Metabolome Profiling of Marrubium peregrinum L. and Marrubium friwaldskyanum Boiss Reveals Their Potential as Sources of Plant-Based Pharmaceuticals
Abstract
:1. Introduction
2. Results
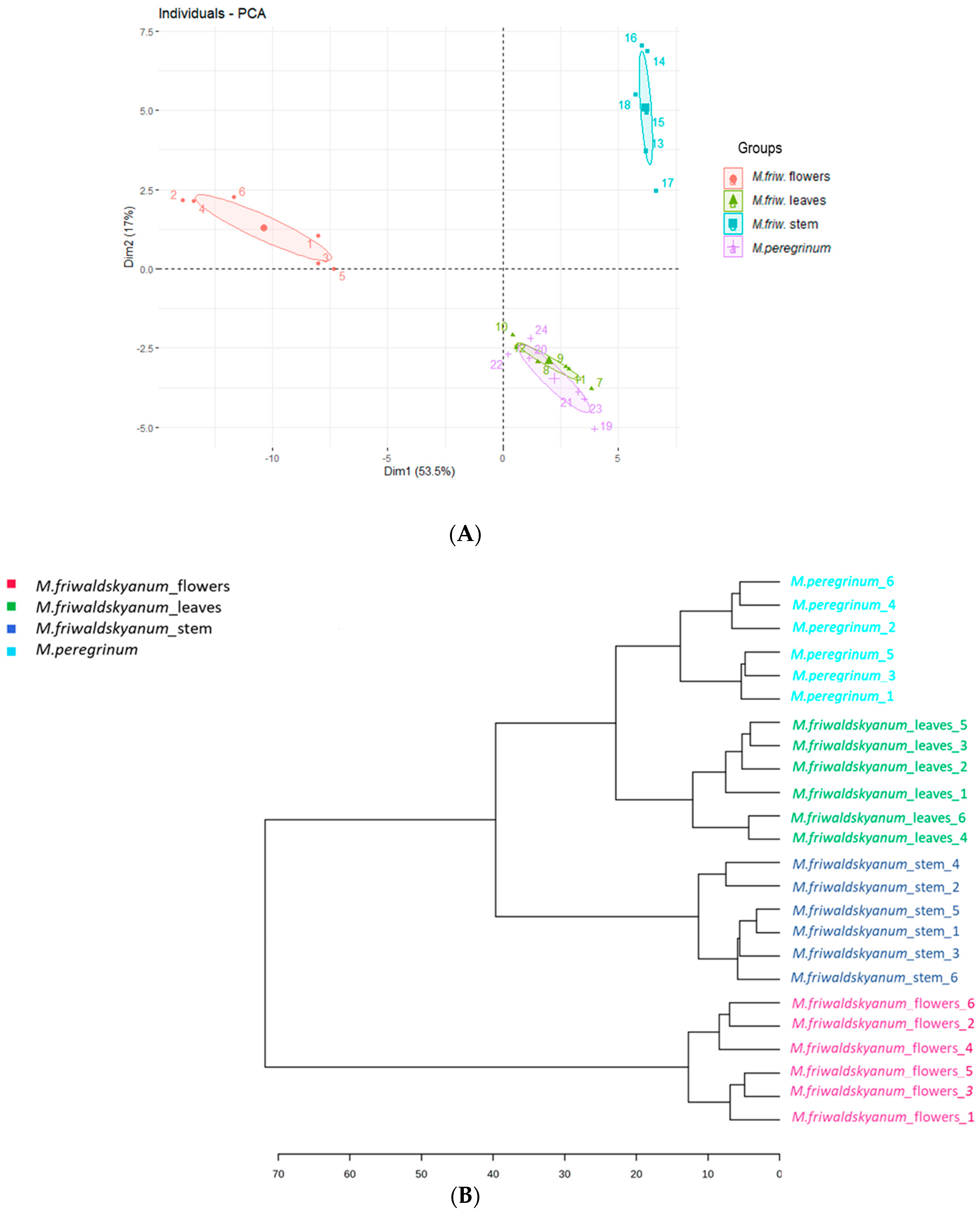
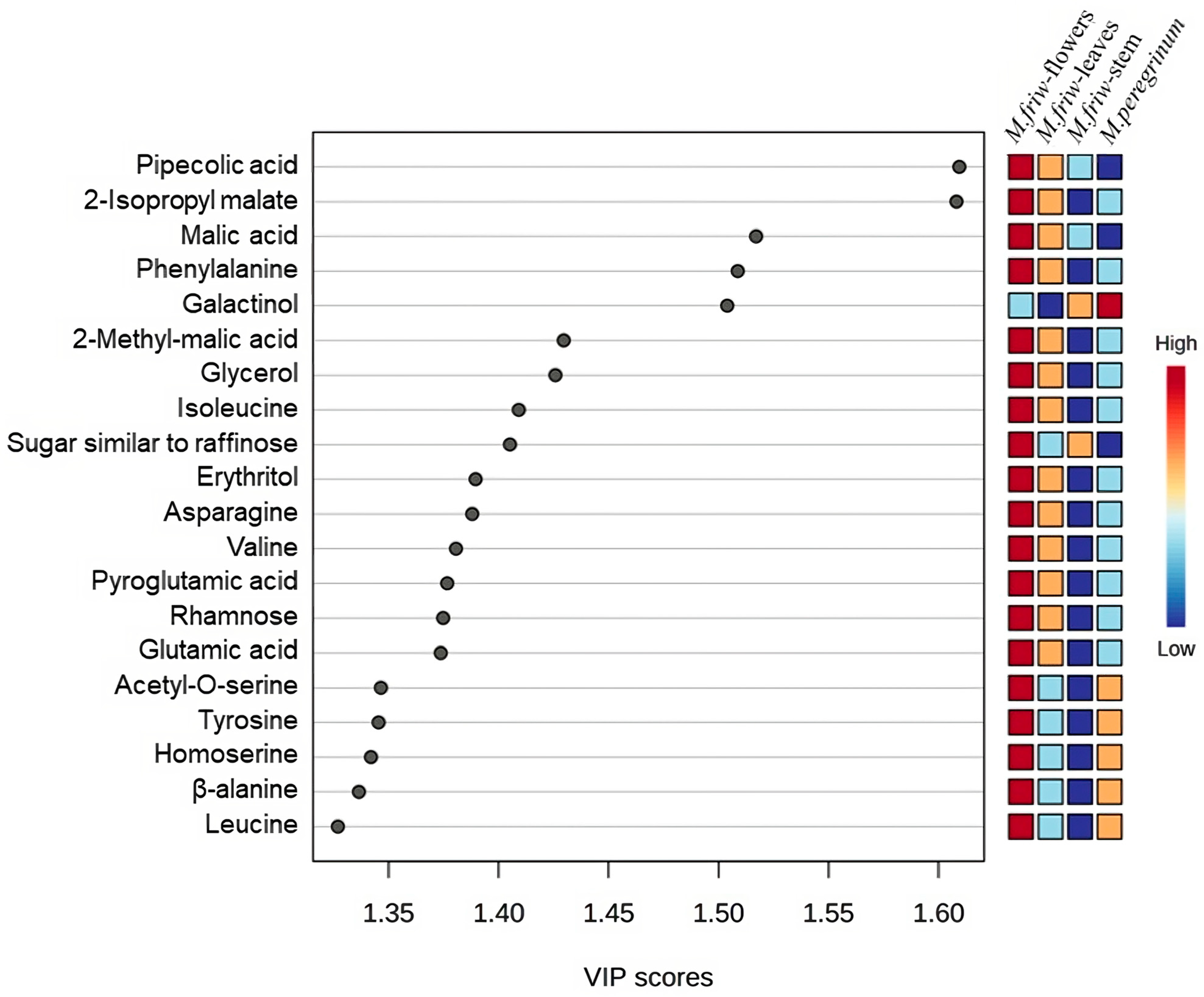
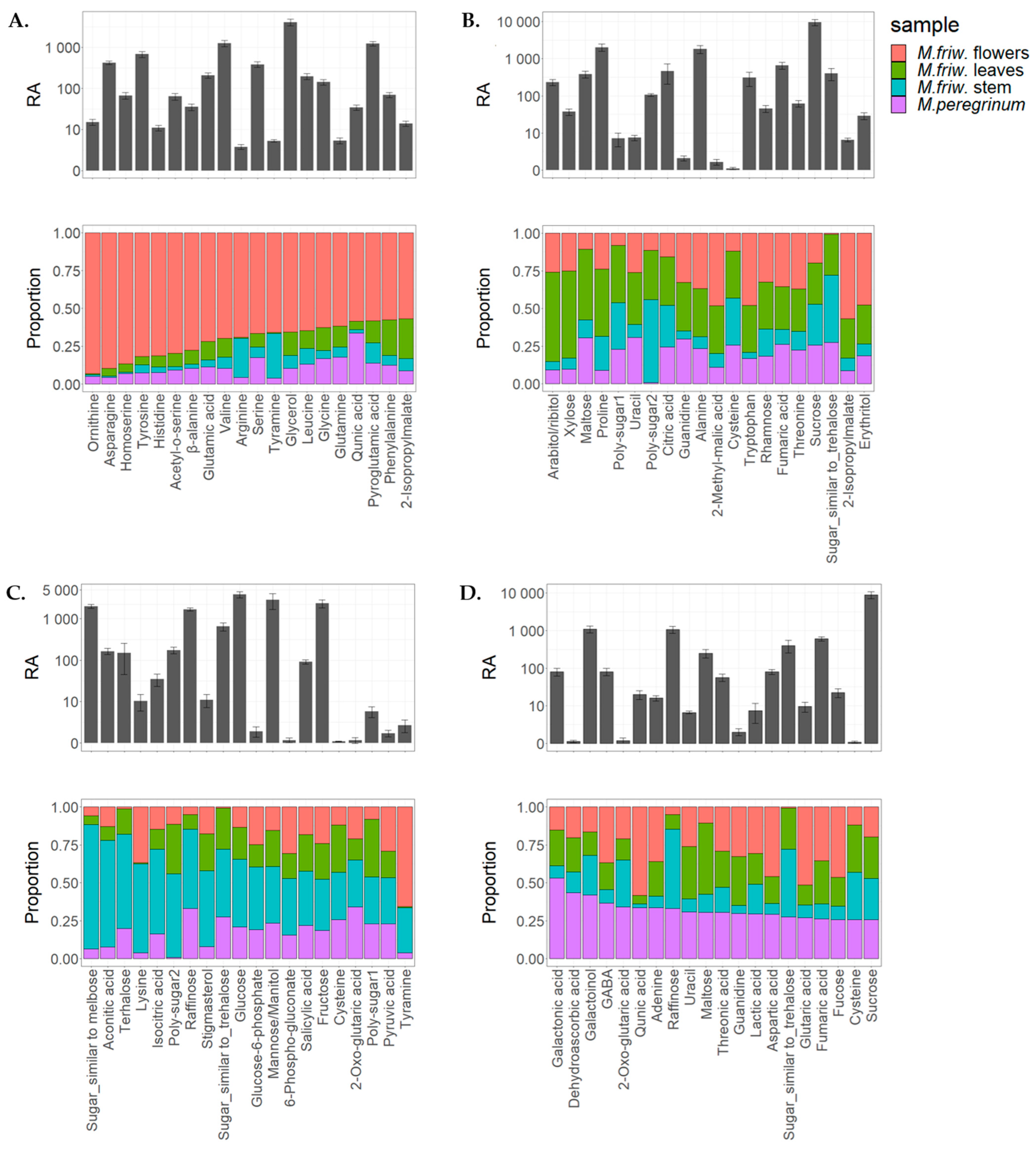
2.1. GC-MS Analysis of Primary Metabolites
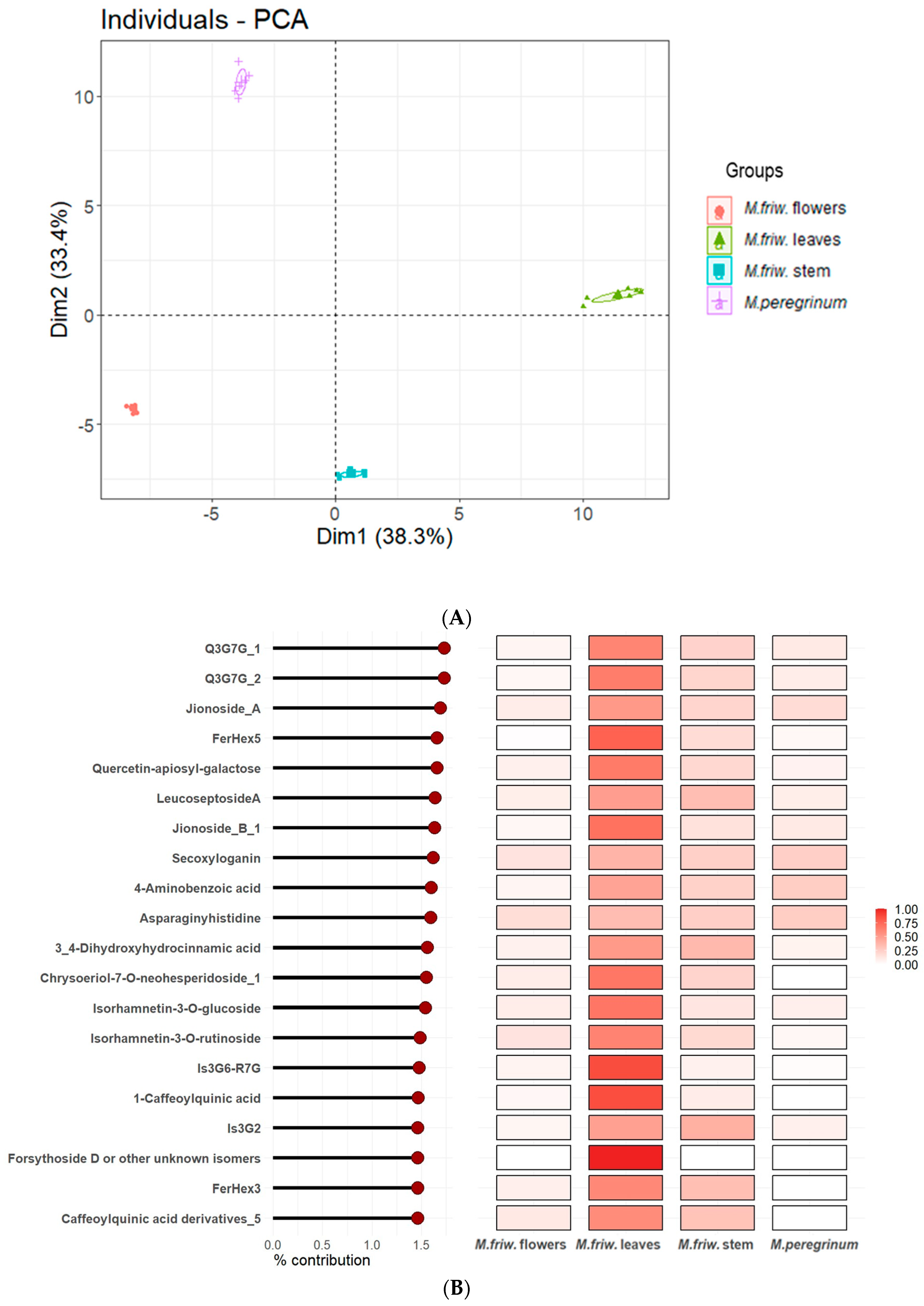
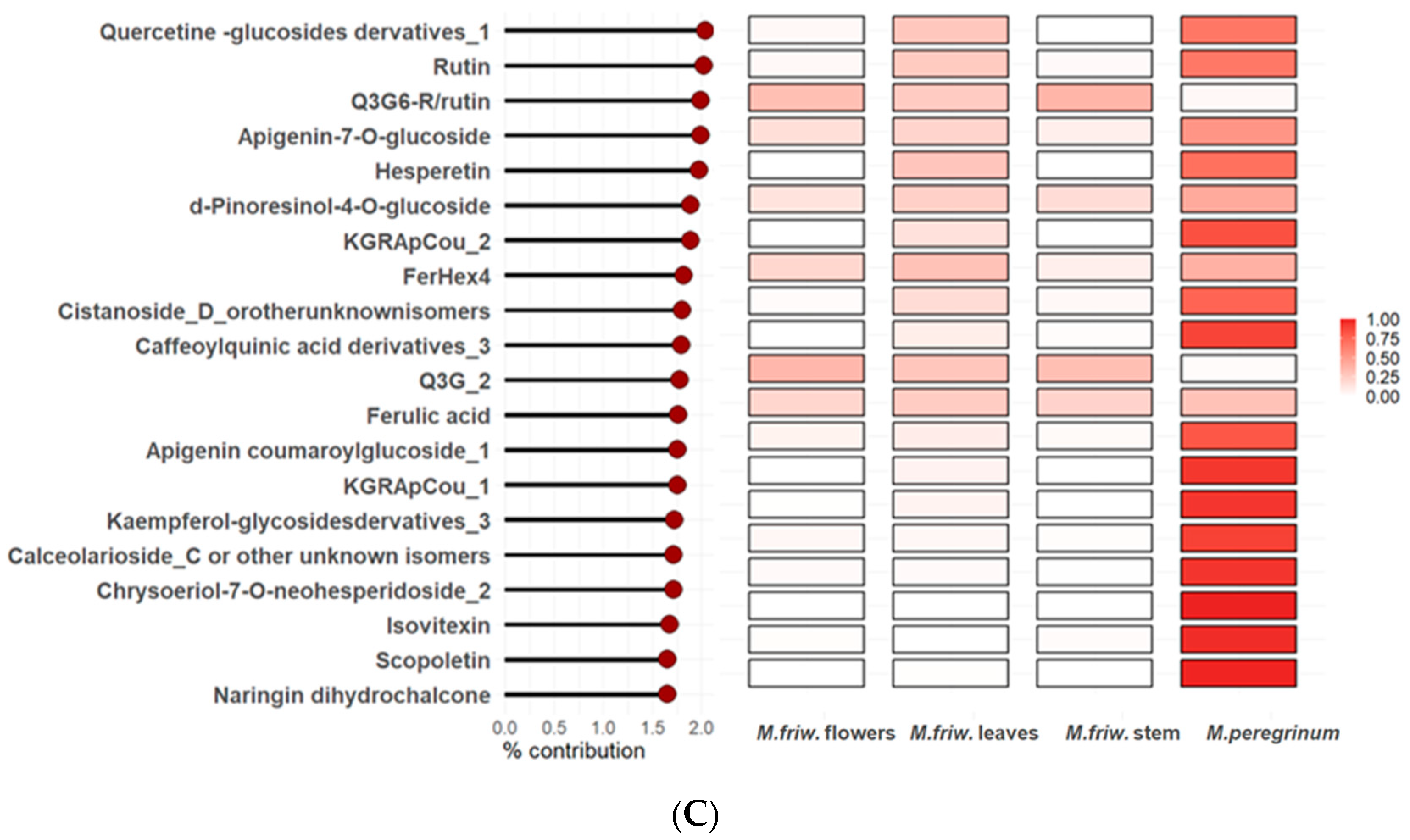
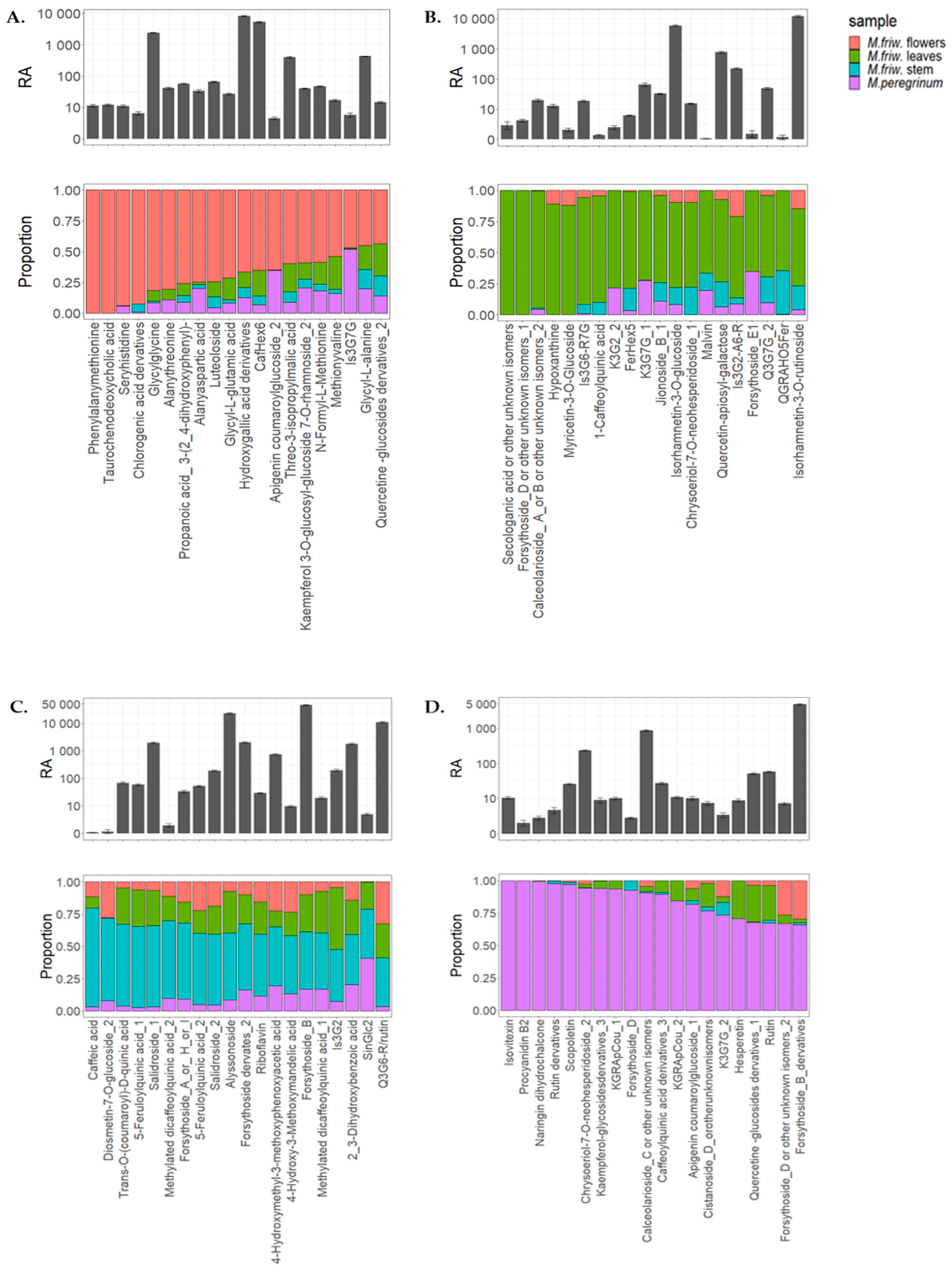
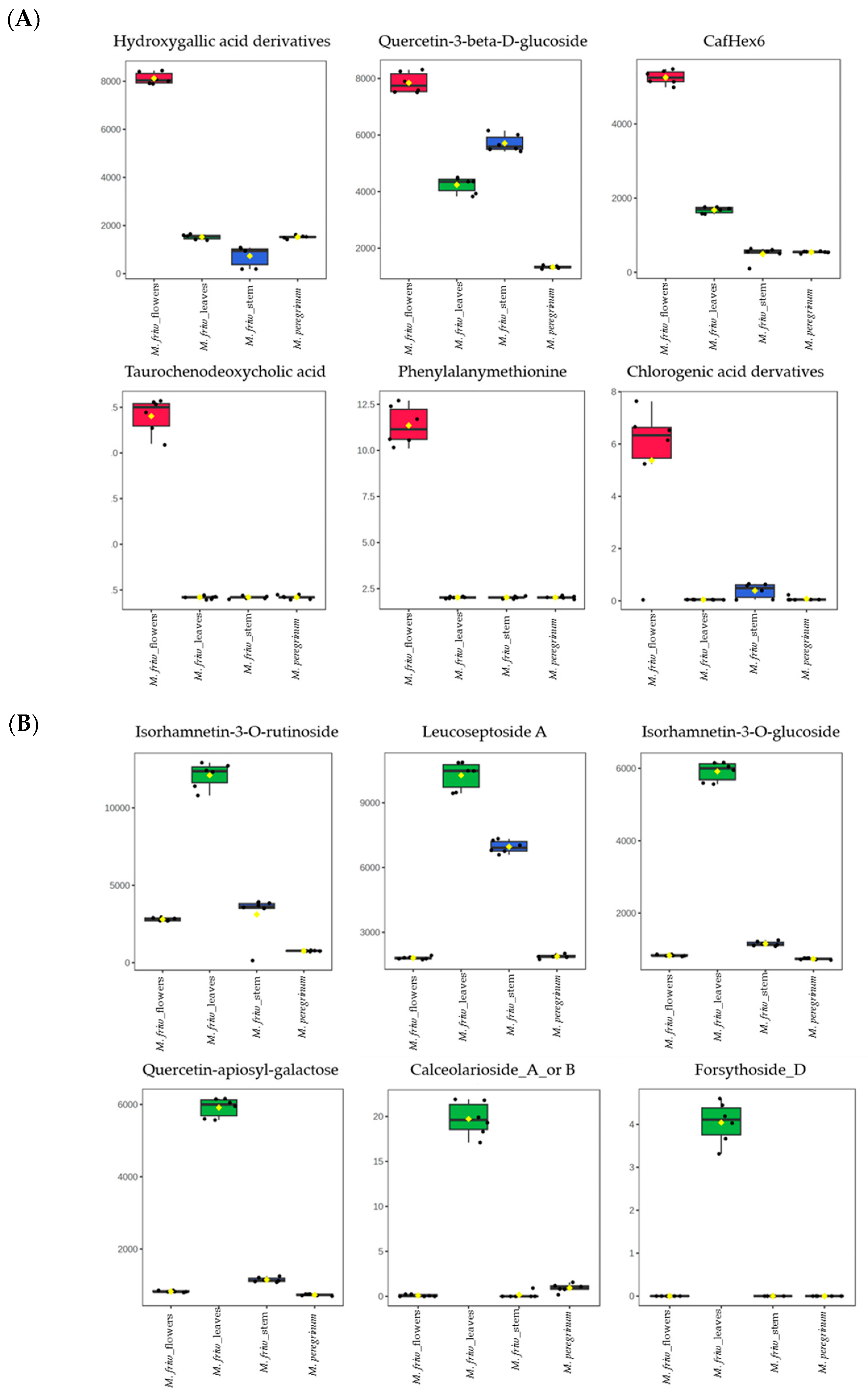
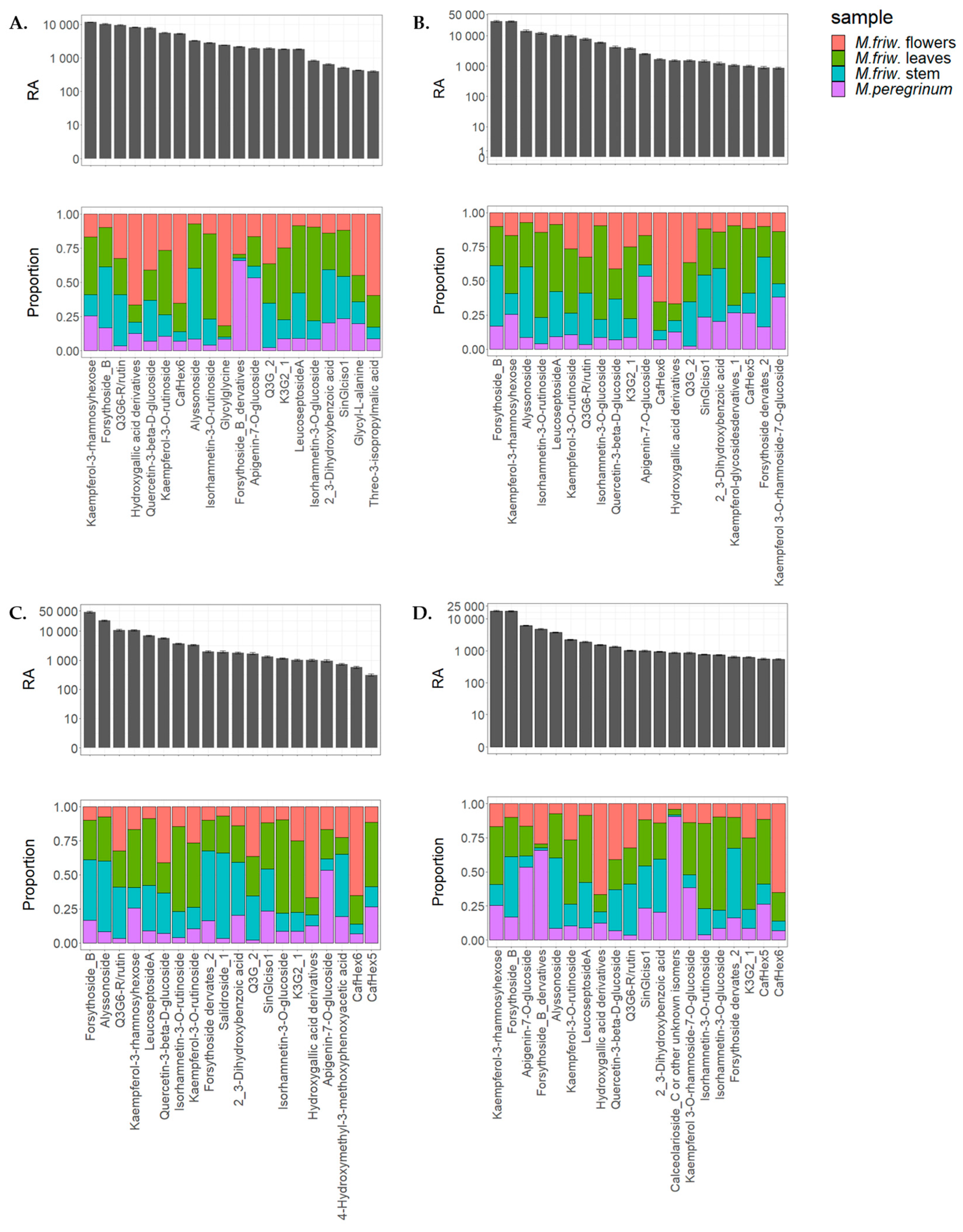
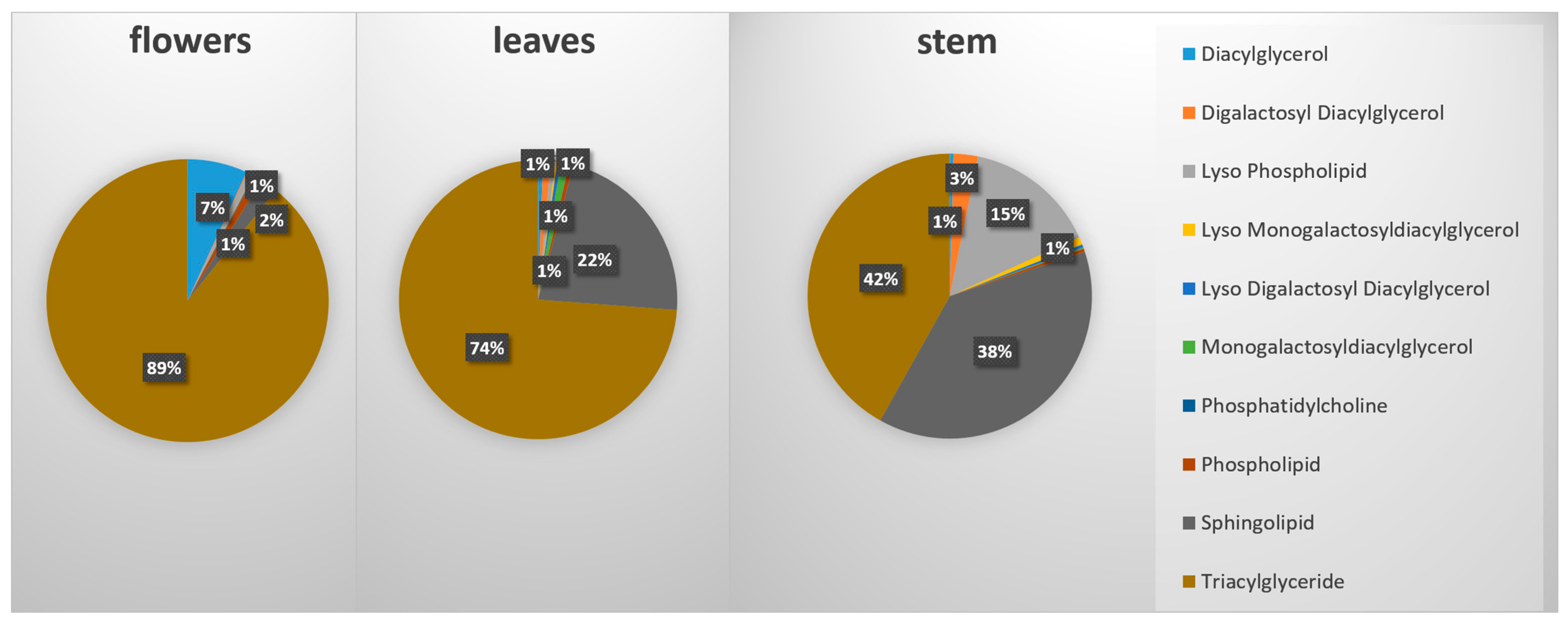
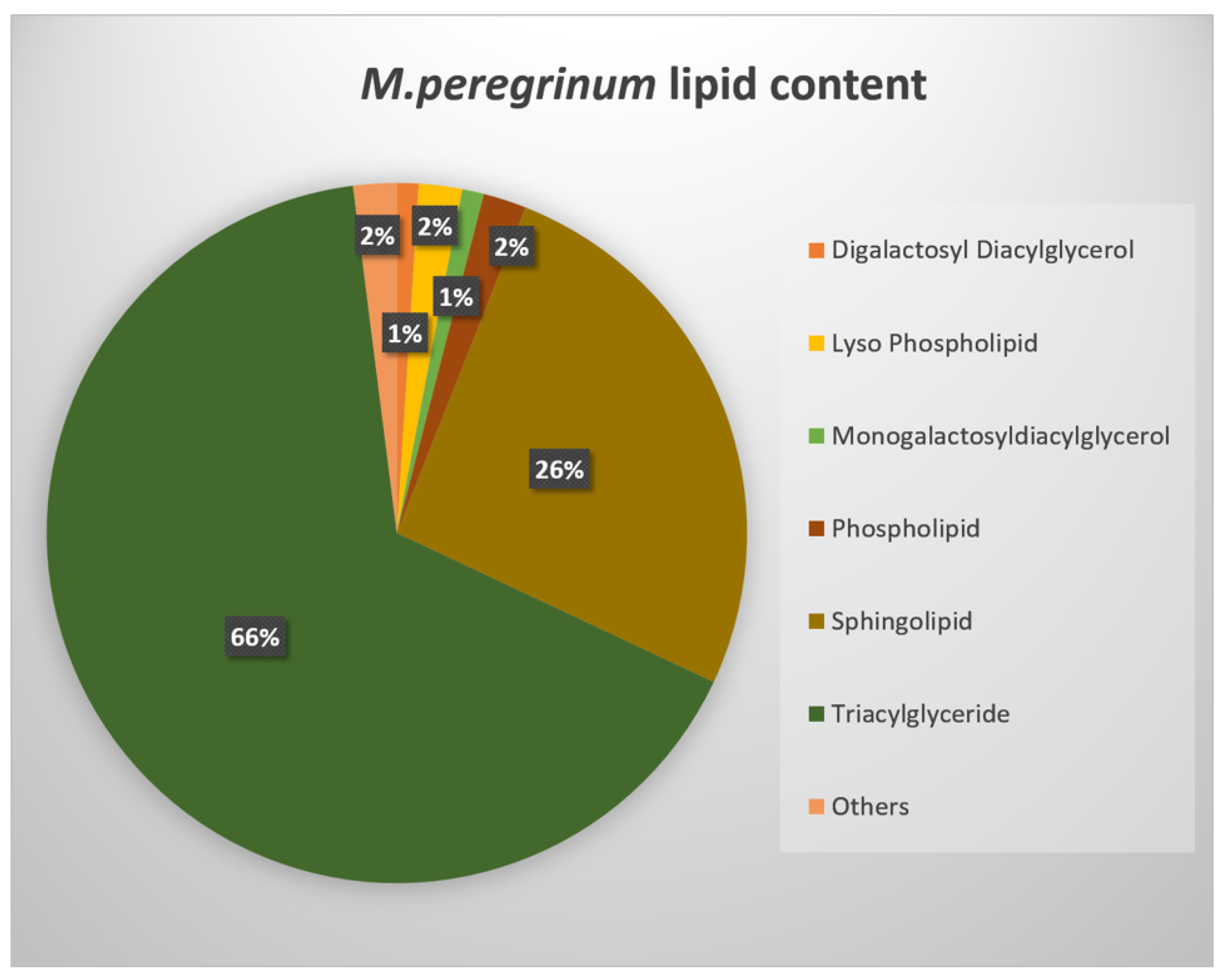
2.2. UPLC-MS/MS Analysis of Secondary Metabolites
2.3. Mineral Contents
3. Discussion
3.1. M. friwaldskyanum Flowers
3.2. M. friwaldskyanum Leaves
3.3. M. friwaldskyanum Stem
3.4. M. peregrinum
3.5. Mineral Content
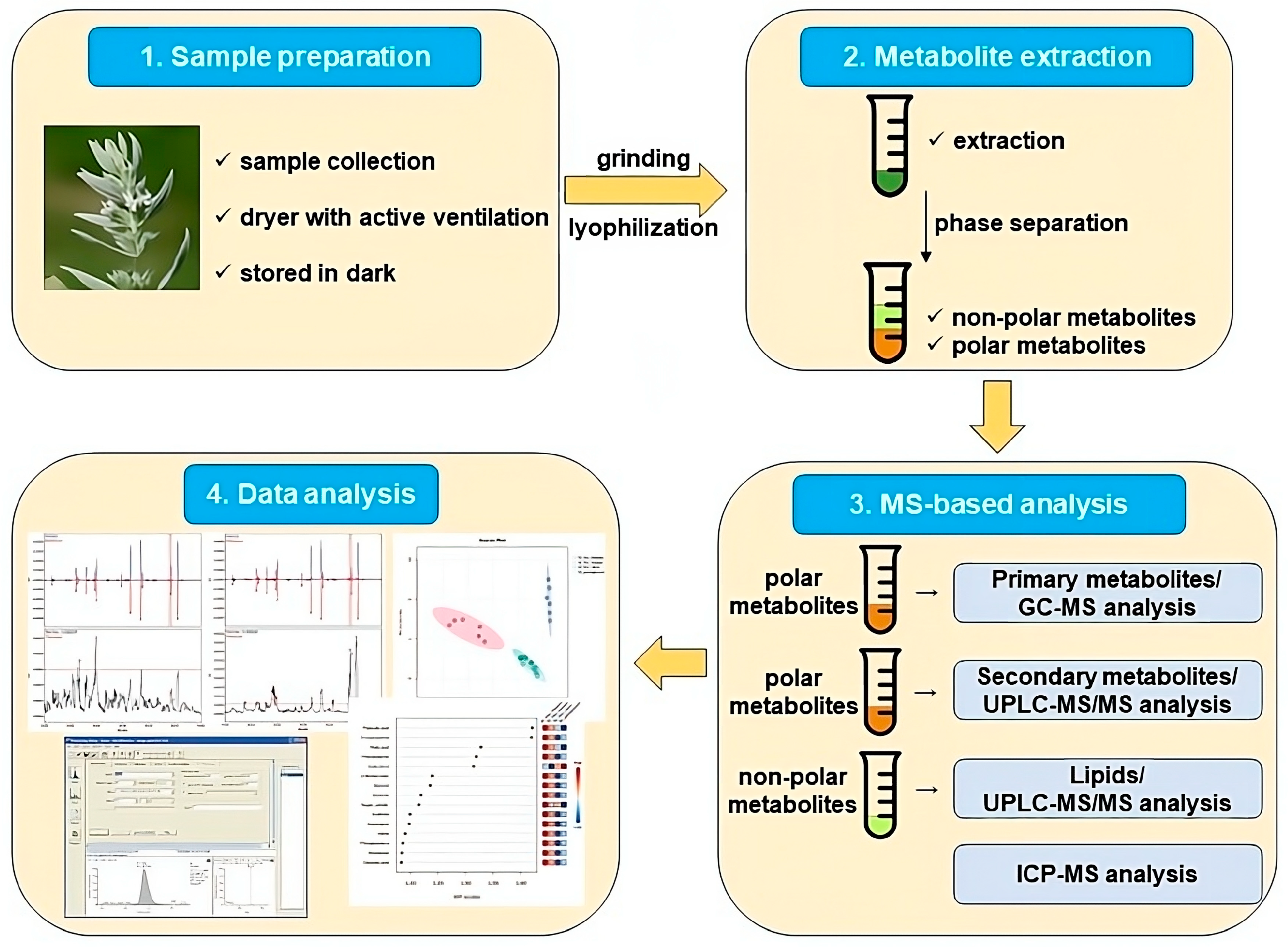
4. Materials and Methods
4.1. Plant Material
4.2. Metabolite Extraction and Metabolite Measurements
4.3. Compound Annotation
4.4. Determination of Mineral Contents
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alarcon-Barrera, J.C.; Kostidis, S.; Ondo-Mendez, A.; Giera, M. Recent Advances in Metabolomics Analysis for Early Drug Development. Drug Discov. Today 2022, 6, 1763–1773. [Google Scholar] [CrossRef]
- Xiao, Q.; Mu, X.; Liu, J.; Li, B.; Liu, H.; Zhang, B.; Xiao, P. Plant Metabolomics: A New Strategy and Tool for Quality Evaluation of Chinese Medicinal Materials. Chin. Med. 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-T. (Ed.) Phytochemical Omics in Medicinal Plants; MDPI—Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Akgül, G.; Ketenoğlu, O.; Pinar, N.M.; Kurt, L. Pollen and Seed Morphology of the Genus Marrubium (Lamiaceae) in Turkey. Ann. Bot. Fenn. 2008, 45, 1–10. [Google Scholar] [CrossRef]
- Meyre-Silva, C.; Cechinel-Filho, V. A Review of the Chemical and Pharmacological Aspects of the Genus Marrubium. Curr. Pharm. Des. 2010, 16, 3503–3518. [Google Scholar] [CrossRef]
- Göğer, F.; Özek, G.; Tekin, M.; Yur, S.; Özek, T. Phytochemical Profiling and Evaluation of Marrubium Sivasense Aytaç, Akgül & Ekici for Antioxidant Activity and Inhibition Effects on α-Amylase, Lipoxygenase, Xanthine Oxidase and Tyrosinase Enzymes. J. Turkish Chem. Soc. Sect. Chem. 2019, 6, 281–292. [Google Scholar] [CrossRef]
- Andreev, N.; Anchev, M.; Asenov, I.; Delipavlov, D.; Koeva, J.; Kozhuharov, S.; Kuzmanov, B.; Harley, R.; Markova, M.; Peev, D.; et al. Flora of the Republic of Bulgaria; Bulgarian Academy of Sciences Publishing House: Sofia, Bulgaria, 1989; Volume 9, pp. 365–369. (In Bulgarian) [Google Scholar]
- Gyuzeleva, D.; Stoyanov, P.; Bivolarska, A.; Mladenov, R.; Mladenova, T.; Petkov, V.; Todorov, K. Anatomical Investigation of Marrubium friwaldskyanum Boiss. and Marrubium peregrinum L. (Lamiaceae) from Bulgaria. Ecol. Balk. 2022, 14, 87–101. [Google Scholar]
- Zheljazkov, V.; Semerdjieva, I.; Stevens, J.; Wu, W.; Cantrell, C.; Yankova-Tsvetkova, E.; Koleva-Valkova, L.; Stoyanova, A.; Astatkie, T. Phytochemical investigation and reproductive capacity of the Bulgarian endemic plant Marrubium friwaldskyanum Boiss. (Lamiaceae). Plants 2022, 11, 114. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vlaisavljevic, S.; Popovic, M.; Vastag, D.; Djurendic-Brenesel, M. Antioxidant Properties of Marrubium peregrinum L. (Lamiaceae) Essential Oil. Molecules 2010, 15, 5943–5955. [Google Scholar] [CrossRef]
- Kozyra, M.; Korga, A.; Ostrowska, M.; Humeniuk, E.; Adamczuk, G.; Gieroba, R.; Makuch-Kocka, A.; Dudka, J. Cytotoxic Activity of Methanolic Fractions of Different Marrubium spp. Against Melanoma Cells Is Independent of Antioxidant Activity and Total Phenolic Content. FEBS Open Bio 2019, 10, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Zlinska, J. In vitro effect of Marrubium peregrinum L. (Lamiaceae) leaves extract. Fresenius Enviromental Bull. 2011, 20, 3152–3315. [Google Scholar]
- Radojević, I.; Stanković, M.; Stefanović, O.; Čomić, L.; Marina, T.; Vasić, S.; Nikolić, M. Exploring Antimicrobial Activity of Horehound, Marrubium peregrinum L. Extracts. Phytochemistry 2013, 35, 99–106. [Google Scholar]
- Al-Snafi, A.E.; Al-Saedy, H.A.; Talab, T.A.; Majid, W.J.; Batiha, G.E.S.; Abofazi, J.S. The bioactive ingredients and therapeutic effects of Marrubium vulgare—A review. Int. J. Biol. Pharm. Sci. Arch. 2021, 01, 009–021. [Google Scholar] [CrossRef]
- Stanković, M. Total Phenolic Content, Flavonoid Concentration and Antioxidant Activity of Marrubium peregrinum L. Extracts. Kragujevac J. Sci. 2011, 33, 63–72. [Google Scholar]
- Piozzi, F.; Bruno, M.; Rosselli, S.; Maggio, A. The Diterpenoids of the Genus Marrubium (Lamiaceae). Nat. Prod. Commun. 2006, 1, 585–592. [Google Scholar] [CrossRef]
- Hennebele, T.; Şahpaz, S.; Skaltsounis, A.; Baileul, F. Phenolic Compounds and Diterpenoids from Marrubium peregrinum. Biochem. Syst. Ecol. 2007, 35, 624–626. [Google Scholar] [CrossRef]
- Jovin, E.; Toth, A.; Beara, I.; Bekvalac, K. Chemical composition and antioxidant capacity of Marrubium peregrinum L. from Vojvodina (Serbia). Planta Medica 2008, 74, PB65. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Abu-Reidah, I.; Quirantes-Pine, R.; Madani, K.; Segura-Carretero, A. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plants growing in Algeria. Ind. Crops Prod. 2014, 61, 120–129. [Google Scholar] [CrossRef]
- Lodhi, S.; Vadnere, G.; Sharma, V.; Usman, M. Marrubium vulgare L.: A Review on Phytochemical and Pharmacological Aspects. J. Intercult. Ethnopharmacol. 2017, 6, 429. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Heiling, S.; Baldwin, I.T.; Gaquerel, E. Illuminating a Plant’s Tissue-Specific Metabolic Diversity Using Computational Metabolomics and Information Theory. Biol. Sci. 2016, 113, 7610–7618. [Google Scholar] [CrossRef] [PubMed]
- Pélabon, C.; Hennet, L.; Strimbeck, R.; Johnson, H.; Armbruster, W.S. Blossom Colour Change after Pollination Provides Carbon for Developing Seeds. Funct. Ecol. 2015, 29, 1137–1143. [Google Scholar] [CrossRef]
- Borghi, M.; Fernie, A.R. Floral Metabolism of Sugars and Amino Acids: Implications for Pollinators’ Preferences and Seed and Fruit Set. Plant Physiol. 2017, 175, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Borrego, E.J.; Savka, M.A.; Dobson, R.C.J.; Hudson, A.O. Amino Acid–Derived Defense Metabolites from Plants: A Potential Source to Facilitate Novel Antimicrobial Development. J. Biol. Chem. 2021, 296, 100438. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Safronova, N.A. Non-Proteinogenic Amino Acid β-N-Methylamino-L-Alanine (BMAA): Bioactivity and Ecological Significance. Toxins 2022, 14, 539. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Shikimate and Phenylalanine Biosynthesis in the Green Lineage. Front. Plant Sci. 2013, 4, 62. [Google Scholar] [CrossRef]
- Moreno, J.; Rojas, B.E.; Vicente, R.; Gorka, M.; Matz, T.W.; Chodasiewicz, M.; Peralta-Ariza, J.S.; Zhang, Y.; Alseekh, S.; Childs, D.; et al. Tyr-Asp Inhibition of Glyceraldehyde 3-Phosphate Dehydrogenase Affects Plant Redox Metabolism. EMBO J. 2021, 40, e106800. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and By-Products: A Review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Wawrzyniak-Gramacka, E.; Książek, A.; Zagrodna, A.; Kopeć, W.; Słowińska-Lisowska, M. Dipeptide Extract Modulates the Oxi-Antioxidant Response to Intense Physical Exercise. Nutrients 2022, 14, 2402. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Shi, L.; Duan, G.; Ma, Y.; Li, P. Taurochenodeoxycholic Acid Increases CAMP Content via Specially Interacting with Bile Acid Receptor TGR5. Molecules 2021, 26, 7066. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Piazza, F.; Baraldini, M.; Speroni, E.; Guerra, M.C.; Cerré, C.; Cantelli Forti, G. Taurohyodeoxycholic Acid Protects against Taurochenodeoxycholic Acid-Induced Cholestasis in the Rat. Hepatology 1998, 27, 520–525. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L.; Huang, J.; Xu, P.; Zhang, Z.; Yin, D.; Liu, J.; He, H.; He, M. Luteoloside Attenuates Anoxia/Reoxygenation-Induced Cardiomyocytes Injury via Mitochondrial Pathway Mediated by 14-3-3η Protein. Phytother. Res. 2018, 32, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ding, Y.; Ke, Z.; Cao, L.; Li, N.; Ding, G.; Wang, Z.; Xiao, W. Luteoloside Acts as 3C Protease Inhibitor of Enterovirus 71 In Vitro. PLoS ONE 2016, 11, 0148693. [Google Scholar] [CrossRef]
- Fan, S.H.; Wang, Y.; Lu, J.; Zheng, Y.L.; Wu, D.M.; Li, M.Q.; Hu, B.; Zhang, Z.F.; Cheng, W.; Shan, Q. Luteoloside Suppresses Proliferation and Metastasis of Hepatocellular Carcinoma Cells by Inhibition of NLRP3 Inflammasome. PLoS ONE 2014, 9, 89961. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-Glucoside Inhibits IL-22/STAT3 Pathway, Reducing Proliferation, Acanthosis, and Inflammation in Keratinocytes and in Mouse Psoriatic Model. Cell Death Dis. 2016, 7, 2344. [Google Scholar] [CrossRef]
- Cartron, E.; Carbonneau, M.A.; Fouret, G.; Descomps, B.; Léger, C.L. Specific Antioxidant Activity of Caffeoyl Derivatives and Other Natural Phenolic Compounds: LDL Protection against Oxidation and Decrease in the Proinflammatory Lysophosphatidylcholine Production. J. Nat. Prod. 2001, 64, 480–486. [Google Scholar] [CrossRef]
- Sayed, S.M.A.; Alseekh, S.; Siems, K.; Fernie, A.R.; Luyten, W.; Schmitz-Linneweber, C.; Saul, N. Identification of a Hydroxygallic Acid Derivative, Zingibroside R1 and a Sterol Lipid as Potential Active Ingredients of Cuscuta Chinensis Extract That Has Neuroprotective and Antioxidant Effects in Aged Caenorhabditis Elegans. Nutrients 2022, 14, 4199. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Baira, E.; Iosifidou, S.; Manea-Karga, E.; Tsipi, D.; Gounari, S.; Theologidis, I.; Barmpouni, T.; Danieli, P.P.; Lazzari, F.; et al. Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS. Molecules 2023, 28, 3967. [Google Scholar] [CrossRef]
- Yang, H.X.; Liu, Q.P.; Zhou, Y.X.; Chen, Y.Y.; An, P.; Xing, Y.Z.; Zhang, L.; Jia, M.; Zhang, H. Forsythiasides: A Review of the Pharmacological Effects. Front. Cardiovasc. Med. 2022, 9, 971491. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tuo, Q.; Li, D.; Wang, X.; Li, X.; Zhang, Y.; Zhao, G.; Lin, F. Antioxidant effects of Salidroside in the Cardiovascular system. Evid. Based Complement. Altern. Med. 2020, 2020, 9568647. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Yang, Z.; Huang, Y.; Yuan, H.; Lin, C.; Wang, T.; Zhao, Z.; Zhou, Y.; Zhu, C. Natural phenylethanoid glycosides isolated from Callicarpa kwangtungensis suppressed lipopolysaccharide-mediated inflammatory response via activating Keap1/Nrf2/HO-1 pathway in RAW 264.7 macrophages cell. J. Ethnopharmacol. 2020, 258, 112857. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Tomar, S.K.; Singh, A.K.; Mandal, S.; Arora, S. Riboflavin and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2017, 57, 3650–3660. [Google Scholar] [CrossRef]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, C.; Chen, K. Nutritional and composition of fruit cultivars: Loquat (Eriobotrya japonica Lindl). In Nutritional Composition of Fruit Cultivars; Academic Press: London, UK, 2016; pp. 371–394. [Google Scholar] [CrossRef]
- Kamimura, A.; Takahashi, T. Procyanidin B-2, extracted from apples, promotes, hair growth: A laboratory study. Br. J. Dermatol. 2002, 146, 41–51. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-kB signaling in inflammation. Signal Transduct Targer Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.K.; Pooniya, V.; Suri, V.K.; Singh, U. Soil Factors Associated with Micronutrient Acquisition in Crops- Biofortification Perspective. In Biofortification of Food Crops, 1st ed.; Singh, U., Praharaj, C., Singh, S., Singh, N., Eds.; Springer: New York, NY, USA, 2016; pp. 159–176. [Google Scholar] [CrossRef]
- Güleryüz, G.; Erdemir, Ü.S.; Arslan, H.; Güçer, Ş. Elemental composition of Marrubium astracanicum Jacq. growing in tungsten-contaminated sites. Environ. Sci. Pollut. Res. 2016, 23, 18332–18342. [Google Scholar] [CrossRef]
- Rezgui, M.; Basma, M.; Neng, N.; Nogueira, J.M.; Bettaieb Ben-Kaab, L.; Machado Araújo, M.E. Evaluation of Marrubium vulgare Growing Wild in Tunisia for Its Potential as a Dietary Supplement. Foods 2021, 10, 2864. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Giavalisco, P.; Li, Y.; Matthes, A.; Eckhardt, A.; Hubberten, H.M.; Hesse, H.; Segu, S.; Hummel, J.; Köhl, K.; Willmitzer, L. Elemental formula annotation of polar and lipophilic metabolites using (13) C, (15) N and (34) S isotope labelling, in combination with high-resolution mass spectrometry. Plant J. 2011, 68, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Yoshida, T.; Perez de Souza, L.; Alseekh, S.; Bajdzienko, K.; Fernie, A.R.; Giavalisco, P. An improved extraction method enables the comprehensiveanalysis of lipids, proteins, metabolites and phytohormonesfrom a single sample of leaf tissue under water-deficit stress. Plant J. 2020, 103, 1614–1632. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods Perspect. 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef]
- Hummel, C.S.; Lu, C.; Loo, D.D.F.; Hirayama, B.A.; Voss, A.A.; Wright, E.M. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am. J. Physiol. Cell Physiol. 2011, 300, 14–21. [Google Scholar] [CrossRef]
- Miller, O. Microwave Digestion of Plant Tissue in Closed Vessels. In Handbook of Reference Methods for Plant Analysis, 1st ed.; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 69–73. ISBN 978-1-57444-124-6. [Google Scholar]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 11 September 2022).
- Wickham, H. Getting Started with ggplot2. In ggplot2. Use R! Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Kassambra, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 11 September 2022).

| Ca | K | Mg | Na | B | Al | |
|---|---|---|---|---|---|---|
| Marrubium friwaldskyanum flowers | 187.7 (1.8) | 9974.6 (1.9) | 1726.3 (1.1) | 12.4 (3.4) | 3.9 (1.2) | 12.6 (0.3) |
| Marrubium friwaldskyanum leaves | 304.1 (1.2) | 8212.9 (0.8) | 2576.7 (0.8) | 8.9 (1.1) | 3.1 (0.6) | 27.4 (0.1) |
| Marrubium friwaldskyanum stems | 98.1 (1.0) | 8945.9 (0.6) | 824.3 (0.9) | 6.9 (2.3) | 4.1 (2.5) | 4.7 (0.8) |
| Marrubium peregrinum | 247.6 (1.5) | 8787.8 (1.2) | 1548.6 (2.1) | 14.1 (2.4) | 7.5 (3.7) | 29.0 (1.0) |
| Mn | Fe | Cu | Zn | Sr | Ba | |
| Marrubium friwaldskyanum flowers | 16.3 (0.8) | 32.1 (0.7) | 4.4 (2.2) | 581.3 (2.4) | 9.0 (1.8) | 11.1 (0.7) |
| Marrubium friwaldskyanum leaves | 26.3 (2.4) | 73.9 (1.8) | 2.7 (0.8) | 559.8 (1.0) | 9.4 (1.8) | 10.4 (0.5) |
| Marrubium friwaldskyanum stems | 9.1 (2.7) | 9.4 (0.3) | 2.3 (1.5) | 472.9 (1.5) | 7.8 (3.8) | 14.3 (2.5) |
| Marrubium peregrinum | 9.7 (1.5) | 57.3 (1.8) | 2.0 (1.7) | 475.7 (3.7) | 3.6 (3.4) | 2.2 (1.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyuzeleva, D.; Benina, M.; Ivanova, V.; Vatov, E.; Alseekh, S.; Mladenova, T.; Mladenov, R.; Todorov, K.; Bivolarska, A.; Stoyanov, P. Metabolome Profiling of Marrubium peregrinum L. and Marrubium friwaldskyanum Boiss Reveals Their Potential as Sources of Plant-Based Pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 17035. https://doi.org/10.3390/ijms242317035
Gyuzeleva D, Benina M, Ivanova V, Vatov E, Alseekh S, Mladenova T, Mladenov R, Todorov K, Bivolarska A, Stoyanov P. Metabolome Profiling of Marrubium peregrinum L. and Marrubium friwaldskyanum Boiss Reveals Their Potential as Sources of Plant-Based Pharmaceuticals. International Journal of Molecular Sciences. 2023; 24(23):17035. https://doi.org/10.3390/ijms242317035
Chicago/Turabian StyleGyuzeleva, Donika, Maria Benina, Valentina Ivanova, Emil Vatov, Saleh Alseekh, Tsvetelina Mladenova, Rumen Mladenov, Krasimir Todorov, Anelia Bivolarska, and Plamen Stoyanov. 2023. "Metabolome Profiling of Marrubium peregrinum L. and Marrubium friwaldskyanum Boiss Reveals Their Potential as Sources of Plant-Based Pharmaceuticals" International Journal of Molecular Sciences 24, no. 23: 17035. https://doi.org/10.3390/ijms242317035
APA StyleGyuzeleva, D., Benina, M., Ivanova, V., Vatov, E., Alseekh, S., Mladenova, T., Mladenov, R., Todorov, K., Bivolarska, A., & Stoyanov, P. (2023). Metabolome Profiling of Marrubium peregrinum L. and Marrubium friwaldskyanum Boiss Reveals Their Potential as Sources of Plant-Based Pharmaceuticals. International Journal of Molecular Sciences, 24(23), 17035. https://doi.org/10.3390/ijms242317035







