Toxicity of Large and Small Surface-Engineered Upconverting Nanoparticles for In Vitro and In Vivo Bioapplications
Abstract
1. Introduction
2. Results and Discussion
2.1. Internalization of UCNPs in Cell Cytoplasm
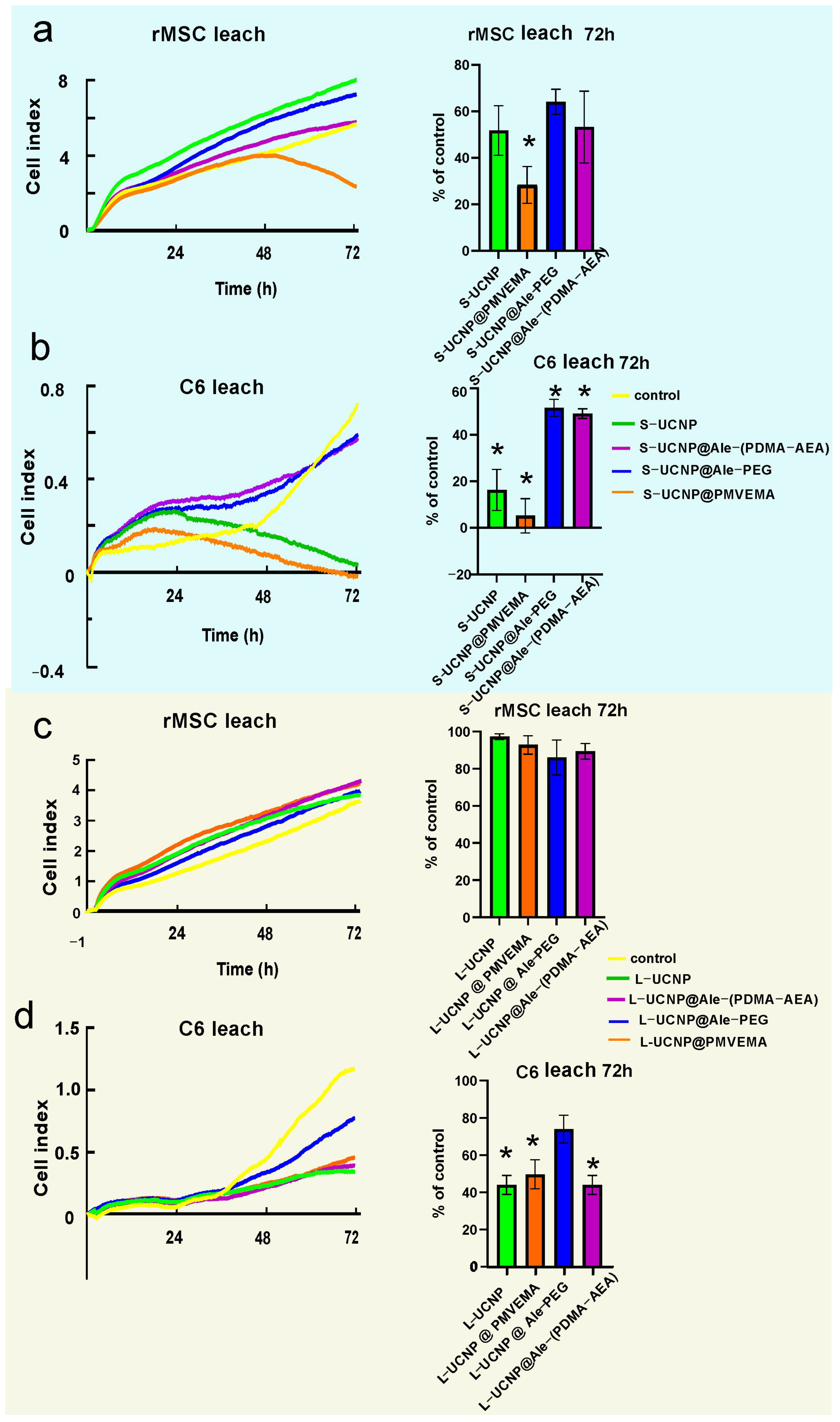
2.2. Cytotoxicity by xCELLigence Assay
2.3. Genotoxicity by Comet Assay
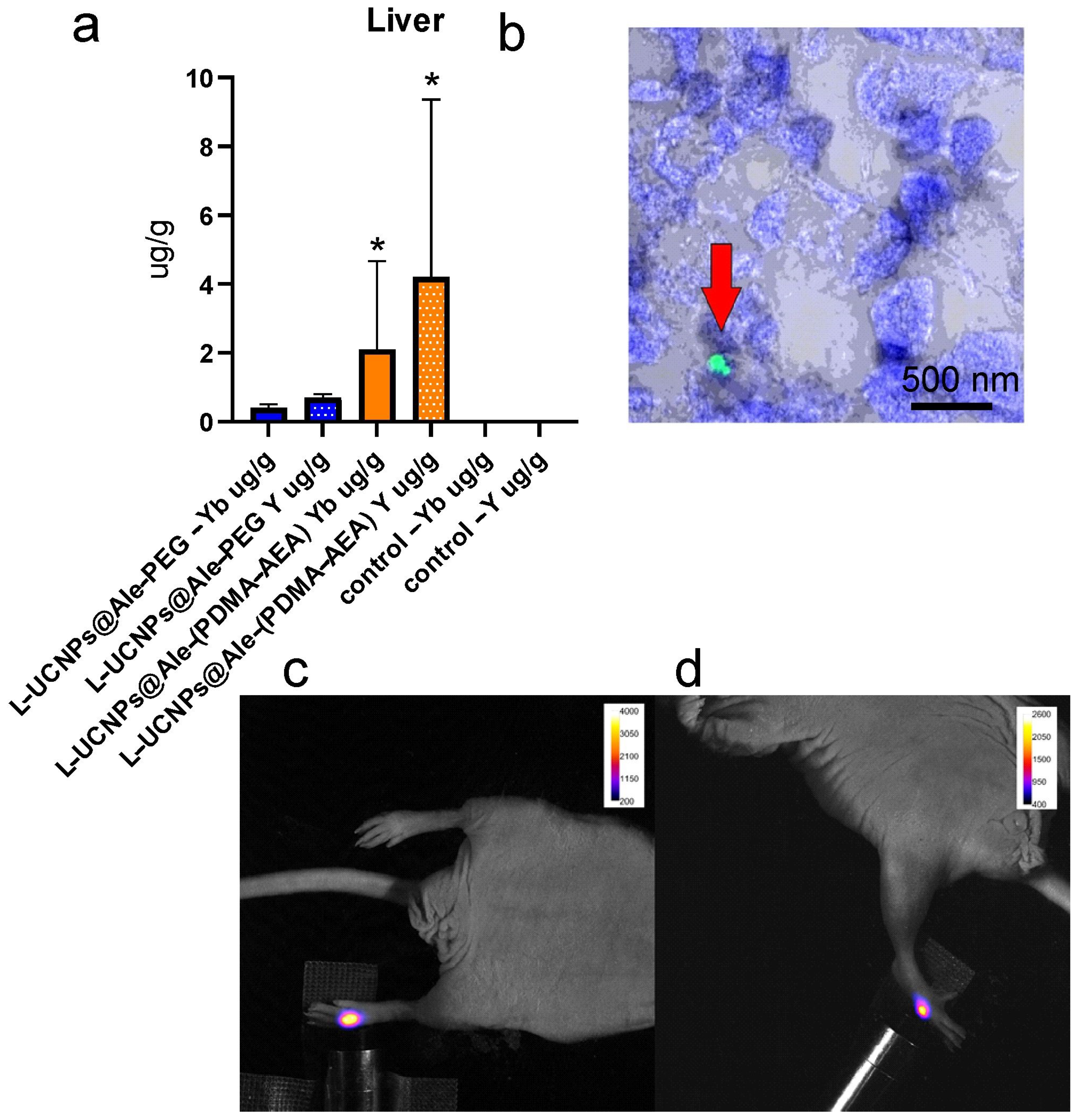
2.4. In Vivo Study
3. Materials and Methods
3.1. Preparation of Leachates
3.2. C6 Cell Line
3.3. Mesenchymal Stem Cells
3.4. UCNPS Treatment
3.5. Electron Microscopy
3.6. Real Time Proliferation Assay
3.7. Determination of Oxidative Damage of DNA by Comet Assay
3.8. Pilot In Vivo Imaging
3.9. Laser Scanning Confocal Microscopy
3.10. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
3.11. Statistical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, C.W.; Park, D.J. Development of Er3+, Yb3+ Co-Doped Y2O3 NPs According to Yb3+ Concentration by LP-PLA Method: Potential Further Biosensor. Biosensors 2021, 11, 150. [Google Scholar] [CrossRef]
- Loo, J.F.C.; Chien, Y.H.; Yin, F.; Kong, S.K.; Ho, H.P.; Yong, K.T. Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coordin. Chem. Rev. 2019, 400, 213042. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef]
- Qin, X.; Xu, J.H.; Wu, Y.M.; Liu, X.G. Energy-Transfer Editing in Lanthanide-Activated Upconversion Nanocrystals: A Toolbox for Emerging Applications. ACS Cent. Sci. 2019, 5, 29–42. [Google Scholar] [CrossRef]
- All, A.H.; Zeng, X.; Teh, D.B.L.; Yi, Z.G.; Prasad, A.; Ishizuka, T.; Thakor, N.; Hiromu, Y.; Liu, X.G. Expanding the Toolbox of Upconversion Nanoparticles for In Vivo Optogenetics and Neuromodulation. Adv. Mater. 2019, 31, 1803474. [Google Scholar] [CrossRef]
- Chen, G.Y.; Qju, H.L.; Prasad, P.N.; Chen, X.Y. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Shikha, S.; Liu, J.L.; Zhang, J.; Mei, Q.S.; Zhang, Y. Upconversion Nanoprobes: Recent Advances in Sensing Applications. Anal. Chem. 2019, 91, 548–568. [Google Scholar] [CrossRef]
- Duan, C.C.; Liang, L.E.; Li, L.; Zhang, R.; Xu, Z.P. Recent progress in upconversion luminescence nanomaterials for biomedical applications. J. Mater. Chem. B 2018, 6, 192–209. [Google Scholar] [CrossRef]
- Maynard, A.D.; Warheit, D.B.; Philbert, M.A. The New Toxicology of Sophisticated Materials: Nanotoxicology and Beyond. Toxicol. Sci. 2011, 120, S109–S129. [Google Scholar] [CrossRef]
- Nampi, P.P.; Vakurov, A.; Saha, S.; Jose, G.; Millner, P.A. Surface modified hexagonal upconversion nanoparticles for the development of competitive assay for biodetection. Biomater. Adv. 2022, 136, 212763. [Google Scholar] [CrossRef]
- Lahtinen, S.; Lyytikäinen, A.; Päkkilä, H.; Hömppi, E.; Perälä, N.; Lastusaari, M.; Soukka, T. Disintegration of Hexagonal NaYF4:Yb3+,Er3+ Upconverting Nanoparticles in Aqueous Media: The Role of Fluoride in Solubility Equilibrium. J. Phys. Chem. C 2017, 121, 656–665. [Google Scholar] [CrossRef]
- Lisjak, D.; Plohl, O.; Ponikvar-Svet, M.; Majaron, B. Dissolution of upconverting fluoride nanoparticles in aqueous suspensions. RSC Adv. 2015, 5, 27393–27397. [Google Scholar] [CrossRef]
- Lisjak, D.; Plohl, O.; Vidmar, J.; Majaron, B.; Ponikvar-Svet, M. Dissolution Mechanism of Upconverting AYF:Yb,Tm (A = Na or K) Nanoparticles in Aqueous Media. Langmuir 2016, 32, 8222–8229. [Google Scholar] [CrossRef] [PubMed]
- Plohl, O.; Kralj, S.; Majaron, B.; Fröhlich, E.; Ponikvar-Svet, M.; Makovec, D.; Lisjak, D. Amphiphilic coatings for the protection of upconverting nanoparticles against dissolution in aqueous media. Dalton Trans. 2017, 46, 6975–6984. [Google Scholar] [CrossRef]
- Dukhno, O.; Przybilla, F.; Muhr, V.; Buchner, M.; Hirsch, T.; Mély, Y. Time-dependent luminescence loss for individual upconversion nanoparticles upon dilution in aqueous solution. Nanoscale 2018, 10, 15904–15910. [Google Scholar] [CrossRef]
- Ding, Y.C.; Tian, Y.T.; Zeng, Z.Y.; Shuai, P.; Lan, H.Y.; Zhu, X.S.; Zhong, Y.; Wu, L.H.; Fan, X.N. YCl3 Promotes Neuronal Cell Death by Inducing Apoptotic Pathways in Rats. BioMed Res. Int. 2017, 2017, 2183658. [Google Scholar] [CrossRef]
- Qian, H.S.; Zhang, Y. Synthesis of Hexagonal-Phase Core-Shell NaYF4 Nanocrystals with Tunable Upconversion Fluorescence. Langmuir 2008, 24, 12123–12125. [Google Scholar] [CrossRef]
- Boyer, J.C.; Cuccia, L.A.; Capobianco, J.A. Synthesis of colloidal upconverting NaYF4: Er3+/Yb3+ and Tm3+/Yb3+ monodisperse nanocrystals. Nano Lett. 2007, 7, 847–852. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.Q.; Shi, Y.F.; Xie, S.H.; Li, Y.G.; Xu, L.; Tu, B.; Zhao, D.Y. Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence. Angew. Chem. Int. Ed. 2007, 46, 7976–7979. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Tilley, R.D.; Nann, T. Size and shape evolution of upconverting nanoparticles using microwave assisted synthesis. Crystengcomm 2010, 12, 1993–1996. [Google Scholar] [CrossRef]
- Shan, S.N.; Wang, X.Y.; Jia, N.Q. Synthesis of NaYF4:Yb3+, Er3+ upconversion nanoparticles in normal microemulsions. Nanoscale Res. Lett. 2011, 6, 539. [Google Scholar] [CrossRef]
- Chen, G.Y.; Ohulchanskyy, T.Y.; Liu, S.; Law, W.C.; Wu, F.; Swihart, M.T.; Ågren, H.; Prasad, P.N. Core/shell NaGdF4:Nd3+/NaGdF4 nanocrystals with efficient near-infrared to near-infrared downconversion photoluminescence for bioimaging applications. ACS Nano 2012, 6, 2969–2977. [Google Scholar] [CrossRef]
- Bastos, V.; Oskoei, P.; Andresen, E.; Saleh, M.I.; Rühle, B.; Resch-Genger, U.; Oliveira, H. Stability, dissolution, and cytotoxicity of NaYF4-upconversion nanoparticles with different coatings. Sci. Rep. 2022, 12, 3770. [Google Scholar] [CrossRef]
- Wang, X.T.; Yang, Y.B.; Liu, C.; Guo, H.L.; Chen, Z.F.; Xia, J.Y.; Liao, Y.G.; Tang, C.Y.; Law, W.C. Photo- and pH-responsive drug delivery nanocomposite based on -nitrobenzyl functionalized upconversion nanoparticles. Polymer 2021, 229, 123961. [Google Scholar] [CrossRef]
- Sun, L.N.; Wei, R.Y.; Feng, J.; Zhang, H.J. Tailored lanthanide-doped upconversion nanoparticles and their promising bioapplication prospects. Coordin. Chem. Rev. 2018, 364, 10–32. [Google Scholar] [CrossRef]
- Andresen, E.; Resch-Genger, U.; Schäferling, M. Surface Modifications for Photon-Upconversion-Based Energy-Transfer Nanoprobes. Langmuir 2019, 35, 5093–5113. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Guo, Z.; Zhang, X.; Gong, L.J.; Dong, X.H.; Fu, Y.Y.; Wang, Q.; Gu, Z.J. Mass production of poly(ethylene glycol) monooleate-modified core-shell structured upconversion nanoparticles for bio-imaging and photodynamic therapy. Sci. Rep. 2019, 9, 5212. [Google Scholar] [CrossRef]
- Cui, S.S.; Chen, H.Y.; Zhu, H.Y.; Tian, J.M.; Chi, X.M.; Qian, Z.Y.; Achilefu, S.; Gu, Y.Q. Amphiphilic chitosan modified upconversion nanoparticles for photodynamic therapy induced by near-infrared light. J. Mater. Chem. 2012, 22, 4861–4873. [Google Scholar] [CrossRef]
- Xue, Z.L.; Zeng, S.J.; Hao, J.H. Non-invasive through-skull brain vascular imaging and small tumor diagnosis based on NIR-II emissive lanthanide nanoprobes beyond 1500 nm. Biomaterials 2018, 171, 153–163. [Google Scholar] [CrossRef]
- Näreoja, T.; Deguchi, T.; Christ, S.; Peltomaa, R.; Prabhakar, N.; Fazeli, E.; Perälä, N.; Rosenholm, J.M.; Arppe, R.; Soukka, T.; et al. Ratiometric Sensing and Imaging of Intracellular pH Using Polyethylenimine-Coated Photon Upconversion Nanoprobes. Anal. Chem. 2017, 89, 1501–1508. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Sangeetha, N.M.; Boyer, J.C.; van Veggel, F.C.J.M. Facile ligand-exchange with polyvinylpyrrolidone and subsequent silica coating of hydrophobic upconverting β-NaYF4:Yb3+/Er3+ nanoparticles. Nanoscale 2010, 2, 771–777. [Google Scholar] [CrossRef]
- Zhao, J.W.; Yang, H.; Li, J.L.; Wang, Y.J.; Wang, X. Fabrication of pH-responsive PLGA(UCNPs/DOX) nanocapsules with upconversion luminescence for drug delivery. Sci. Rep. 2017, 7, 18014. [Google Scholar] [CrossRef]
- Wilhelm, S.; Kaiser, M.; Würth, C.; Heiland, J.; Carrillo-Carrion, C.; Muhr, V.; Wolfbeis, O.S.; Parak, W.J.; Resch-Genger, U.; Hirsch, T. Water dispersible upconverting nanoparticles: Effects of surface modification on their luminescence and colloidal stability. Nanoscale 2015, 7, 1403–1410. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Chen, Y.H.; Tawfik, S.A.; Wen, S.H.; Parviz, M.; Shimoni, O.; Jin, D.Y. Systematic investigation of functional ligands for colloidal stable upconversion nanoparticles. RSC Adv. 2018, 8, 4842–4849. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Iversen, T.G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Nahorniak, M.; Oleksa, V.; Vasylyshyn, T.; Pop-Georgievski, O.; Rydvalová, E.; Filipová, M.; Horák, D. Cytotoxicity Evaluation of Photosensitizer-Conjugated Hexagonal Upconverting Nanoparticles. Nanomaterials 2023, 13, 1535. [Google Scholar] [CrossRef]
- Nahorniak, M.; Patsula, V.; Mareková, D.; Matous, P.; Shapoval, O.; Oleksa, V.; Vosmanská, M.; Urdziková, L.M.; Jendelová, P.; Herynek, V.; et al. Chemical and Colloidal Stability of Polymer-Coated NaYF4:Yb,Er Nanoparticles in Aqueous Media and Viability of Cells: The Effect of a Protective Coating. Int. J. Mol. Sci. 2023, 24, 2724. [Google Scholar] [CrossRef]
- Patsula, V.; Mareková, D.; Jendelová, P.; Nahorniak, M.; Shapoval, O.; Matous, P.; Oleksa, V.; Konefal, R.; Vosmanská, M.; Machová-Urdziková, L.; et al. Polymer-coated hexagonal upconverting nanoparticles: Chemical stability and cytotoxicity. Front. Chem. 2023, 11, 1207984. [Google Scholar] [CrossRef]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Oleksa, V.; Macková, H.; Engstová, H.; Patsula, V.; Shapoval, O.; Velychkivska, N.; Jezek, P.; Horák, D. Poly(N,N-dimethylacrylamide)-coated upconverting NaYF4:Yb,Er@NaYF4:Nd core-shell nanoparticles for fluorescent labeling of carcinoma cells. Sci. Rep. 2021, 11, 21373. [Google Scholar] [CrossRef]
- Andresen, E.; Würth, C.; Prinz, C.; Michaelis, M.; Resch-Genger, U. Time-resolved luminescence spectroscopy for monitoring the stability and dissolution behaviour of upconverting nanocrystals with different surface coatings. Nanoscale 2020, 12, 12589–12601. [Google Scholar] [CrossRef]
- Saleh, M.I.; Rühle, B.; Wang, S.; Radnik, J.; You, Y.; Resch-Genger, U. Assessing the protective effects of different surface coatings on NaYF4:Yb3+, Er3+ upconverting nanoparticles in buffer and DMEM. Sci. Rep. 2020, 10, 19318. [Google Scholar] [CrossRef]
- Jin, J.F.; Gu, Y.J.; Man, C.W.Y.; Cheng, J.P.; Xu, Z.H.; Zhang, Y.; Wang, H.S.; Lee, V.H.Y.; Cheng, S.H.; Wong, W.T. Polymer-Coated NaYF4:Yb3+, Er3+ Upconversion Nanoparticles for Charge-Dependent Cellular Imaging. ACS Nano 2011, 5, 7838–7847. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, L.; Zhong, M.L.; Xiao, L.H.; Li, H.W.; Wang, J.F. The morphology and surface charge-dependent cellular uptake efficiency of upconversion nanostructures revealed by single-particle optical microscopy. Chem. Sci. 2018, 9, 5260, Correction in Chem. Sci. 2022, 13, 3610. [Google Scholar] [CrossRef]
- Zhang, Z.; Rahmat, J.N.; Mahendran, R.; Zhang, Y. Controllable Assembly of Upconversion Nanoparticles Enhanced Tumor Cell Penetration and Killing Efficiency. Adv. Sci. 2020, 7, 2001831. [Google Scholar] [CrossRef]
- Jalil, R.A.; Zhang, Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials 2008, 29, 4122–4128. [Google Scholar] [CrossRef]
- Chatteriee, D.K.; Rufalhah, A.J.; Zhang, Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials 2008, 29, 937–943. [Google Scholar] [CrossRef]
- Das, G.K.; Stark, D.T.; Kennedy, I.M. Potential Toxicity of Up-Converting Nanoparticles Encapsulated with a Bilayer Formed by Ligand Attraction. Langmuir 2014, 30, 8167–8176. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity Assessment of Silica Coated Iron Oxide Nanoparticles and Biocompatibility Improvement by Surface Engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Kueznik, T.; Bösch, M.; Roblegg, E.; Fröhlich, E. Intracellular calcium levels as screening tool for nanoparticle toxicity. J. Appl. Toxicol. 2015, 35, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, L.; Cuddapah, S.; Costa, M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Curr. Pharmacol. Rep. 2016, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.; Zhang, Y.N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Wang, H.; Ma, Z.G.; Wu, B.J. Effects of pharmaceutical PEGylation on drug metabolism and its clinical concerns. Expert. Opin. Drug Met. 2014, 10, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Z.; Ge, X.Q.; Ke, D.M.; Tang, H.; Zhang, J.Z.; Calvaresi, M.; Gao, B.; Sun, L.N.; Su, Q.Q.; Wang, H.F. The Bioavailability, Biodistribution, and Toxic Effects of Silica-Coated Upconversion Nanoparticles. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Jendelova, P.; Herynek, V.; DeCroos, J.; Glogarova, K.; Andersson, B.; Hajek, M.; Sykova, E. Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn. Reson. Med. 2003, 50, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Novotna, B.; Pelclova, D.; Rossnerova, A.; Zdimal, V.; Ondracek, J.; Lischkova, L.; Vlckova, S.; Fenclova, Z.; Klusackova, P.; Zavodna, T.; et al. The genotoxic effects in the leukocytes of workers handling nanocomposite materials. Mutagenesis 2020, 35, 331–340. [Google Scholar] [CrossRef]
- Novotna, B.; Topinka, J.; Solansky, I.; Chvatalova, I.; Lnenickova, Z.; Sram, R.J. Impact of air pollution and genotype variability on DNA damage in Prague policemen. Toxicol. Lett. 2007, 172, 37–47. [Google Scholar] [CrossRef]

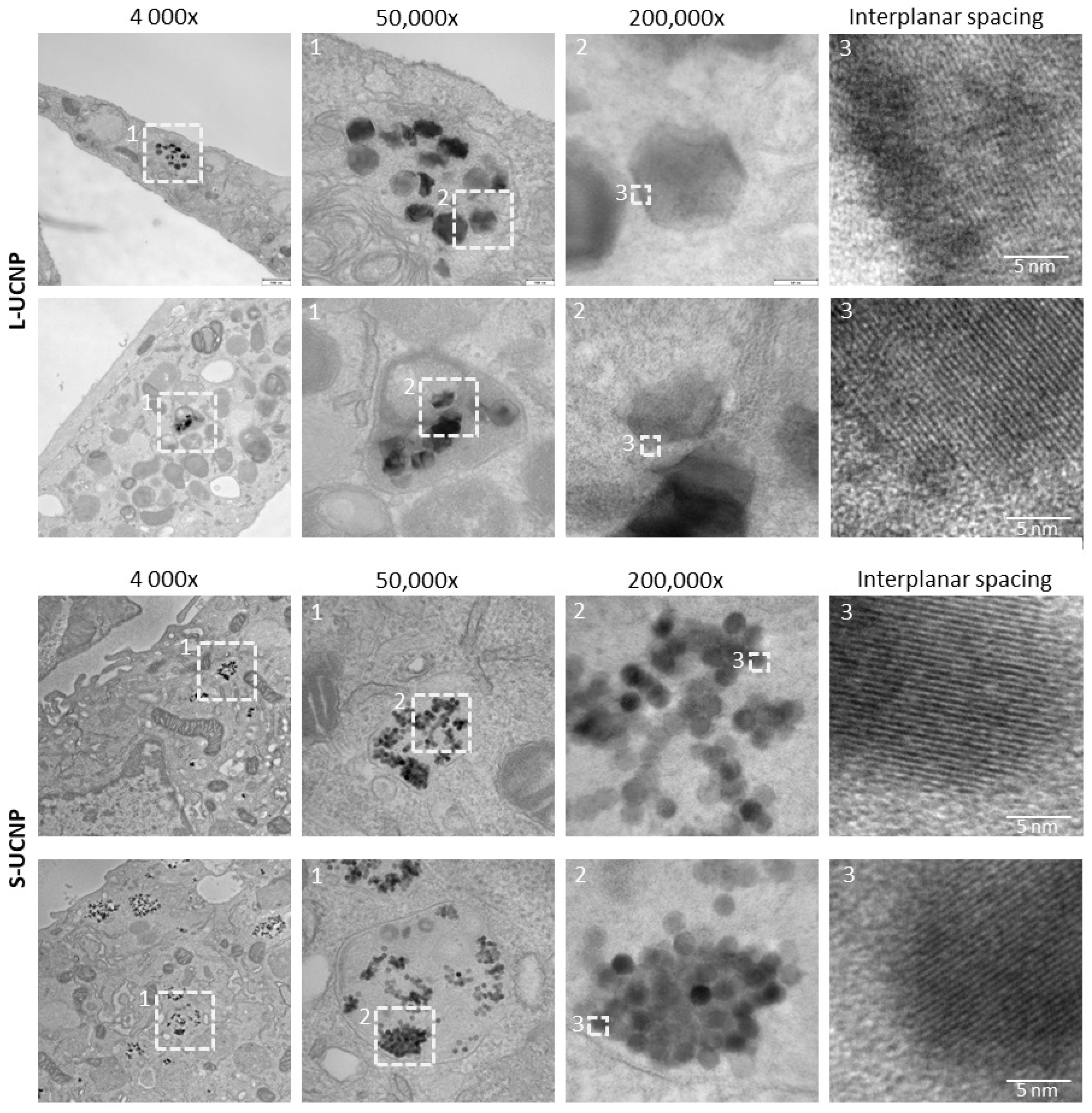


| S-UCNPs | L-UCNPs | |||||||
|---|---|---|---|---|---|---|---|---|
| Coating | Dn (nm) | Đ | Dh (nm) | ζ-Potential (mV) | Dn (nm) | Đ | Dh (nm) | ζ-Potential (mV) |
| - | 25 | 1.01 | 101 | 30 ± 5 | 121 | 1.01 | 174 | 28 ± 3 |
| Ale-PEG | 25 | 1.01 | 68 | 8 ± 1 | 119 | 1.02 | 158 | 4 ± 1 |
| Ale-(PDMA-AEA) | 25 | 1.01 | 102 | 27 ± 3 | 122 | 1.01 | 160 | 22 ± 2 |
| PMVEMA | 25 | 1.01 | 112 | −32 ± 2 | 119 | 1.01 | 234 | −46 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machová Urdzíková, L.; Mareková, D.; Vasylyshyn, T.; Matouš, P.; Patsula, V.; Oleksa, V.; Shapoval, O.; Vosmanská, M.; Liebl, D.; Benda, A.; et al. Toxicity of Large and Small Surface-Engineered Upconverting Nanoparticles for In Vitro and In Vivo Bioapplications. Int. J. Mol. Sci. 2024, 25, 5294. https://doi.org/10.3390/ijms25105294
Machová Urdzíková L, Mareková D, Vasylyshyn T, Matouš P, Patsula V, Oleksa V, Shapoval O, Vosmanská M, Liebl D, Benda A, et al. Toxicity of Large and Small Surface-Engineered Upconverting Nanoparticles for In Vitro and In Vivo Bioapplications. International Journal of Molecular Sciences. 2024; 25(10):5294. https://doi.org/10.3390/ijms25105294
Chicago/Turabian StyleMachová Urdzíková, Lucia, Dana Mareková, Taras Vasylyshyn, Petr Matouš, Vitalii Patsula, Viktoriia Oleksa, Oleksandr Shapoval, Magda Vosmanská, David Liebl, Aleš Benda, and et al. 2024. "Toxicity of Large and Small Surface-Engineered Upconverting Nanoparticles for In Vitro and In Vivo Bioapplications" International Journal of Molecular Sciences 25, no. 10: 5294. https://doi.org/10.3390/ijms25105294
APA StyleMachová Urdzíková, L., Mareková, D., Vasylyshyn, T., Matouš, P., Patsula, V., Oleksa, V., Shapoval, O., Vosmanská, M., Liebl, D., Benda, A., Herynek, V., Horák, D., & Jendelová, P. (2024). Toxicity of Large and Small Surface-Engineered Upconverting Nanoparticles for In Vitro and In Vivo Bioapplications. International Journal of Molecular Sciences, 25(10), 5294. https://doi.org/10.3390/ijms25105294








