Evaluation of Polyphenols Synthesized in Mature Seeds of Common Bean (Phaseolus vulgaris L.) Advanced Mutant Lines
Abstract
1. Introduction
2. Results
2.1. Profiles of Anthocyanins Acquired by HPLC-DAD-MS/MS
2.1.1. Chromatographic Analysis
2.1.2. MS Spectroscopy
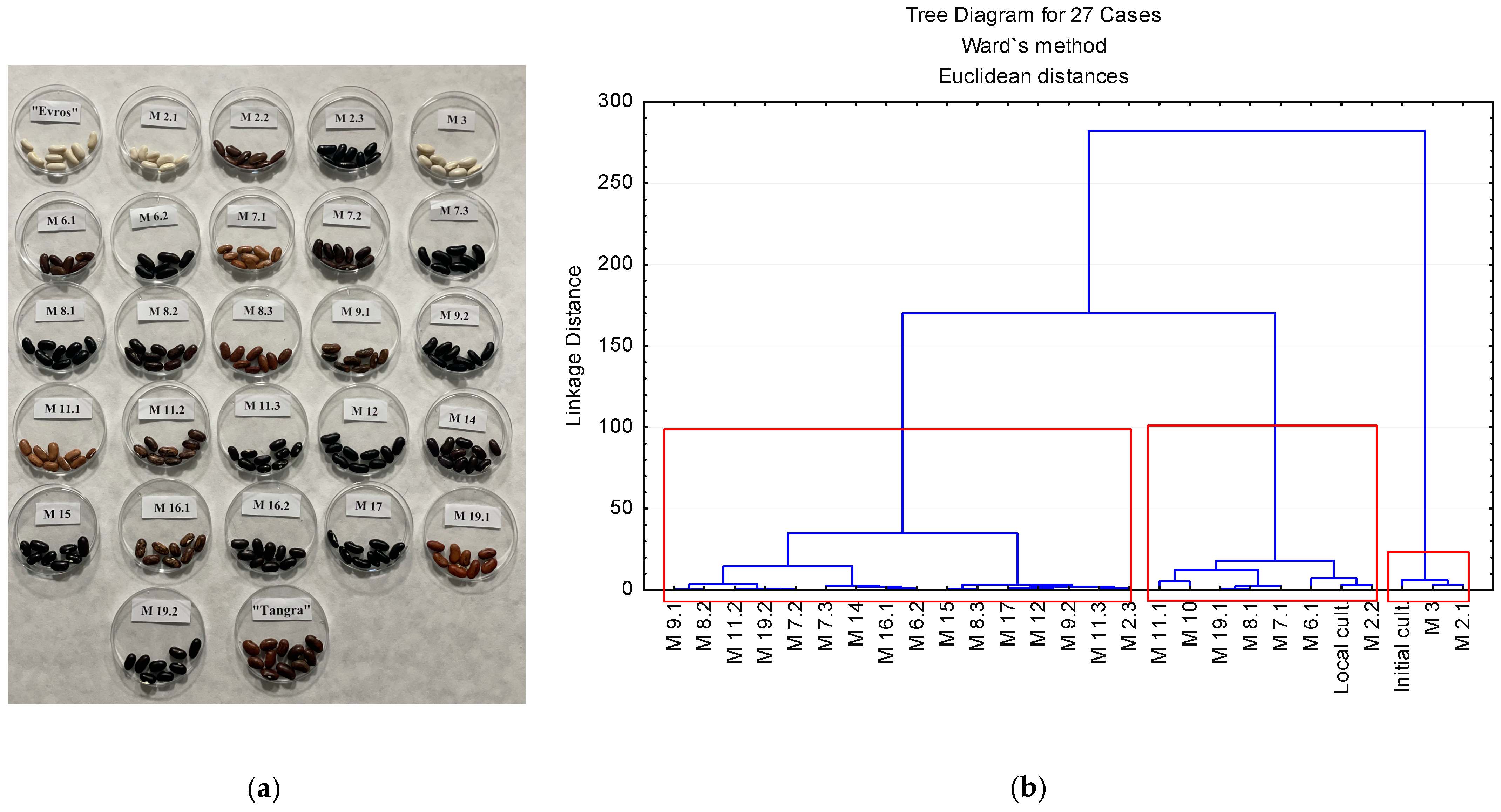
2.2. Seed Color Evaluation
2.2.1. Cluster Analysis—Seed Clusters Based on Coat Color
2.2.2. Hierarchical Cluster
2.2.3. Mean Cluster Values
2.2.4. Analysis of Variance
2.2.5. Phenolic Acid and Flavonoid Profile by HPLC-MS/MS Analysis (QTRAP 5500 Ion Trap Mass Spectrometer)
3. Discussion
3.1. Anthocyanin Concentration in Common Bean
3.2. Seed Color Evaluation
3.3. Phenolic Acid and Flavonoid Profile by HPLC-MS/MS Analysis (QTRAP 5500 Ion Trap Mass Spectrometer)
4. Materials and Methods
4.1. Materials
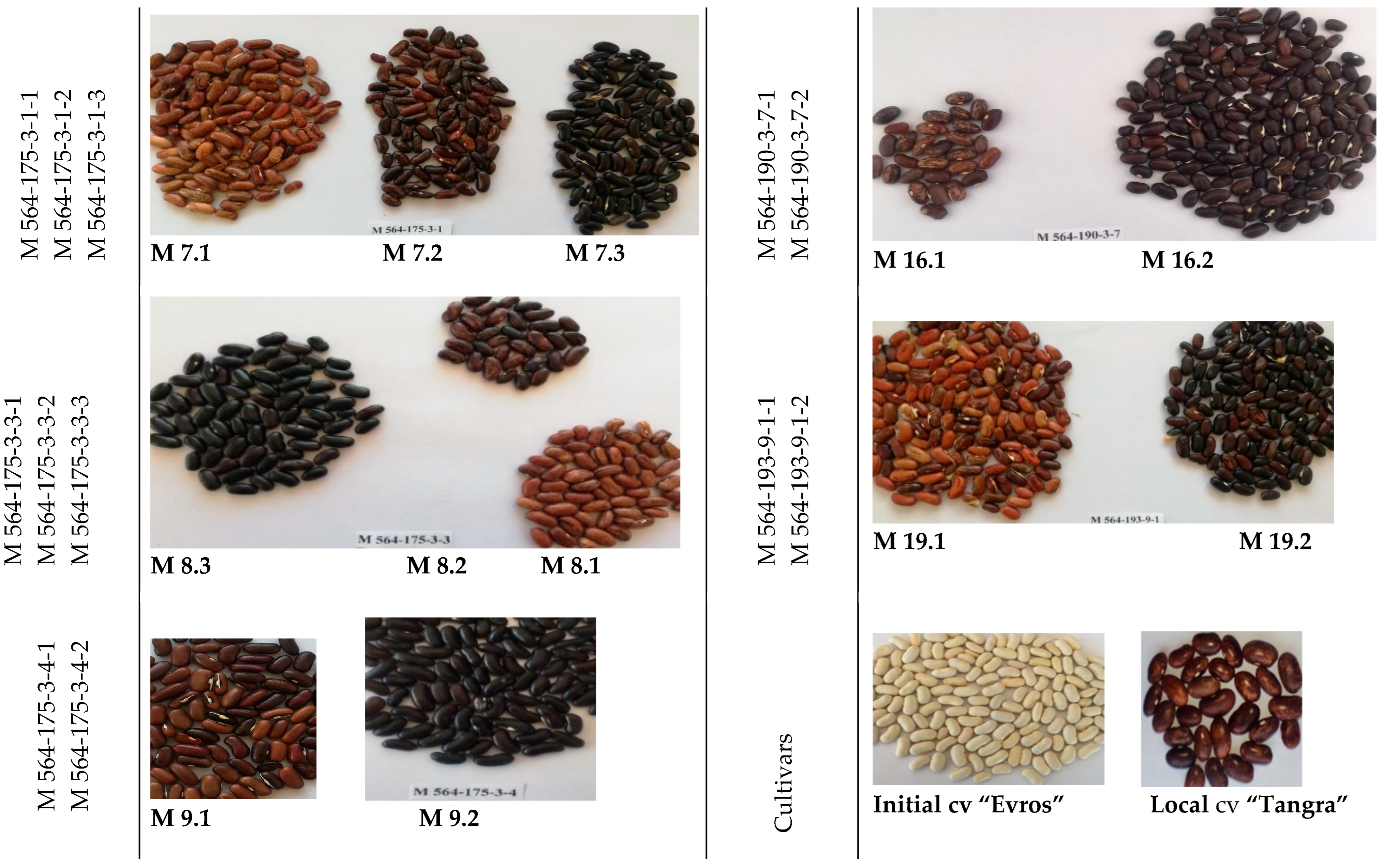
4.1.1. Plant Material
4.1.2. Description of Mutant Lines M 564 in Comparison with the IP 564 (Cultivar “Evros”) Initial Line
4.1.3. Chemicals Used
4.2. Methods
4.2.1. Extraction of Anthocyanins
4.2.2. Chromatographic Analysis of Anthocyanins
4.2.3. MS Spectroscopy
4.2.4. Determination of Free and Conjugated Flavonoids and Phenolic Acids
Extraction of Phenolic Acids and Flavonoids
Evaluation of Phenolic Acids and Flavonoids by HPLC-MS/MS Measurements
4.2.5. Seed Color Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Ron, A.M.; Papa, R.; Bitocchi, E.; González, A.M.; Debouck, D.G.; Brick, M.A.; Fourie, D.; Marsolais, F.; Beaver, J.; Geffroy, V.; et al. Common Bean. In Grain Legumes. Handbook of Plant Breeding; De Ron, A., Ed.; Springer: New York, NY, USA, 2015; Volume 10. [Google Scholar] [CrossRef]
- Harlen, W.C.; Jati, I.R.A. Chapter 8—Antioxidant activity of anthocyanins in common legume grains. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 81–92. [Google Scholar]
- Mihaylova, D.; Dimitrova-Dimova, M.; Popova, A. Dietary Phenolic Compounds—Wellbeing and Perspective Applications. Int. J. Mol. Sci. 2024, 25, 4769. [Google Scholar] [CrossRef] [PubMed]
- Tomlekova, N.; Sofkova-Bobcheva, S.; Sarsu, F.; Baudoin, J.P. Genetic diversity of Bulgarian Phaseolus vulgaris L. based on phaseolin type and seed-coat colour. Bulg. J. Agric. Sci. 2016, 22, 447–451. [Google Scholar]
- Sofkova-Bobcheva, S.; Pantchev, I.; Kiryakov, I.; Chavdarov, P.; Muhovski, Y.; Sarsu, F.; Tomlekova, N. Induced mutagenesis for improvement of bean (Phaseolus vulgaris L.) production in Bulgaria. In Mutation Breeding, Genetic Diversity and Crop Adaptation to Climate Change; CAB International: Wallingford, UK, 2021; pp. 178–193. [Google Scholar]
- Svetleva, D.; Velcheva, M.; and Bhowmik, G. Biotechnology as a useful tool in common bean (Phaseolus vulgaris L.) improvement. Euphytica 2003, 131, 189–200. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Mateus, N.; De Freitas, V. Anthocyanins. Plant pigments and beyond. J. Agric. Food Chem. 2014, 62, 6879–6884. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Krzesiński, P.; Kura, M.; Szmigiel, B.; and Błaszczyk, J. Anthocyanins in medicine. Pol. J. Pharmacol. 2003, 55, 699–702. [Google Scholar] [PubMed]
- Madrera, R.R.; Negrillo, A.C.; Suárez Valles, B.; Ferreira Fernández, J.J. Phenolic content and antioxidant activity in seeds of common bean (Phaseolus vulgaris L.). Foods 2021, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, G.; Zhang, Y.; Cui, B.; Yin, W.; Yu, X.; Zhu, Z.; Hu, Z. Anthocyanin composition and expression analysis of anthocyanin biosynthetic genes in kidney bean pod. Plant Physiol. Biochem. 2015, 97, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Sunil, L.; Shetty, N.P. Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, I.D.; Venskutonis, P.R.; Georgiev, M.I.; Clément, C.; et al. Anthocyanins, multi-functional natural products of industrial relevance: Recent biotechnological advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Beta, T. Proximate composition. Phenolic profiles and antioxidant capacity of three common bean varieties (Phaseolus vulgaris L.). J. Food Chem. Nanotechnol. 2016, 2, 147–152. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable concentrations of delphinidin and its metabolite, gallic acid, induce antioxidant protection associated with increased Intracminiellular Glutathione in Cultured Endothelial Cells. Oxidative Med. Cell. Longev. 2017, 2017, 9260701. [Google Scholar] [CrossRef]
- Dzomba, P.; Togarepi, E.; Mupa, M. Anthocyanin content and antioxidant activities of common bean species (Phaseolus vulgaris L.) grown in Mashonaland Central, Zimbabwe. Afr. J. Agric. Res. 2013, 8, 3330–3333. [Google Scholar]
- Ministry of Agriculture. Statistics. Available online: https://www.mzh.government.bg/media/filer_public/2023/06/29/ra_427_publication_crops_2022.pdf (accessed on 23 November 2023).
- Blair, M.W.; Porch, T.; Cichy, K.; Galeano, C.H.; Lariguet, P.; Pankhurst, C.; Broughton, W. Induced mutants in common bean (Phaseolus vulgaris), and their potential use in nutrition quality breeding and gene discovery. Isr. J. Plant Sci. 2007, 55, 191–200. [Google Scholar] [CrossRef]
- Sena, J.S.P.; Barbosa, H.M.; Vieira, C. Induced mutations in the common bean, Phaseolus vulgaris L.; affecting flower colour and seed characteristics. Braz. J. Genet. 1991, 14, 1033–1039. [Google Scholar]
- Amini, M. Ethyl Methanesulfonate. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 522–524. ISBN 9780123864550. [Google Scholar]
- Kutscher, L.M.; Shaham, S. Forward and reverse mutagenesis in C. elegans. WormBook 2014, 17, 1–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Espinosa-Alonso, L.G.; Lygin, A.; Widholm, J.M.; Valverde, M.E.; Paredes-Lopez, O. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 4436–4444. [Google Scholar] [CrossRef]
- Sammyia, J.; Shah, A.H.; Ul Hassan, M.; Sher, A.; Fiaz, S.; Elesawy, B.H.; Ismail, K.A.; El Askary, A.; Gharib, A.F.; Qayyum, A. Genetic diversity of common bean (Phaseolus vulgaris L.) ecotypes from Pakistan using Simple Sequence Repeats. Saudi J. Biol. Sci. 2022, 29, 103300. [Google Scholar]
- Mojica, L.; Berhow, M.; de Mejia, E.G. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef]
- Takeoka, C.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D.J. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Hall, C. Chapter 9—Sources of natural antioxidants: Oilseeds, nuts, cereals, legumes, animal products and microbial sources. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Antioxidants in Food; Pokorny, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing: Sawston, UK, 2001; pp. 159–209. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Luciano, R.-H.; Sosa-Montes, E.; Pérez-Herrera, P. Anthocyanin composition in black bean (Phaseolus vulgaris L.) varieties grown in México. Agrociencia 2005, 39, 385–394. [Google Scholar]
- Hernández, D.F.; Mojica, L.; Berhow, M.A.; Brownstein, K.; Cervantes, E.L.; de Mejia, E.G. Black and pinto beans (Phaseolus vulgaris L.) unique Mexican varieties exhibit antioxidant and anti-inflammatory potential. Food Res. Int. 2023, 169, 112816. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high-performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and color. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- López, A.; El-Naggar, T.; Dueñas, M.; Ortega, T.; Estrella, I.; Hernández, T.; Gómez-Serranillos, M.P.; Palomino, O.M.; Carretero, M.E. Effect of cooking and germination on phenolic composition and biological properties of dark beans (Phaseolus vulgaris L.). Food Chem. 2013, 138, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Meyer, A.; Berhow, M.A.; González de Mejía, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Galardi, C.; Mulinacci, N.; Benedettelli, S.; Heimler, D. Germplasm characterization of Zolfino landraces (Phaseolus vulgaris L.) by flavonoid content. J. Agric. Food Chem. 2004, 52, 3838–3842. [Google Scholar] [CrossRef]
- Dintcheva, T.; Boteva, H.; Tomlekova, N.; Kalapchieva, S. Selection of perspective early mutant lines of bean (Phaseolus vulgaris L.) in drought conditions. Agric. Sci. J. Agric. Univ. Plovdiv. 2021, pp. 46–52. Available online: https://agrarninauki.au-plovdiv.bg/2021/issue-30/7-30/ (accessed on 17 March 2024).
- Mladenov, P.; Aziz, S.; Topalova, E.; Renaut, J.; Planchon, S.; Raina, A.; Tomlekova, N. Physiological Responses of Common Bean Genotypes to Drought Stress. Agronomy 2023, 13, 1022. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Sawicki, T.; Mitrus, J.; Kasprzykowski, Z.; Horbowicz, M. Pro le of phenolic acids and antioxidant capacity in organs of common buckwheat sprout. Acta Aliment. 2016, 45, 250–257. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Development and validation of ultrasound assisted extraction (UAE) and HPLC-DAD method for determination of polyphenols in dry beans (Phaseolus vulgaris). J. Food Compos. Anal. 2020, 85, 103334. [Google Scholar] [CrossRef]


| Genotype | Delphinidin 3-O-Glucoside [µg/g dw] | Cyanidin 3-O-Glucoside [µg/g dw] | Pelargonidin 3-O-Glucoside [µg/g dw] | Petunidin 3-O-Glucoside [µg/g dw] | Name of the Mutant Line |
|---|---|---|---|---|---|
| M 2.1 | ND | ND | ND | ND | M 564-56-4-3-1 |
| M 2.2 | 171.55 ± 49.35 | 60.47 ± 12.12 | 304.54 ± 3.52 | 35.79 ± 21.80 | M 564-56-4-3-2 |
| M 2.3 | 1887.18 ± 133.34 | 60.40 ± 17.75 | 312.76 ± 11.52 | 24.26 ± 11.88 | M 564-56-4-3-3 |
| M 3 | ND | ND | ND | ND | M 564-110-1-2 |
| M 6.1 | 227.13 ± 10.74 | 63.64 ± 18.76 | 327.44 ± 20.08 | ND | M 564-172-2-1-1 |
| M 6.2 | 475.21 ± 57.08 | 156.86 ± 17.05 | 369.28 ± 19.03 | ND | M 564-172-2-1-2 |
| M 7.1 | ND | ND | ND | ND | M 564-175-3-1-1 |
| M 7.2 | 407.80 ± 45.95 | 220.32 ± 29.71 | 318.59 ± 19.66 | ND | M 564-175-3-1-2 |
| M 7.3 | 884.23 ± 123.00 | 420.62 ± 42.36 | 409.39 ± 4.70 | ND | M 564-175-3-1-3 |
| M 8.1 | ND | ND | ND | ND | M 564-175-3-3-1 |
| M 8.2 | 431.12 ± 50.74 | 303.70 ± 13.68 | 372.56 ± 25.54 | ND | M 564-175-3-3-2 |
| M 8.3 | 1092.44 ± 35.53 | 618.52 ± 24.43 | 441.41 ± 11.78 | ND | M 564-175-3-3-3 |
| M 9.1 | 311.51 ± 42.02 | 122.32 ± 13.18 | 332.98 ± 9.994 | ND | M 564-175-3-4-1 |
| M 9.2 | 688.20 ± 50.13 | 376.58 ± 34.78 | 429.34 ± 11.162 | ND | M 564-175-3-4.2 |
| M 10 | ND | ND | ND | ND | M 564-190-1-1 |
| M 11.1 | ND | ND | ND | ND | M 564-190-3-1-1 |
| M 11.2 | 289.96 ± 7.55 | 66.06 ± 5.02 | 332.43 ± 19.164 | ND | M 564-190-3-1-2 |
| M 11.3 | 618.19 ± 30.83 | 196.20 ± 7.31 | 402.53 ± 12.136 | ND | M 564-190-3-1-3 |
| M 12 | 1023.63 ± 44.10 | 451.75 ± 21.11 | 404.23 ± 58.836 | ND | M 564-190-3-2 |
| M 14 | 532.43 ± 30.95 | 273.47 ± 11.97 | 341.53 ± 8.136 | ND | M 564-190-3-4 |
| M 15 | 1507.83 ± 65.74 | 581.38 ± 14.38 | 390.63 ± 81.736 | ND | M 564-190-3-5 |
| M 16.1 | 281.65 ± 24.43 | 173.07 ± 15.44 | 342.23 ± 12.436 | ND | M 564-190-3-7-1 |
| M 16.2 | 503.55 ± 79.59 | 363.16 ± 18.93 | 401.73 ± 9.936 | ND | M 564-190-3-7-2 |
| M 17 | 1158.97 ± 38.70 | 537.83 ± 17.23 | 449.43 ± 18.136 | ND | M 564-191-1-1 |
| M 19.1 | 163.13 ± 38.70 | 111.41 ± 50.64 | 345.23 ± 23.936 | ND | M 564-193-9-1-1 |
| M 19.2 | 707.11 ± 56.34 | 378.88 ± 18.09 | 410.93 ± 34.036 | ND | M 564-193-9-1-2 |
| “Tangra” | ND | 76.97 ± 11.06 | 326.83 ± 6.836 | ND | Local cultivar |
| “Evros” | ND | ND | ND | ND | Initial cultivar |
| Peak | Compound | tR, min | [M + H]+ | Fragments MS2 | UV-Vis Max |
|---|---|---|---|---|---|
| 1 | Delphinidin 3-O-glucoside | 12.58 | 465.2 | 303.1 | 277; 526 |
| 2 | Cyanidin 3-O-glucoside | 18.19 | 449.2 | 287.1 | 280; 517 |
| 3 | Petunidin 3-O-glucoside chloride | 23.43 | 479.2 | 317.1 | 278, 523 |
| 4 | Pelargonidin 3-O-glucoside | 23.95 | 433.2 | 271.1 | 274; 503 |
| Genotype | Accession | Color Groups [%] | Predominant Color Group | Color |
|---|---|---|---|---|
| M 564-56-4-3-1 | M 2.1 | II = 100 | II | white |
| M 564-56-4-3-2 | M 2.2 | V = 12; VIII = 72; IX = 16 | VIII | beige to light brown |
| M 564-56-4-3-3 | M 2.3 | I = 39.22; III = 3.92; IV = 56.86 | IV | ink |
| M 564-110-1 | M 3 | II = 91.30; VI = 8.70 | II | white |
| M 564-172-2-1-1 | M 6.1 | III = 32.76; V = 1.72; VIII = 15.52; IX = 50 | IX | speckled brown |
| M 564-172-2-1-2 | M 6.2 | I = 58.73; III = 22.22; IV = 17.46; IX = 1.59 | I | dark brown to black |
| M 564-175-3-1-1 | M 7.1 | I = 9.26; III = 1.85; V = 31.48; VII = 14.81; VIII = 42.59 | VIII | beige to light brown |
| M 564-175-3-1-2 | M 7.2 | I = 27.91; III = 51.16; VIII = 4.65; IX = 16.28 | III | light brown to brown |
| M 564-175-3-1-3 | M 7.3 | I = 27.27; III = 2.27; IV = 70.45; | IV | ink |
| M 564-175-3-3-1 | M 8.1 | V = 52.73; VIII = 47.27 | V | dark brown |
| M 564-175-3-3-2 | M 8.2 | I = 17.24; III = 72.41; IV = 3.45; IX = 6.90 | III | light brown to brown |
| M 564-175-3-3-3 | M 8.3 | I = 8.96; IV = 91.04 | IV | ink |
| M 564-175-3-4-1 | M 9.1 | I = 32.14; III = 50; IV = 7.14; IX = 10.71 | III | light brown to brown |
| M 564-175-3-4-2 | M 9.2 | I = 22.64; III = 1.89; IV = 75.47 | IV | ink |
| M 564-190-1-1 | M 10 | V = 48.86; VII = 14.77; VIII = 31.82; IX = 4.55 | V | light brown to dark brown |
| M 564-190-3-1-1 | M 11.1 | V = 19.15; VII = 72.34; VIII = 6.38; IX = 2.13 | VII | beige |
| M 564-190-3-1-2 | M 11.2 | I = 23.26; III = 62.79; IX = 13.95 | III | light brown to brown |
| M 564-190-3-1-3 | M 11.3 | I = 56; III = 4; IV = 40 | I | dark brown to black |
| M 564-190-3-2 | M 12 | I = 28.79; III = 1.52; IV = 69.70 | IV | ink |
| M 564-190-3-4 | M 14 | I = 40.74; III = 17.28; IV = 39.51; IX = 2.47 | I/IV | dark brown to black |
| M 564-190-3-5 | M 15 | I = 4.23; IV = 95.77 | IV | ink |
| M 564-190-3-7-1 | M 16.1 | n/a | n/a | n/a |
| M 564-190-3-7-2 | M 16.2 | I = 67.14; III = 15.71; IV = 17.14 | I | dark brown to black |
| M 564-191-1-1 | M 17 | I = 20; III = 10; IV = 70 | IV | ink |
| M 564-193-9-1-1 | M 19.1 | V = 28.57; VII = 26.19; VIII = 28.57; IX = 16.67 | VII/VIII | beige to light brown |
| M 564-193-9-1-2 | M 19.2 | I = 41.46; III = 14.63; IV = 39.02; IX = 4.88 | I/IV | dark brown to black |
| “Tangra” | Local var. | III = 5.13; V = 7.69; VIII = 30.77; IX = 56.41 | IX | speckled brown |
| “Evros” | Initial var. | II = 13.33; VI = 86.67 | VI | pale yellow |
| Variable | Between SS | Df | Within SS | df | F | Significance p |
|---|---|---|---|---|---|---|
| L | 10,289.70 | 2 | 158.65 | 24 | 778.30 | 0.00 |
| c | 1607.49 | 2 | 300.13 | 24 | 64.27 | 0.00 |
| Name of Genotype, Color Group | Form | Ferulic Acid, μg/g dw | Sinapic Acid, μg/g dw | p-Cou-maric Acid, μg/g dw | Caffeic Acid, μg/g dw | Chlorogenic Acid, μg/g dw | Total Phenolic Acids, μg/g dw | Querceti, μg/g dw | Luteoli, μg/g dw | Epicate-chin, μg/g dw | Total Flavonoid, μg/g dw | Total Polyphenols, μg/g dw | Flavonoid/ Phenolics Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M 2.1; | F | 94.47 | 5.37 | 24.39 | 28.94 | 2.41 | 12.53 | 3.05 | 48.67 | ||||
| M 564-56-4-3-1; | C | 269.19 | 19.61 | 422.55 | 742.93 | ||||||||
| White. II. | sum | 363.66 | 24.98 | 446.94 | 771.87 | 2.41 | 1609.87 | 12.53 | 3.05 | 48.67 | 64.25 | 1674.12 | 0.040 |
| M 2.2; | F | 73.92 | 3.83 | 21.88 | 29.94 | 2.63 | 15.04 | 3.44 | 48.55 | ||||
| M 564-56-4-3-2; | C | 183.22 | 54.33 | 418.98 | 691.29 | ||||||||
| beige to light brown. VIII. | sum | 257.14 | 58.15 | 440.86 | 721.24 | 2.63 | 1480.02 | 15.04 | 3.44 | 48.55 | 67.03 | 1547.05 | 0.045 |
| M 2.3 | F | 98.62 | 6.79 | 29.18 | 38.38 | 2.47 | 12.79 | 3.28 | 86.18 | ||||
| M 564-56-4-3-3 | C | 395.09 | 95.11 | 991.39 | 185.90 | ||||||||
| Ink. IV. | sum | 493.71 | 101.90 | 1020.57 | 224.28 | 2.47 | 1842.93 | 12.79 | 3.28 | 86.18 | 102.26 | 1945.19 | 0.055 |
| M 3 | F | 285.00 | 12.49 | 87.52 | 80.61 | 1.13 | 10.32 | 1.73 | 28.55 | ||||
| M 564-56-4-3-3 | C | 649.84 | 423.05 | 144.02 | 233.00 | ||||||||
| White. II. | sum | 934.84 | 435.55 | 231.55 | 313.60 | 1.13 | 1916.66 | 10.32 | 1.73 | 28.55 | 40.60 | 1957.25 | 0.021 |
| M 6.1 | F | 145.71 | 7.64 | 68.52 | 87.86 | 2.95 | 17.06 | 5.82 | 35.46 | ||||
| M 564-172-2-1-1 | C | 663.32 | 351.91 | 218.08 | 491.49 | ||||||||
| speckled brown. IX. | sum | 809.03 | 359.54 | 286.61 | 579.36 | 2.95 | 2037.48 | 17.06 | 5.82 | 35.46 | 58.34 | 2095.83 | 0.029 |
| M 6.2 | F | 157.22 | 8.24 | 78.00 | 9.53 | 2.71 | 15.82 | 6.22 | 38.02 | ||||
| M 564-172-2-1-2 | C | 659.46 | 110.28 | 225.32 | 331.34 | ||||||||
| dark brown to black. I. | sum | 816.67 | 118.52 | 303.32 | 340.86 | 2.71 | 1582.09 | 15.82 | 6.22 | 38.02 | 60.06 | 1642.15 | 0.038 |
| M 7.1 | F | 122.46 | 4.04 | 63.12 | 10.33 | 1.64 | 25.61 | 9.75 | 50.34 | ||||
| M 564-175-3-1-1 | C | 557.88 | 413.08 | 1238.19 | 366.20 | ||||||||
| beige to light brown. VIII. | sum | 680.34 | 417.13 | 1301.31 | 376.53 | 1.64 | 2776.94 | 25.61 | 9.75 | 50.34 | 85.70 | 2862.64 | 0.031 |
| M 7.2 | F | 101.85 | 3.91 | 9.03 | 26.99 | 0.55 | 44.17 | 9.09 | 11.85 | ||||
| M 564-175-3-1-2 | C | 328.07 | 8.64 | 722.81 | 577.90 | ||||||||
| light brown to brown. III. | sum | 429.92 | 12.55 | 731.84 | 604.88 | 0.55 | 1779.75 | 44.17 | 9.09 | 11.85 | 65.11 | 1844.86 | 0.037 |
| M 7.3 | F | 334.03 | 3.53 | 10.86 | 28.90 | 0.19 | 40.52 | 7.43 | 25.57 | ||||
| M 564-175-3-1-3 | C | 155.54 | 195.85 | 811.03 | 523.97 | ||||||||
| Ink. IV. | sum | 489.57 | 199.38 | 821.89 | 552.87 | 0.19 | 2063.91 | 40.52 | 7.43 | 25.57 | 73.52 | 2137.42 | 0.036 |
| M 8.1 | F | 122.91 | 1.07 | 1.51 | 10.00 | 0.56 | 18.88 | 3.55 | 26.84 | ||||
| M 564-175-3-3-1 | C | 239.07 | 8.55 | 294.35 | 88.55 | ||||||||
| dark brown. V. | sum | 361.98 | 9.62 | 295.86 | 98.56 | 0.56 | 766.58 | 18.88 | 3.55 | 26.84 | 49.28 | 815.86 | 0.064 |
| M 8.2 | F | 86.02 | 1.08 | 4.89 | 34.81 | 3.10 | 66.29 | 2.38 | 62.30 | ||||
| M 564-175-3-3-2 | C | 334.09 | 10.22 | 545.28 | 573.39 | ||||||||
| light brown to brown. III. | sum | 420.11 | 11.30 | 550.17 | 608.20 | 3.10 | 1592.89 | 66.29 | 2.38 | 62.30 | 130.96 | 1723.85 | 0.082 |
| M 8.3 | F | 118.90 | 1.07 | 18.67 | 65.34 | 1.65 | 29.55 | 2.66 | 55.04 | ||||
| M 564-175-3-3-3 | C | 460.90 | 163.92 | 549.23 | 242.33 | ||||||||
| Ink. IV. | sum | 579.80 | 165.00 | 567.90 | 307.67 | 1.65 | 1622.02 | 29.55 | 2.66 | 55.04 | 87.25 | 1709.26 | 0.054 |
| M 9.1 | F | 129.60 | 4.92 | 7.06 | 12.56 | 1.04 | 47.33 | 2.69 | 20.40 | ||||
| M 564-175-3-4-1 | C | 225.07 | 9.71 | 427.18 | 359.38 | ||||||||
| light brown to brown. III. | sum | 354.67 | 14.63 | 434.23 | 371.94 | 1.04 | 1176.52 | 47.33 | 2.69 | 20.40 | 70.43 | 1246.94 | 0.060 |
| M 9.2 | F | 122.49 | 4.93 | 11.19 | 76.40 | 3.29 | 52.91 | 6.75 | 58.20 | ||||
| M 564-175-3-4-2 | C | 502.25 | 24.03 | 770.28 | 108.86 | ||||||||
| Ink. IV | sum | 624.73 | 28.96 | 781.47 | 185.26 | 3.29 | 1623.71 | 52.91 | 6.75 | 58.20 | 117.86 | 1741.57 | 0.073 |
| M 10 | F | 81.09 | 0.91 | 41.46 | 46.26 | 2.98 | 14.29 | 5.80 | 20.43 | ||||
| M 564-190-1-1 | C | 353.82 | 19.34 | 174.25 | 139.33 | ||||||||
| light brown–dark brown. V. | sum | 434.91 | 20.26 | 215.71 | 185.59 | 2.98 | 859.44 | 14.29 | 5.80 | 20.43 | 40.52 | 899.96 | 0.047 |
| M 11.1 | F | 84.49 | 9.42 | 15.05 | 19.07 | 1.46 | 12.44 | 1.77 | 88.46 | ||||
| M 564-190-3-1-1 | C | 255.80 | 5.88 | 130.51 | 488.84 | ||||||||
| Beige. VII. | sum | 340.29 | 15.30 | 145.56 | 507.91 | 1.46 | 1010.52 | 12.44 | 1.77 | 88.46 | 102.67 | 1113.19 | 0.102 |
| M 11.2 | F | 97.80 | 8.40 | 13.57 | 24.38 | 2.90 | 40.16 | 1.46 | 31.18 | ||||
| M 564-190-3-1-2 | C | 461.11 | 303.27 | 498.60 | 186.28 | ||||||||
| light brown to brown. III. | sum | 558.90 | 311.67 | 512.17 | 210.66 | 2.90 | 1596.30 | 40.16 | 1.46 | 31.18 | 72.80 | 1669.11 | 0.046 |
| M 11.3 | F | 69.38 | 10.84 | 12.86 | 34.15 | 2.33 | 34.07 | 1.64 | 41.94 | ||||
| M 564-190-3-1-3 | C | 295.86 | 68.34 | 377.46 | 613.50 | ||||||||
| dark brown to black. I. | sum | 365.24 | 79.19 | 390.32 | 647.64 | 2.33 | 1484.72 | 34.07 | 1.64 | 41.94 | 77.64 | 1562.37 | 0.052 |
| M 12 | F | 101.00 | 13.59 | 14.05 | 35.91 | 0.35 | 50.34 | 0.75 | 22.65 | ||||
| M 564-190-3-2 | C | 464.74 | 100.19 | 492.11 | 89.16 | ||||||||
| Ink. IV. | sum | 565.73 | 113.78 | 506.16 | 125.07 | 0.35 | 1311.10 | 50.34 | 0.75 | 22.65 | 73.75 | 1384.84 | 0.056 |
| M 14 | F | 39.35 | 1.39 | 3.60 | 12.69 | 0.50 | 79.78 | 5.17 | 11.70 | ||||
| M 564-190-3-4 | C | 235.22 | 9.29 | 393.27 | 429.27 | ||||||||
| dark brown to black. I./Ink. IV. | sum | 274.57 | 10.67 | 396.87 | 441.96 | 0.50 | 1124.57 | 79.78 | 5.17 | 11.70 | 96.65 | 1221.22 | 0.086 |
| M 15 | F | 61.40 | 1.25 | 5.74 | 17.62 | 2.57 | 84.19 | 2.78 | 22.87 | ||||
| M 564-190-3-5 | C | 97.47 | 10.39 | 567.01 | 252.42 | ||||||||
| Ink. IV. | sum | 158.87 | 11.64 | 572.75 | 270.04 | 2.57 | 1015.87 | 84.19 | 2.78 | 22.87 | 109.84 | 1125.72 | 0.108 |
| M 17 | F | 63.85 | 7.31 | 10.70 | 33.43 | 1.66 | 28.92 | 1.35 | 10.18 | ||||
| M 564-191-1-1 | C | 269.50 | 26.95 | 389.45 | 271.12 | ||||||||
| Ink. IV. | sum | 333.35 | 34.26 | 400.15 | 304.55 | 1.66 | 1073.98 | 28.92 | 1.35 | 10.18 | 40.46 | 1114.43 | 0.038 |
| M 19.1 | F | 69.29 | 0.64 | 3.89 | 24.93 | 1.19 | 17.89 | 14.23 | 18.72 | ||||
| M 564-193-9-1-1 | C | 105.11 | 7.96 | 1051.36 | 155.07 | ||||||||
| beige to light brown. VIII. | sum | 174.41 | 8.60 | 1055.25 | 180.00 | 1.19 | 1419.44 | 17.89 | 14.23 | 18.72 | 50.83 | 1470.27 | 0.036 |
| M 19.2 | F | 66.24 | 0.61 | 4.85 | 11.33 | 1.13 | 4.15 | 0.34 | 22.97 | ||||
| M 564-193-9-1-2 | C | 108.01 | 18.71 | 427.87 | 57.48 | ||||||||
| dark brown to black/ink. I/IV. | sum | 174.25 | 19.31 | 432.72 | 68.81 | 1.13 | 696.23 | 4.15 | 0.34 | 22.97 | 27.46 | 723.68 | 0.039 |
| Evros cv. | F | 32.41 | 1.40 | 11.40 | 9.51 | 0.17 | 4.82 | 0.47 | 8.00 | ||||
| Initial cv | C | 124.18 | 39.76 | 31.84 | 43.59 | ||||||||
| Pale yellow. VI | sum | 156.59 | 41.16 | 43.24 | 53.10 | 0.17 | 294.26 | 4.82 | 0.47 | 8.00 | 13.29 | 307.55 | 0.045 |
| Compound | Rt, min | [M]+ (m/z) | MS/MS (m/z) |
|---|---|---|---|
| Phenolic acids | |||
| Ferulic acid | 1.15 | 193 | 178/134 |
| p-coumaric acid | 1.22 | 163 | 119/93 |
| Chlorogenic acid | 1.00 | 353 | 191/179 |
| Caffeic acid | 1.04 | 179 | 135/107 |
| Sinapic acid | 1.13 | 223 | 208/179/164 |
| Flavonoids | |||
| Luteolin | 1.26 | 285 | 151/133 |
| Epicatechin | 1.04 | 289 | 245/203/109 |
| Quercetin | 1.27 | 301 | 179/151 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaneva, T.G.; Wiczkowski, W.; Marchev, A.S.; Iserliyska, D.; Georgiev, M.I.; Tomlekova, N.B. Evaluation of Polyphenols Synthesized in Mature Seeds of Common Bean (Phaseolus vulgaris L.) Advanced Mutant Lines. Int. J. Mol. Sci. 2024, 25, 5638. https://doi.org/10.3390/ijms25115638
Yaneva TG, Wiczkowski W, Marchev AS, Iserliyska D, Georgiev MI, Tomlekova NB. Evaluation of Polyphenols Synthesized in Mature Seeds of Common Bean (Phaseolus vulgaris L.) Advanced Mutant Lines. International Journal of Molecular Sciences. 2024; 25(11):5638. https://doi.org/10.3390/ijms25115638
Chicago/Turabian StyleYaneva, Teodora G., Wieslaw Wiczkowski, Andrey S. Marchev, Dida Iserliyska, Milen I. Georgiev, and Nasya B. Tomlekova. 2024. "Evaluation of Polyphenols Synthesized in Mature Seeds of Common Bean (Phaseolus vulgaris L.) Advanced Mutant Lines" International Journal of Molecular Sciences 25, no. 11: 5638. https://doi.org/10.3390/ijms25115638
APA StyleYaneva, T. G., Wiczkowski, W., Marchev, A. S., Iserliyska, D., Georgiev, M. I., & Tomlekova, N. B. (2024). Evaluation of Polyphenols Synthesized in Mature Seeds of Common Bean (Phaseolus vulgaris L.) Advanced Mutant Lines. International Journal of Molecular Sciences, 25(11), 5638. https://doi.org/10.3390/ijms25115638








