkot, Z., Strzałkowska, Z., Wiśniewski, J., Nowak, T., Bogdan, J., Anusz, K., & Pławińska-Czarnak, J. (2024). Does Salmonella diarizonae 58:r:z53 Isolated from a Mallard Duck Pose a Threat to Human Health? International Journal of Molecular Sciences, 25(11), 5664. https://doi.org/10.3390/ijms25115664






