Voltage Sensors Embedded in G Protein-Coupled Receptors
Abstract
1. Introduction
2. Voltage Dependence of Muscarinic Receptors
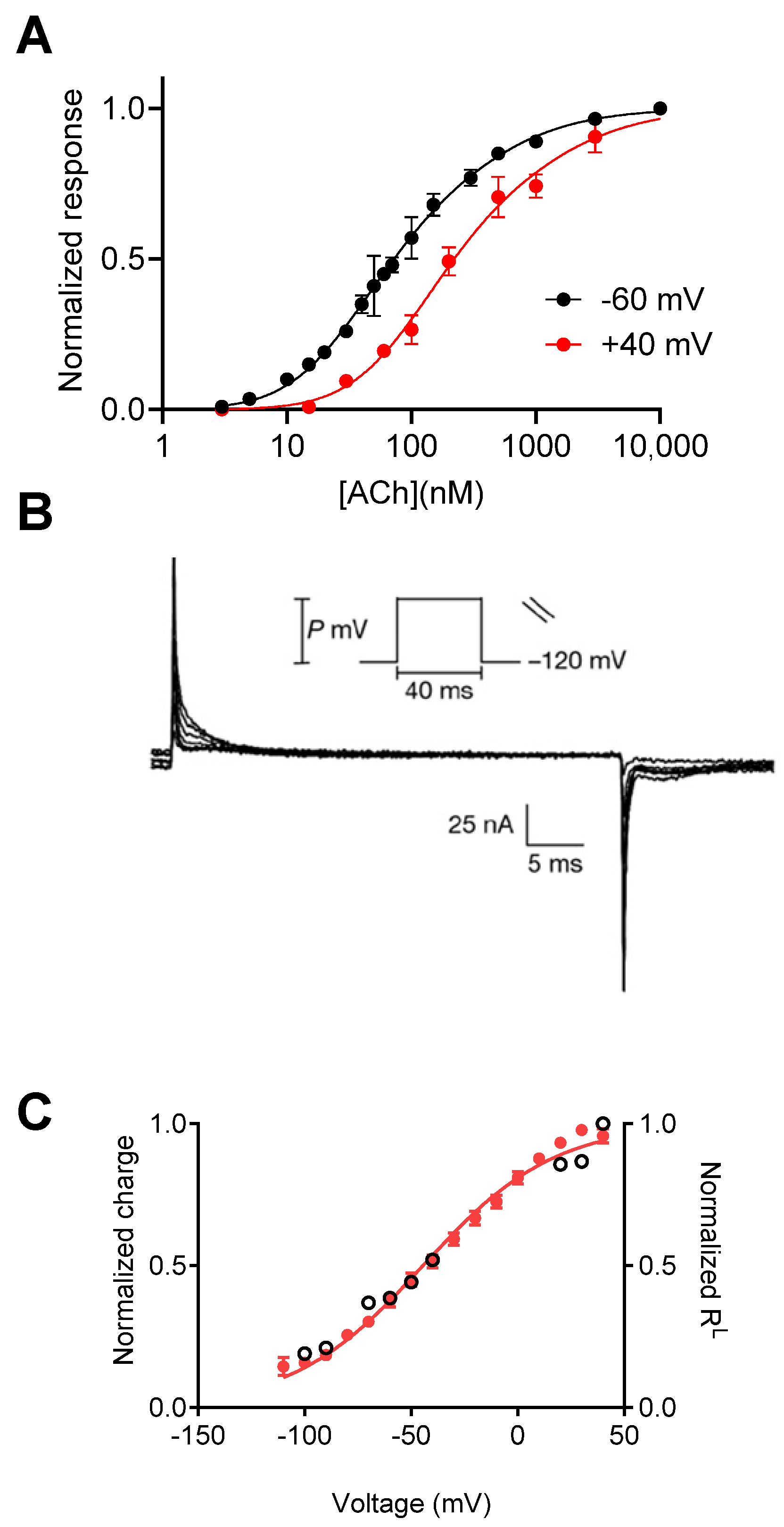
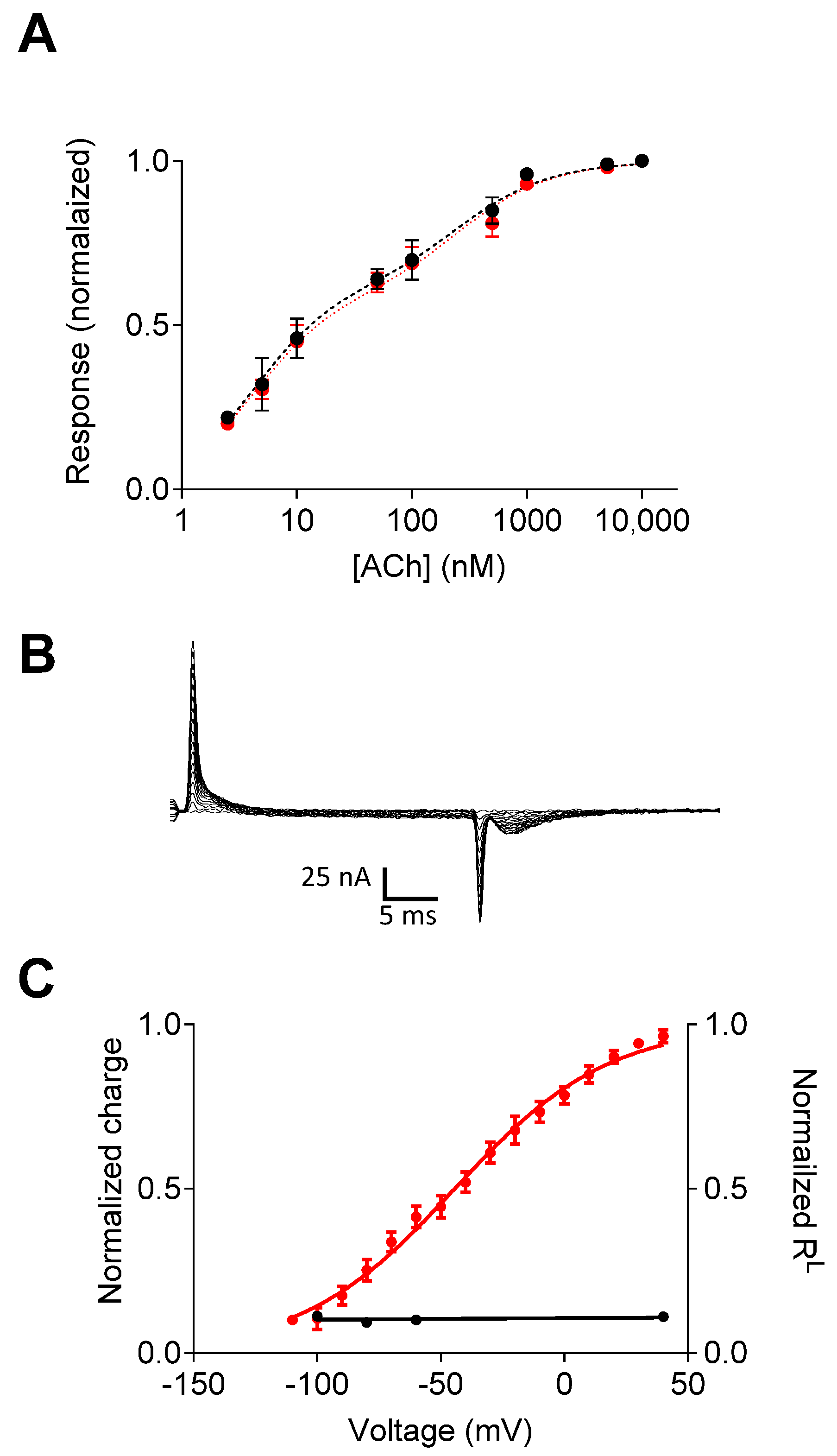
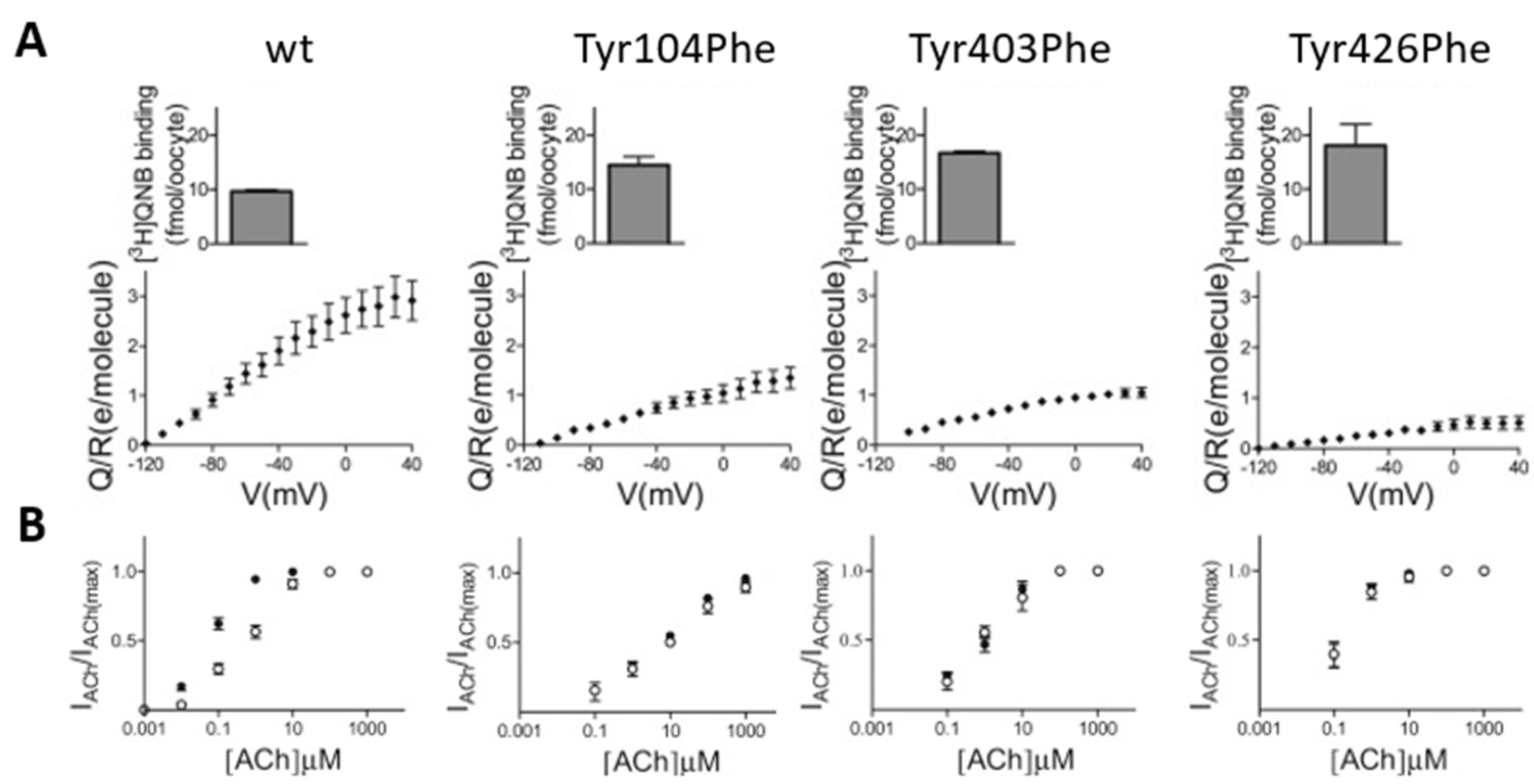
3. The Molecular Mechanism That Underlies Muscarinic Receptors’ Voltage Dependence
4. Alternative Mechanism for Muscarinic Receptors’ Voltage Dependence
5. Physiological Implications of Voltage Dependence of Muscarinic Receptors and Other GPCRs
Funding
Conflicts of Interest
References
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, V.V.; Gurevich, E.V. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front. Pharmacol. 2019, 10, 00125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qiao, Y.; Li, Z. New Insights into Modes of GPCR Activation. Trends Pharmacol. Sci. 2018, 39, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Kadir, L.A.; Stacey, M.; Barrett-Jolley, R. Emerging Roles of the Membrane Potential: Action Beyond the Action Potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Armon, M.; Sokolovsky, M. Depolarization-induced changes in the muscarinic receptor in rat brain and heart are mediated by pertussis-toxin-sensitive G-proteins. J. Biol. Chem. 1991, 266, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Armon, M.; Garty, H.; Sokolovsky, M. G-protein mediates voltage regulation of agonist binding to muscarinic receptors: Effects on receptor-sodium channel interaction. Biochemistry 1988, 27, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Linial, M.; Ilouz, N.; Parnas, H. Voltage-Dependent Interaction Between the Muscarinic ACh Receptor and Proteins of the Exocytic Machinery. J. Physiol. 1997, 504, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Ilouz, N.; Branski, L.; Parnis, J.; Parnas, H.; Linial, M. Depolarization Affects the Binding Properties of Muscarinic Acetylcholine Receptors and Their Interaction with Proteins of the Exocytic Apparatus. J. Biol. Chem. 1999, 274, 29519–29528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marty, A.; Tan, Y.P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J. Physiol. 1989, 419, 665–687. [Google Scholar] [CrossRef]
- Ong, B.H.; Ohsaga, A.; Sato, K.; Oshiro, T.; Shirato, K.; Maruyama, Y. G protein modulation of voltage-sensitive muscarinic receptor signalling in mouse pancreatic acinar cells. Pflügers Archiv 2001, 441, 604–610. [Google Scholar] [CrossRef]
- Kupchik, Y.M.; Barchad-Avitzur, O.; Wess, J.; Ben-Chaim, Y.; Parnas, I.; Parnas, H. A novel fast mechanism for GPCR-mediated signal transduction—Control of neurotransmitter release. J. Cell Biol. 2011, 192, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chaim, Y.; Tour, O.; Dascal, N.; Parnas, I.; Parnas, H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J. Biol. Chem. 2003, 278, 22482–22491. [Google Scholar] [CrossRef] [PubMed]
- Chaim, Y.B.; Bochnik, S.; Parnas, I.; Parnas, H. Voltage Affects the Dissociation Rate Constant of the m2 Muscarinic Receptor. PLoS ONE 2013, 8, e74354. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Bezanilla, F. Currents Related to Movement of the Gating Particles of the Sodium Channels. Nature 1973, 242, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. Gating currents. J. Gen. Physiol. 2018, 150, 911–932. [Google Scholar] [CrossRef] [PubMed]
- Ben-Chaim, Y.; Chanda, B.; Dascal, N.; Bezanilla, F.; Parnas, I.; Parnas, H. Movement of “gating charge” is coupled to ligand binding in a G-protein-coupled receptor. Nature 2006, 444, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F.; Villalba-Galea, C.A. The gating charge should not be estimated by fitting a two-state model to a Q-V curve. J. Gen. Physiol. 2013, 142, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron 2010, 67, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Dekel, N.; Priest, M.F.; Parnas, H.; Parnas, I.; Bezanilla, F. Depolarization induces a conformational change in the binding site region of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 285–290. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [Google Scholar] [CrossRef]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. The Voltage Sensor in Voltage-Dependent Ion Channels. Physiol. Rev. 2000, 80, 555–592. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F.; Perozo, E. The voltage sensor and the gate in ion channels. In Advances in Protein Chemistry; Academic Press: New York, NY, USA, 2003; pp. 211–241. [Google Scholar] [CrossRef]
- Navarro-Polanco, R.A.; Galindo, E.G.M.; Ferrer-Villada, T.; Arias, M.; Rigby, J.R.; Sánchez-Chapula, J.A.; Tristani-Firouzi, M. Conformational changes in the M2 muscarinic receptor induced by membrane voltage and agonist binding. J. Physiol. 2011, 589, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, X.; Cao, X.; Rao, K.; Hong, L. Trp207 regulation of voltage-dependent activation of human Hv1 proton channel. J. Biol. Chem. 2024, 300, 105674. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Lou, X.J.; Horrigan, F.T. Role of charged residues in the S1-S4 voltage sensor of BK channels. J. Gen. Physiol. 2006, 127, 309–328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ågren, R.; Sahlholm, K.; Nilsson, J.; Århem, P. Point mutation of a conserved aspartate, D69, in the muscarinic M2 receptor does not modify voltage-sensitive agonist potency. Biochem. Biophys. Res. Commun. 2018, 496, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Barchad-Avitzur, O.; Priest, M.F.; Dekel, N.; Bezanilla, F.; Parnas, H.; Ben-Chaim, Y. A Novel Voltage Sensor in the Orthosteric Binding Site of the M2 Muscarinic Receptor. Biophys. J. 2018, 111, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Momany, F.A.; McGuire, R.F.; Burgess, A.W.; Scheraga, H.A. Energy parameters in polypeptides. VII. Geometric parameters, partial atomic charges, nonbonded interactions, hydrogen bond interactions, and intrinsic torsional potentials for the naturally occurring amino acids. J. Phys. Chem. 1975, 79, 2361–2381. [Google Scholar] [CrossRef]
- Kruse, A.C.; Ring, A.M.; Manglik, A.; Hu, J.; Hu, K.; Eitel, K.; Hübner, H.; Pardon, E.; Valant, C.; Sexton, P.M.; et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 2013, 504, 101–106. [Google Scholar] [CrossRef]
- Gregory, K.J.; Hall, N.E.; Tobin, A.B.; Sexton, P.M.; Christopoulos, A. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 2010, 285, 7459–7474. [Google Scholar] [CrossRef]
- DeVree, B.T.; Mahoney, J.P.; Vélez-Ruiz, G.A.; Rasmussen, S.G.F.; Kuszak, A.J.; Edwald, E.; Fung, J.-J.; Manglik, A.; Masureel, M.; Du, Y.; et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature 2016, 535, 182. [Google Scholar] [CrossRef]
- Rinne, A.; Mobarec, J.C.; Mahaut-Smith, M.; Kolb, P.; Bünemann, M. The mode of agonist binding to a G protein–coupled receptor switches the effect that voltage changes have on signaling. Sci. Signal 2015, 8, ra110. [Google Scholar] [CrossRef]
- Kirchhofer, S.B.; Lim, V.J.Y.; Ernst, S.; Karsai, N.; Ruland, J.G.; Canals, M.; Kolb, P.; Bünemann, M. Differential interaction patterns of opioid analgesics with µ opioid receptors correlate with ligand-specific voltage sensitivity. Elife 2023, 12, e91291. [Google Scholar] [CrossRef]
- Hoppe, A.; Marti-Solano, M.; Drabek, M.; Bünemann, M.; Kolb, P.; Rinne, A. The allosteric site regulates the voltage sensitivity of muscarinic receptors. Cell Signal 2018, 42, 114–126. [Google Scholar] [CrossRef]
- Cohen-Armon, M. Are Voltage Sensors Really Embedded in Muscarinic Receptors? Int. J. Mol. Sci. 2023, 24, 7538. [Google Scholar] [CrossRef]
- Cohen-Armon, M.; Sokolovsky, M. Interactions between the muscarinic receptors, sodium channels, and guanine nucleotide-binding protein(s) in rat atria. J. Biol. Chem. 1986, 261, 12498–12505. [Google Scholar] [CrossRef]
- Cohen-Armon, M.; Kloog, Y.; Henis, Y.I.; Sokolovsky, M. Batrachotoxin changes the properties of the muscarinic receptor in rat brain and heart: Possible interaction(s) between muscarinic receptors and sodium channels. Proc. Natl. Acad. Sci. USA 1985, 82, 3524–3527. [Google Scholar] [CrossRef]
- Anis, Y.; Nürnberg, B.; Visochek, L.; Reiss, N.; Naor, Z.; Cohen-Armon, M. Activation of Go-proteins by Membrane Depolarization Traced by in Situ Photoaffinity Labeling of Gαo-proteins with [α32P]GTP-azidoanilide*. J. Biol. Chem. 1999, 274, 7431–7440. [Google Scholar] [CrossRef]
- Cohen-Armon, M.; Sokolovsky, M. Evidence for involvement of the voltage-dependent Na+ channel gating in depolarization-induced activation of G-proteins. J. Biol. Chem. 1993, 268, 9824–9838. [Google Scholar] [CrossRef] [PubMed]
- Siegl, P.K.S.; Garcia, M.L.; King, V.F.; Scott, A.L.; Morgan, G.; Kaczorowski, G.J. Interactions of DPI 201-106, a novel cardiotonic agent, with cardiac calcium channels. Naunyn Schmiedebergs Arch. Pharmacol. 1988, 338, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Groschner, K.; Ulle, P.; Brunner, F.; Kukovetz, W.R. Interaction of DPI 201-106 with cardiac muscarinic receptors. Eur. J. Pharmacol. 1989, 159, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Katritch, V.; Fenalti, G.; Abola, E.E.; Roth, B.L.; Cherezov, V.; Stevens, R.C. Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci. 2014, 39, 233–244. [Google Scholar] [CrossRef]
- Liu, W.; Chun, E.; Thompson, A.A.; Chubukov, P.; Xu, F.; Katritch, V.; Han, G.W.; Roth, C.B.; Heitman, L.H.; IJzerman, A.P. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.; Tauber, M.; Ben-chaim, Y. Sodium ions allosterically modulate the M2 muscarinic receptor. Sci. Rep. 2020, 10, 11177. [Google Scholar] [CrossRef] [PubMed]
- Vickery, O.N.; Machtens, J.-P.; Zachariae, U. Membrane potentials regulating GPCRs: Insights from experiments and molecular dynamics simulations. Curr. Opin. Pharmacol. 2016, 30, 44–50. [Google Scholar] [CrossRef]
- Vickery, O.N.; Machtens, J.-P.; Tamburrino, G.; Seeliger, D.; Zachariae, U. Structural Mechanisms of Voltage Sensing in G Protein-Coupled Receptors. Structure 2016, 24, 997–1007. [Google Scholar] [CrossRef]
- Moreno-Galindo, E.G.; Alamilla, J.; Sanchez-Chapula, J.A.; Tristani-Firouzi, M.; Navarro-Polanco, R.A. The agonist-specific voltage dependence of M2 muscarinic receptors modulates the deactivation of the acetylcholine-gated K+ current IKACh). Pflugers Arch. 2016, 468, 1207–1214. [Google Scholar] [CrossRef]
- Ruland, J.G.; Kirchhofer, S.B.; Klindert, S.; Bailey, C.P.; Bünemann, M. Voltage modulates the effect of μ-receptor activation in a ligand-dependent manner. Br. J. Pharmacol. 2020, 177, 3489–3504. [Google Scholar] [CrossRef]
- Sahlholm, K.; Marcellino, D.; Nilsson, J.; Fuxe, K.; Århem, P. Voltage-sensitivity at the human dopamine D2S receptor is agonist-specific. Biochem. Biophys. Res. Commun. 2008, 377, 1216–1221. [Google Scholar] [CrossRef]
- Tauber, M.; Chaim, Y.B. The activity of the serotonergic 5-HT1A receptor is modulated by voltage and sodium levels. J. Biol. Chem. 2022, 298, 101978. [Google Scholar] [CrossRef]
- Goldberger, E.; Tauber, M.; Ben-Chaim, Y. Voltage dependence of the cannabinoid CB1 receptor. Front. Pharmacol. 2022, 13, 1022275. [Google Scholar] [CrossRef]
- Ohana, L.; Barchad, O.; Parnas, I.; Parnas, H. The Metabotropic Glutamate G-protein-coupled Receptors mGluR3 and mGluR1a Are Voltage-sensitive. J. Biol. Chem. 2006, 281, 24204–24215. [Google Scholar] [CrossRef]
- Boutonnet, M.; Carpena, C.; Bouquier, N.; Chastagnier, Y.; Font-Ingles, J.; Moutin, E.; Tricoire, L.; Chemin, J.; Perroy, J. Voltage tunes mGlu5 receptor function, impacting synaptic transmission. Br. J. Pharmacol. 2024, 181, 1793–1811. [Google Scholar] [CrossRef]
- Ågren, R.; Sahlholm, K. Voltage-Dependent Dopamine Potency at D1-Like Dopamine Receptors. Front. Pharmacol. 2020, 11, 1615. [Google Scholar] [CrossRef]
- Sahlholm, K.; Nilsson, J.; Marcellino, D.; Fuxe, K.; Århem, P. Voltage-dependence of the human dopamine D2 receptor. Synapse 2008, 62, 476–480. [Google Scholar] [CrossRef]
- Sahlholm, K.; Marcellino, D.; Nilsson, J.; Fuxe, K.; Århem, P. Differential voltage-sensitivity of D2-like dopamine receptors. Biochem. Biophys. Res. Commun. 2008, 374, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Birk, A.; Rinne, A.; Bünemann, M. Membrane Potential Controls the Efficacy of Catecholamine-induced β1-Adrenoceptor Activity. J. Biol. Chem. 2015, 290, 27311–27320. [Google Scholar] [CrossRef]
- Rinne, A.; Birk, A.; Bünemann, M. Voltage regulates adrenergic receptor function. Proc. Natl. Acad. Sci. USA 2013, 110, 1536. [Google Scholar] [CrossRef] [PubMed]
- Sahlholm, K.; Nilsson, J.; Marcellino, D.; Fuxe, K.; Århem, P. Voltage sensitivities and deactivation kinetics of histamine H3 and H4 receptors. Biochim. Et Biophys. Acta Biomembr. 2012, 1818, 3081–3089. [Google Scholar] [CrossRef]
- Kurz, M.; Krett, A.-L.; Bünemann, M. Voltage Dependence of Prostanoid Receptors. Mol. Pharmacol. 2020, 97, 267. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Galindo, E.G.; Sánchez-Chapula, J.A.; Sachse, F.B.; Rodríguez-Paredes, J.A.; Tristani-Firouzi, M.; Navarro-Polanco, R.A. Relaxation gating of the acetylcholine-activated inward rectifier K+ current is mediated by intrinsic voltage sensitivity of the muscarinic receptor. J. Physiol. 2011, 589, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Fajardo, P.D.; Aréchiga-Figueroa, I.A.; López-Serrano, A.L.; Rodriguez-Elias, J.C.; Alamilla, J.; Sánchez-Chapula, J.A.; Tristani-Firouzi, M.; Navarro-Polanco, R.A.; Moreno-Galindo, E.G. The voltage-sensitive cardiac M2 muscarinic receptor modulates the inward rectification of the G protein-coupled, ACh-gated K+ current. Pflugers Arch. 2018, 470, 1765–1776. [Google Scholar] [CrossRef]
- Parnas, H.; Parnas, I. The chemical synapse goes electric: Ca2+- and voltage-sensitive GPCRs control neurotransmitter release. Trends Neurosci. 2007, 30, 54–61. [Google Scholar] [CrossRef]
- Parnas, I.; Parnas, H. Control of neurotransmitter release: From Ca2+ to voltage dependent G-protein coupled receptors. Pflugers Arch. 2010, 460, 975–990. [Google Scholar] [CrossRef]
- Parnas, H.; Segel, L.; Dudel, J.; Parnas, I. Autoreceptors, membrane potential and the regulation of transmitter release. Trends Neurosci. 2000, 23, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, B.; Li, Y.; Yin, L.; Younus, M.; Jiang, X.; Lin, Z.; Sun, X.; Huang, R.; Liu, B.; et al. Regulating quantal size of neurotransmitter release through a GPCR voltage sensor. Proc. Natl. Acad. Sci. USA 2020, 117, 26985–26995. [Google Scholar] [CrossRef]
- Rozenfeld, E.; Tauber, M.; Ben-Chaim, Y.; Parnas, M. GPCR voltage dependence controls neuronal plasticity and behavior. Nat. Commun. 2021, 12, 7252. [Google Scholar] [CrossRef] [PubMed]
- Mahaut-Smith, M.P.; Martinez-Pinna, J.; Gurung, I.S. A role for membrane potential in regulating GPCRs? Trends Pharmacol. Sci. 2008, 29, 421–429. [Google Scholar] [CrossRef]
- David, D.; Bentulila, Z.; Tauber, M.; Ben-Chaim, Y. G Protein-Coupled Receptors Regulated by Membrane Potential. Int. J. Mol. Sci. 2022, 23, 13988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauber, M.; Ben-Chaim, Y. Voltage Sensors Embedded in G Protein-Coupled Receptors. Int. J. Mol. Sci. 2024, 25, 5295. https://doi.org/10.3390/ijms25105295
Tauber M, Ben-Chaim Y. Voltage Sensors Embedded in G Protein-Coupled Receptors. International Journal of Molecular Sciences. 2024; 25(10):5295. https://doi.org/10.3390/ijms25105295
Chicago/Turabian StyleTauber, Merav, and Yair Ben-Chaim. 2024. "Voltage Sensors Embedded in G Protein-Coupled Receptors" International Journal of Molecular Sciences 25, no. 10: 5295. https://doi.org/10.3390/ijms25105295
APA StyleTauber, M., & Ben-Chaim, Y. (2024). Voltage Sensors Embedded in G Protein-Coupled Receptors. International Journal of Molecular Sciences, 25(10), 5295. https://doi.org/10.3390/ijms25105295




