Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ingredient Characterization
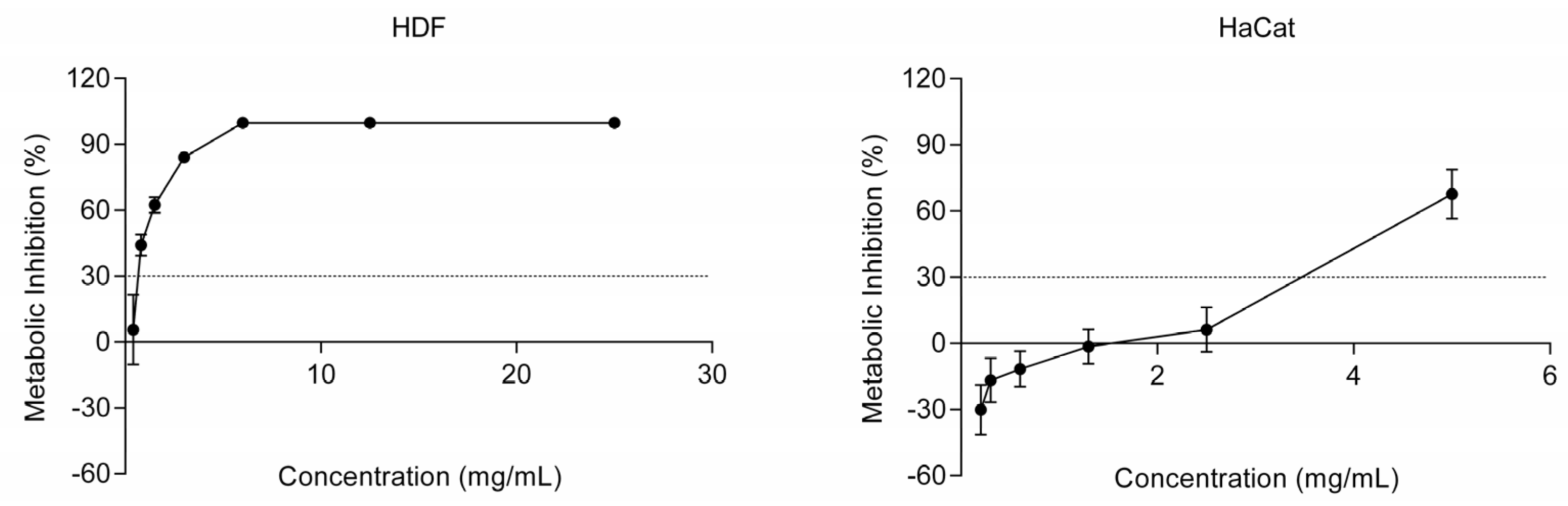
2.2. Ingredient Safety
2.3. Skin Aging-Related Enzymes Inhibitory Capacity
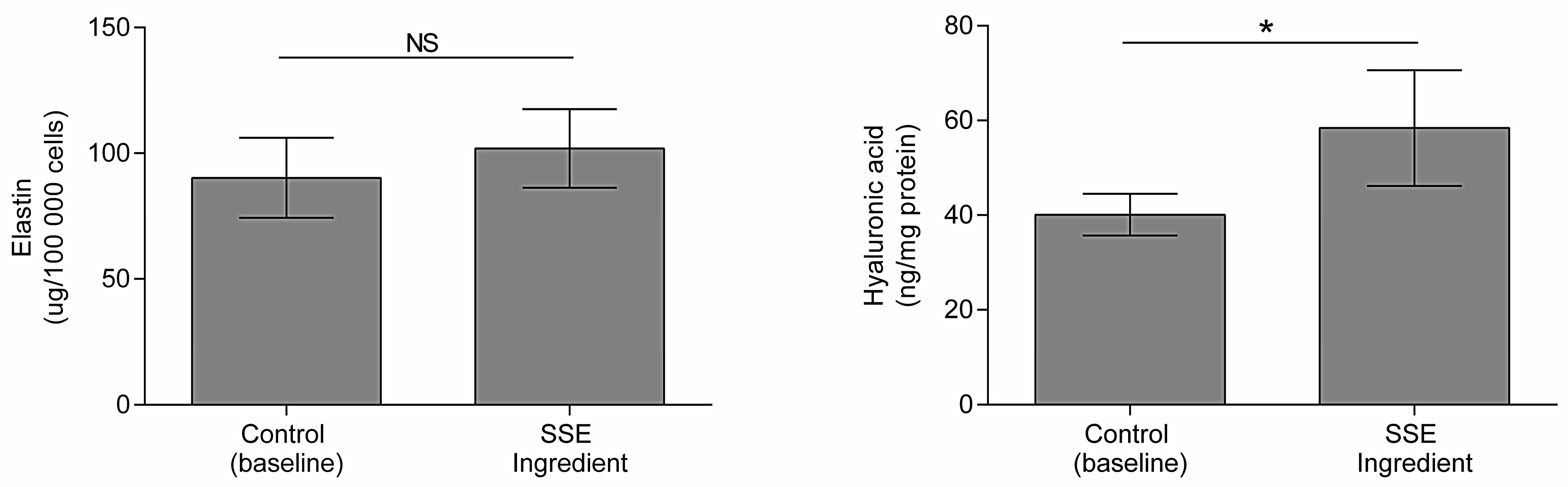
2.4. Impact on Hyaluronic Acid and Elastin Production
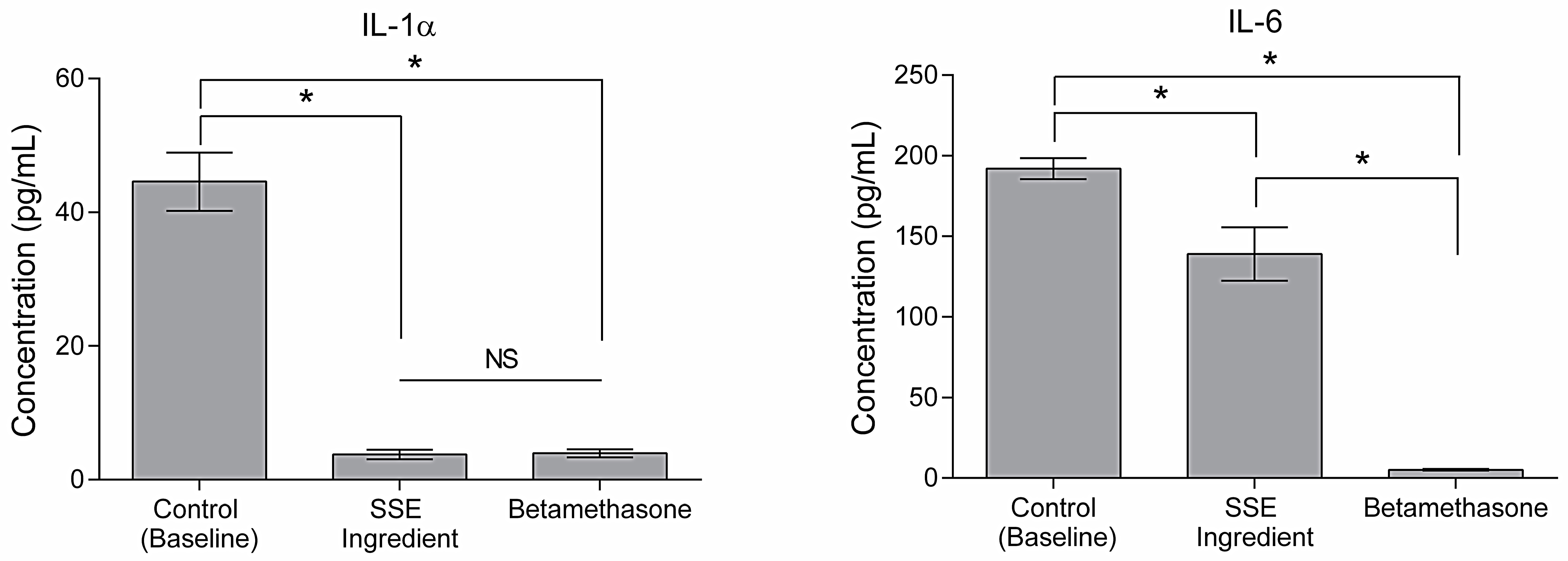
2.5. Cellular Response to Urban Particulate Matter
2.6. Permeability
3. Materials and Methods
3.1. Material
3.2. Phenolic Compounds Identification and Quantification Using LC-ESI-QqTOF-HRMS
3.3. Cytotoxicity
3.4. Direct Peptide Reactivity Assay
3.5. Evaluation of the Inhibition of Skin Aging-Related Enzymes
3.5.1. Collagenase
3.5.2. Elastase
3.5.3. Tyrosinase
3.6. Hyaluronic Acid Quantification
3.7. Elastin Quantification
3.8. Exposure to Urban Particle Matter
3.9. In Vitro Penetration with a Synthetic Membrane
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive Compounds from Natural Resources against Skin Aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia Zerumbet Components on Antioxidant and Skin Diseases-Related Enzymes. BMC Complement. Altern. Med. 2012, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Birch-Machin, M. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Costa, J.R.; Pedrosa, S.S.; Pintado, M.; Oliveira, A.L.S.; Madureira, A.R. Sugarcane Straw Extracts: Production and Purification by Amberlite XAD-2. Food Bioprod. Process. 2023, 140, 189–199. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Carvalho, M.J.; Oliveira, D.L.; Costa, E.; Pintado, M.; Madureira, A.R. Sugarcane Straw Polyphenols as Potential Food and Nutraceutical Ingredient. Foods 2022, 11, 4025. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Oliveira, A.L.; Pedrosa, S.S.; Pintado, M.; Madureira, A.R. Potential of Sugarcane Extracts as Cosmetic and Skincare Ingredients. Ind. Crops Prod. 2021, 169, 113625. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In Vitro Determination of the Anti-Aging Potential of Four Southern African Medicinal Plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.A.; Park, B.; Hwang, E.; Park, S.Y.; Yi, T.H. Borago officinalis L. Attenuates UVB-Induced Skin Photodamage via Regulation of AP-1 and Nrf2/ARE Pathway in Normal Human Dermal Fibroblasts and Promotion of Collagen Synthesis in Hairless Mice. Exp. Gerontol. 2018, 107, 178–186. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, Antioxidant and Anti-Inflammatory Phenolic Activities in Extra Virgin Olive Oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L.G. Plant Polyphenols Differentially Modulate Inflammatory Responses of Human Keratinocytes by Interfering with Activation of Transcription Factors NFκB and AhR and EGFR–ERK Pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms Extracts and Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics-A Review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant Activity and Polyphenol Composition of Sugarcane Molasses Extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Brochier, B.; de Medeiros, J.K.; Marczak, L.D.F.; Mercali, G.D. Phenolic Profile of Sugarcane Juice: Effects of Harvest Season and Processing by Ohmic Heating and Ultrasound. Food Chem. 2021, 347, 129058. [Google Scholar] [CrossRef]
- Charoenprasert, S.; Mitchell, A. Factors Influencing Phenolic Compounds in Table Olives (Olea europaea). J. Agric. Food Chem. 2012, 60, 7081–7095. [Google Scholar] [CrossRef]
- Souza, T.P.; Dias, R.O.; Silva-Filho, M.C. Defense-Related Proteins Involved in Sugarcane Responses to Biotic Stress. Genet. Mol. Biol. 2017, 40, 360–372. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant Activity of Selected Phenols Estimated by ABTS and FRAP Methods. Postepy Hig. Med. Dosw. 2013, 67, 958–963. [Google Scholar] [CrossRef]
- Adamska-szewczyk, A.; Zgórka, G. Plant Polyphenols in Cosmetics—A Review. Eur. J. Med. Technol. 2019, 3, 1–10. [Google Scholar]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic Acids and Their Derivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a Review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Garg, C.; Khurana, P.; Garg, M. Molecular Mechanisms of Skin Photoaging and Plant Inhibitors. Int. J. Green Pharm. 2017, 11, S217–S232. [Google Scholar]
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic Structure and Function. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1134–1144. [Google Scholar]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009; p. 34.
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin Anti-Aging Assays of Proanthocyanidin Rich Red Rice Extract, Oryzanol and Other Phenolic Compounds. Nat. Prod. Commun. 2018, 13, 967–972. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef]
- Hemming, J.D.C.; Hosford, M.; Shafer, M.M. Application of the Direct Peptide Reactivity Assay (DPRA) to Inorganic Compounds: A Case Study of Platinum Species. Toxicol. Res. 2019, 8, 802–814. [Google Scholar] [CrossRef]
- Urbisch, D.; Becker, M.; Honarvar, N.; Kolle, S.N.; Mehling, A.; Teubner, W.; Wareing, B.; Landsiedel, R. Assessment of Pre- and Pro-Haptens Using Nonanimal Test Methods for Skin Sensitization. Chem. Res. Toxicol. 2016, 29, 901–913. [Google Scholar] [CrossRef]
- Kreiling, R.; Gehrke, H.; Broschard, T.H.; Dreeßen, B.; Eigler, D.; Hart, D.; Höpflinger, V.; Kleber, M.; Kupny, J.; Li, Q.; et al. In Chemico, in Vitro and in Vivo Comparison of the Skin Sensitizing Potential of Eight Unsaturated and One Saturated Lipid Compounds. Regul. Toxicol. Pharmacol. 2017, 90, 262–276. [Google Scholar] [CrossRef]
- Kimber, I.; Basketter, D.A.; Gerberick, G.F.; Dearman, R.J. Allergic Contact Dermatitis. Int. Immunopharmacol. 2002, 2, 201–211. [Google Scholar] [CrossRef]
- Natsch, A.; Emter, R. Nrf2 Activation as a Key Event Triggered by Skin Sensitisers: The Development of the Stable KeratinoSens Reporter Gene Assay. ATLA Altern. Lab. Anim. 2016, 44, 443–451. [Google Scholar] [CrossRef]
- Kolle, S.N.; Flach, M.; Kleber, M.; Basketter, D.A.; Wareing, B.; Mehling, A.; Hareng, L.; Watzek, N.; Bade, S.; Funk-Weyer, D.; et al. Plant Extracts, Polymers and New Approach Methods: Practical Experience with Skin Sensitization Assessment. Regul. Toxicol. Pharmacol. 2023, 138, 105330. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/Peptide Binding and Precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a Hundred Plant Extracts as Tyrosinase and Elastase Inhibitors, Two Enzymatic Targets of Cosmetic Interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Ameer, F.; Iwuoha, E.I.; Hussein, A.A. Inhibition of Oxidative Stress and Skin Aging-Related Enzymes by Prenylated Chalcones and Other Flavonoids from Helichrysum Teretifolium. Molecules 2015, 20, 7143–7155. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase Inhibition and Antioxidant Properties of Asphodelus Microcarpus Extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef]
- Wittenauer, J.; MäcKle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic Acid: A Key Molecule in Skin Aging. Dermatoendocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin Structure and Its Involvement in Skin Photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.H.; Karsdal, M.A. Elastin. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–201. [Google Scholar]
- Chowdhury, A.; Nosoudi, N.; Karamched, S.; Parasaram, V.; Vyavahare, N. Polyphenol Treatments Increase Elastin and Collagen Deposition by Human Dermal Fibroblasts; Implications to Improve Skin Health. J. Dermatol. Sci. 2021, 102, 94–100. [Google Scholar] [CrossRef]
- Mistry, N. Guidelines for Formulating Anti-Pollution Products. Cosmetics 2017, 4, 57. [Google Scholar] [CrossRef]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Park, S.; Seok, J.K.; Kwak, J.Y.; Suh, H.-J.; Kim, Y.M.; Boo, Y.C. Anti-Inflammatory Effects of Pomegranate Peel Extract in THP-1 Cells Exposed to Particulate Matter PM10. Evid.-Based Complement. Altern. Med. 2016, 2016, 6836080. [Google Scholar] [CrossRef]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol Is the Major Anti-Inflammatory Compound in Aqueous Olive Extracts and Impairs Cytokine and Chemokine Production in Macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Balupillai, A.; Prasad, R.N.; Ramasamy, K.; Muthusamy, G.; Shanmugham, M.; Govindasamy, K.; Gunaseelan, S. Caffeic Acid Inhibits UVB-Induced Inflammation and Photocarcinogenesis Through Activation of Peroxisome Proliferator-Activated Receptor-γ in Mouse Skin. Photochem. Photobiol. 2015, 91, 1458–1468. [Google Scholar] [CrossRef]
- Staniforth, V. Caffeic Acid Suppresses UVB Radiation-Induced Expression of Interleukin-10 and Activation of Mitogen-Activated Protein Kinases in Mouse. Carcinogenesis 2006, 27, 1803–1811. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Hasenkopf, K.; Eisner, P.; Kerscher, M. Antioxidant Activity, Lipophilicity and Extractability of Polyphenols from Pig Skin—Development of Analytical Methods for Skin Permeation Studies. Biomed. Chromatogr. 2013, 27, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Heenatigala Palliyage, G.; Singh, S.; Ashby, C.R.; Tiwari, A.K.; Chauhan, H. Pharmaceutical Topical Delivery of Poorly Soluble Polyphenols: Potential Role in Prevention and Treatment of Melanoma. AAPS PharmSciTech 2019, 20, 250. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Hung, C.-F.; Aljuffali, I.A.; Huang, Y.-L.; Liao, W.-C.; Fang, J.-Y. Methylation and Esterification of Magnolol for Ameliorating Cutaneous Targeting and Therapeutic Index by Topical Application. Pharm. Res. 2016, 33, 2152–2167. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pla, J.J.; Martín-Biosca, Y.; Sagrado, S.; Villanueva-Camañas, R.M.; Medina-Hernández, M.J. Biopartitioning Micellar Chromatography to Predict Skin Permeability. Biomed. Chromatogr. 2003, 17, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Romes, N.B.; Abdul Wahab, R.; Abdul Hamid, M.; Oyewusi, H.A.; Huda, N.; Kobun, R. Thermodynamic Stability, in-Vitro Permeability, and In-Silico Molecular Modeling of the Optimal Elaeis Guineensis Leaves Extract Water-in-Oil Nanoemulsion. Sci. Rep. 2021, 11, 20851. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Verma, A.K.; Pratap, R. The Biological Potential of Flavones. Nat. Prod. Rep. 2010, 27, 1571–1593. [Google Scholar] [CrossRef]
- Diniz, A.; Escuder-Gilabert, L.; Lopes, N.P.; Gobbo-Neto, L.; Villanueva-Camañas, R.M.; Sagrado, S.; Medina-Hernández, M.J. Permeability Profile Estimation of Flavonoids and Other Phenolic Compounds by Biopartitioning Micellar Capillary Chromatography. J. Agric. Food Chem. 2007, 55, 8372–8379. [Google Scholar] [CrossRef]
- Oliveira, A.L.S.; Valente, D.; Moreira, H.R.; Pintado, M.; Costa, P. Effect of Squalane-Based Emulsion on Polyphenols Skin Penetration: Ex Vivo Skin Study. Colloids Surf. B Biointerfaces 2022, 218, 112779. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of Nanoparticles and Cell-Penetrating Peptides with Skin for Transdermal Drug Delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the Temperature and Oxygen Exposure in Red Port Wine: A Kinetic Approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guideline for the Testing of Chemicals: In Chemico Skin Sensitisation: Direct Peptide Reactivity Assay (DPRA); Organisation for Economic Co-Operation and Development: Paris, France, 2015; Volume 422C, pp. 1–19. [Google Scholar]
- OECD. OECD Guideline for the Testing of Chemicals: Skin Absorption: In Vitro Method; Organisation for Economic Co-Operation and Development: Paris, France, 2004. [Google Scholar]

| Tentative Identification | Formula-H | Retention Time (min) | m/z Measured [M-H] | MS/MS Fragments (m/z) | Concentration (µg/g dw Extract) |
|---|---|---|---|---|---|
| Hydroxybenzoic acids | |||||
| 1-O-Vanilloyl-β-D-glucose | C14H17O9 | 6.4 | 329.0 | 167 | 109.7 ± 5.7 |
| Vanillic acid | C8H7O4 | 7.5 | 167.0 | 108, 119, 152 | 28.03 ± 6.27 |
| Protocatechuic acid | C7H5O4 | 5.5 | 153.0 | 109, 153 | 14.01 ± 0.69 |
| 2,5-Dihydrobenzoic acid | C7H5O4 | 7.8 | 153.0 | 109, 153 | 10.31 ± 0.75 |
| 2,4-Dihydrobenzoic acid | C7H5O4 | 8.9 | 153.0 | 109, 153 | 441.7 ± 38.3 |
| Gentisic acid 2-O-β-glucoside | C13H15O9 | 5.4 | 315.1 | 108, 152 | 21.51 ± 4.71 |
| Gentisic acid 5-O-β-glucoside | C13H15O9 | 5.9 | 315.1 | 109, 153 | 23.11 ± 3.28 |
| 4-Hydroxybenzoic acid | C7H5O3 | 6.9 | 137.0 | 137 | 12.44 ± 0.38 |
| 3,4-Dihydroxybenzaldehyde | C7H5O3 | 7.3 | 137.0 | 93, 137 | 28.20 ± 0.95 |
| 4-Hydroxybenzaldehyde | C7H5O2 | 8.7 | 121.0 | 121 | 33.66 ± 1.39 |
| Hydroxycinnamic acids | |||||
| Neochlorogenic acid | C16H17O9 | 6.3 | 353.1 | 135, 179, 191 | 250.9 ± 18.5 |
| Chlorogenic acid | C16H17O9 | 7.8 | 353.1 | 191 | 262.4 ± 12.1 |
| 4-Caffeoylquinic acid isomer 1 | C16H17O9 | 8 | 353.1 | 135, 173, 179, 191 | 122.9 ± 5.4 |
| 4-Caffeoylquinic acid isomer 2 | C16H17O9 | 9.1 | 353.1 | 191 | 25.63 ± 2.25 |
| 3-O-Feruloylquinic acid | C17H19O9 | 8 | 367.1 | 134, 193 | 208.6 ± 6.6 |
| trans-3-Feruloylquinic acid | C17H19O9 | 9.7 | 367.1 | 173 | 100.9 ± 2.0 |
| Caffeic acid | C9H8O4 | 8.2 | 179.0 | 135, 179 | 18.82 ± 0.62 |
| Isoferulic acid | C10H9O4 | 11.1 | 193.0 | 134, 161, 193 | 21.01 ± 0.48 |
| p-Coumaric acid | C9H7O3 | 10.3 | 163.0 | 119 | 145.4 ± 5.7 |
| 1,3-Dicaffeoylquinic acid | C25H23O12 | 12.1 | 515.1 | 173, 179, 191, 335, 353 | 13.61 ± 0.59 |
| Flavones | |||||
| Apigenin-8-C-glucoside isomer 1 | C21H19O10 | 8.6 | 431.1 | 89, 179 | 2.773 ± 0.269 |
| Apigenin-8-C-glucoside isomer 2 | C21H19O10 | 9 | 431.1 | 311, 341, 431 | 10.46 ± 0.48 |
| Apigenin-6-C-glucoside | C21H19O10 | 11 | 431.1 | 311, 341 | 6.094 ± 0.352 |
| Isoschaftoside | C26H27O14 | 10.1 | 563.1 | 353, 473 | 5.643 ± 0.334 |
| Luteolin-8-C-glucoside | C21H19O11 | 10.3 | 447.1 | 327, 357 | 16.45 ± 0.62 |
| Vitexin 2″-O-beta-L-rhamnoside | C27H29O14 | 11.2 | 577.2 | 293, 413 | 4.153 ± 0.159 |
| Apigenin 7-O-neohesperidoside isomer 2 | C27H29O14 | 13.9 | 577.2 | 293, 413, 474 | 6.410 ± 0.180 |
| Tricin-O-neohesperoside isomer 2 | C29H33O16 | 13.3 | 637.2 | 329 | 2.748 ± 0.137 |
| Tricin-7-O-glucoside | C25H31O10 | 12.4 | 491.2 | 329 | 6.420 ± 0.095 |
| Tricin | C17H13O7 | 17.7 | 329.1 | 299 | 7.949 ± 0.236 |
| Sample | mg/mL | % Cysteine and Lysine Peptides Depletion | Reactivity (Cysteine and Lysine) | Based on the Mean of Cysteine and Lysine Prediction Model | Potential Sensitizer |
|---|---|---|---|---|---|
| Positive Control (Cinnamic aldehyde) | 0.1 M | 78.8 | High reactivity | Positive | Yes |
| SSE ingredient | 12.5 | 29.3 | Moderate | Positive | Yes |
| 6.25 | 16.0 | Low | Positive | Yes | |
| 3.15 | 8.7 | Low | Positive | Yes | |
| 1.60 | 4.3 | Minimal | Negative | No |
| Not Absorbed (%) | Strat-M™ (%) | Receptor Cell (%) | |
|---|---|---|---|
| Hydroxybenzoic acids | 59.03 | 17.06 | 23.91 |
| Hydroxycinnamic acids | 67.10 | 18.70 | 17.60 |
| Flavones | 66.70 | 25.10 | 8.20 |
| Applied Dose (µg) | Not Absorbed (µg) | Strat-M™ (µg) | Receptor Cell (µg) | |
|---|---|---|---|---|
| Hydroxybenzoic acids | 24.27 ± 3.89 a | 14.60 ± 1.97 b | 4.45 ± 1.80 c | 7.56 ± 1.53 c |
| 1-O-Vanilloyl-β-D-glucose | 6.13 ± 1.18 a | 6.04 ± 0.23 a | 1.29 ± 0.69 b | 0.78 ± 0.26 b |
| 2,5-Dihydroxybenzoic acid | 6.04 ± 0.99 a | 3.39 ± 0.45 b | 1.15 ± 0.27 c | 1.35 ± 0.64 c |
| 4-Hydroxybenzaldehyde | 6.32 ± 0.80 a | 1.59 ± 0.85 b | 1.71 ± 0.61 b | 2.41 ± 1.44 b |
| Hydroxycinnamic acids | 48.04 ± 8.09 a | 41.00 ± 1.29 a | 8.22 ± 2.92 b | 10.32 ± 1.65 b |
| Chlorogenic acid | 8.15 ± 1.57 a | 7.40 ± 0.16 a | 1.86 ± 0.99 b | 0.78 ± 0.13 b |
| Ferulic acid | 1.21 ± 0.11 a | 0.65 ± 0.14 b | 0.49 ± 0.16 b | 0.66 ± 0.35 ab |
| p-Coumaric acid | 4.43 ± 0.63 a | 1.90 ± 0.78 b | 1.74 ± 0.59 b | 2.86 ± 1.38 ab |
| Flavones | 44.51 ± 2.65 a | 43.09 ± 0.37 a | 14.25 ± 2.07 b | 6.64 ± 1.16 c |
| Apigenin-8-C-glucoside | 5.76 ± 0.81 a | 5.34 ± 0.11 a | 0.92 ± 0.58 b | 0.78 ± 0.15 b |
| Isoschaftoside | 5.16 ± 0.60 a | 5.05 ± 0.10 a | 1.40 ± 0.86 b | 0.67 ± 0.13 b |
| Luteolin-8-C-glucoside | 3.13 ± 0.17 a | 3.31 ± 0.03 a | 1.85 ± 0.65 b | 0.63 ± 0.20 c |
| Mean of Cysteine and Lysine % Depletion | Reactivity Class | DPRA Prediction |
|---|---|---|
| 0% ≤ Mean % Depletion ≤ 6.38% | No or Minimal Reactivity | Negative |
| 6.38% < Mean % Depletion ≤ 22.62% | Low Reactivity | Positive |
| 22.62% < Mean % Depletion ≤ 42.47% | Moderate Reactivity | |
| 42.47% < Mean % Depletion ≤ 100% | High Reactivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, M.J.; Pedrosa, S.S.; Mendes, A.; Azevedo-Silva, J.; Fernandes, J.; Pintado, M.; Oliveira, A.L.S.; Madureira, A.R. Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE). Int. J. Mol. Sci. 2024, 25, 21. https://doi.org/10.3390/ijms25010021
Carvalho MJ, Pedrosa SS, Mendes A, Azevedo-Silva J, Fernandes J, Pintado M, Oliveira ALS, Madureira AR. Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE). International Journal of Molecular Sciences. 2024; 25(1):21. https://doi.org/10.3390/ijms25010021
Chicago/Turabian StyleCarvalho, Maria João, Sílvia Santos Pedrosa, Adélia Mendes, João Azevedo-Silva, João Fernandes, Manuela Pintado, Ana L. S. Oliveira, and Ana Raquel Madureira. 2024. "Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE)" International Journal of Molecular Sciences 25, no. 1: 21. https://doi.org/10.3390/ijms25010021
APA StyleCarvalho, M. J., Pedrosa, S. S., Mendes, A., Azevedo-Silva, J., Fernandes, J., Pintado, M., Oliveira, A. L. S., & Madureira, A. R. (2024). Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE). International Journal of Molecular Sciences, 25(1), 21. https://doi.org/10.3390/ijms25010021









