The Potential of Campanula takesimana Callus Extract to Enhance Skin Barrier Function
Abstract
:1. Introduction
2. Results
2.1. Induction of Calluses and the HPLC Analysis of the Callus Extract
2.2. Decreased Expression of FLG, ZO-1, and CLDN-1 by Th2 Cytokines Was Reversed by the C. takesimana Callus Extract
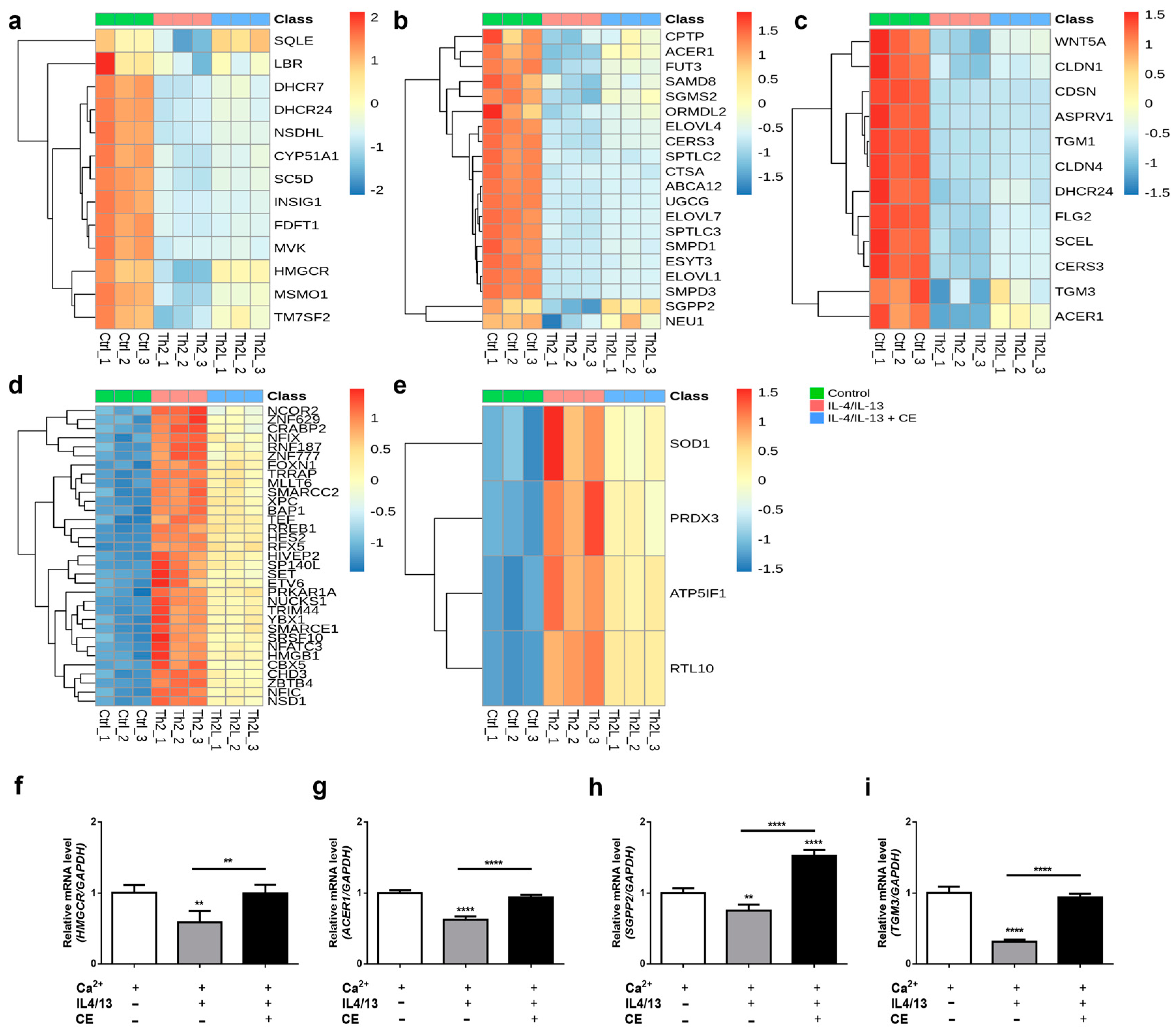
2.3. The C. takesimana Callus Extract Restores Lipid Biosynthesis and the Epidermal Development Pathway
3. Discussion
4. Materials and Methods
4.1. Induction of the Callus and Optimization of Culture Medium
4.2. Preparing Test Samples from C. takesimana Callus and Leaf
4.3. HPLC Analysis of the Samples
4.4. Cell Culture
4.5. The Cell Viability and Proliferation Assay
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. RNA Sequencing Data Analysis
4.8. Producing a 3D-Reconstructed Human Skin Equivalent
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidinger, S.; O’Sullivan, M.; Illig, T.; Baurecht, H.; Depner, M.; Rodriguez, E.; Ruether, A.; Klopp, N.; Vogelberg, C.; Weiland, S.K.; et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J. Allergy Clin. Immunol. 2008, 121, 1203–1209.e1201. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y.M. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007, 120, 150–155. [Google Scholar] [CrossRef]
- Brown, S.J.; Kroboth, K.; Sandilands, A.; Campbell, L.E.; Pohler, E.; Kezic, S.; Cordell, H.J.; McLean, W.H.I.; Irvine, A.D. Intragenic Copy Number Variation within Filaggrin Contributes to the Risk of Atopic Dermatitis with a Dose-Dependent Effect. J. Investig. Dermatol. 2012, 132, 98–104. [Google Scholar] [CrossRef]
- Pellerin, L.; Henry, J.; Hsu, C.-Y.; Balica, S.; Jean-Decoster, C.; Méchin, M.-C.; Hansmann, B.; Rodriguez, E.; Weindinger, S.; Schmitt, A.-M.; et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J. Allergy Clin. Immunol. 2013, 131, 1094–1102. [Google Scholar] [CrossRef]
- Flohr, C.; England, K.; Radulovic, S.; McLean, W.H.I.; Campbell, L.E.; Barker, J.; Perkin, M.; Lack, G. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br. J. Dermatol. 2010, 163, 1333–1336. [Google Scholar] [CrossRef]
- Kezic, S.; Kemperman, P.M.J.H.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, I.W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-Function Mutations in the Filaggrin Gene Lead to Reduced Level of Natural Moisturizing Factor in the Stratum Corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef]
- Cork, M.J.; Robinson, D.A.; Vasilopoulos, Y.; Ferguson, A.; Moustafa, M.; MacGowan, A.; Duff, G.W.; Ward, S.J.; Tazi-Ahnini, R. New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene–environment interactions. J. Allergy Clin. Immunol. 2006, 118, 3–21. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Roelandt, T.; Schürer, N.; Pu, X.; Fluhr, J.; Giddelo, C.; Man, M.-Q.; Crumrine, D.; Roseeuw, D.; Feingold, K.R.; et al. Acute Acidification of Stratum Corneum Membrane Domains Using Polyhydroxyl Acids Improves Lipid Processing and Inhibits Degradation of Corneodesmosomes. J. Investig. Dermatol. 2010, 130, 500–510. [Google Scholar] [CrossRef]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e777. [Google Scholar] [CrossRef]
- De Benedetto, A.; Slifka, M.K.; Rafaels, N.M.; Kuo, I.H.; Georas, S.N.; Boguniewicz, M.; Hata, T.; Schneider, L.C.; Hanifin, J.M.; Gallo, R.L.; et al. Reductions in claudin-1 may enhance susceptibility to herpes simplex virus 1 infections in atopic dermatitis. J. Allergy Clin. Immunol. 2011, 128, 242–246.e245. [Google Scholar] [CrossRef]
- Gruber, R.; Elias, P.M.; Crumrine, D.; Lin, T.-K.; Brandner, J.M.; Hachem, J.-P.; Presland, R.B.; Fleckman, P.; Janecke, A.R.; Sandilands, A.; et al. Filaggrin Genotype in Ichthyosis Vulgaris Predicts Abnormalities in Epidermal Structure and Function. Am. J. Pathol. 2011, 178, 2252–2263. [Google Scholar] [CrossRef]
- Gutowska-Owsiak, D.; Schaupp, A.L.; Salimi, M.; Taylor, S.; Ogg, G.S. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br. J. Dermatol. 2011, 165, 492–498. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef]
- Cubero, J.L.; Isidoro-García, M.; Segura, N.; Benito Pescador, D.; Sanz, C.; Lorente, F.; Dávila, I.; Colás, C. Filaggrin gene mutations and new SNPs in asthmatic patients: A cross-sectional study in a Spanish population. Allergy Asthma Clin. Immunol. 2016, 12, 1–10. [Google Scholar] [CrossRef]
- Kubo, A.; Nagao, K.; Amagai, M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J. Clin. Investig. 2012, 122, 440–447. [Google Scholar] [CrossRef]
- Kodama, M.; Asano, K.; Oguma, T.; Kagawa, S.; Tomomatsu, K.; Wakaki, M.; Takihara, T.; Ueda, S.; Ohmori, N.; Ogura, H.; et al. Strain-Specific Phenotypes of Airway Inflammation and Bronchial Hyperresponsiveness Induced by Epicutaneous Allergen Sensitization in BALB/c and C57BL/6 Mice. Int. Arch. Allergy Immunol. 2010, 152, 67–74. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Demehri, S.; Morimoto, M.; Holtzman, M.J.; Kopan, R. Skin-Derived TSLP Triggers Progression from Epidermal-Barrier Defects to Asthma. PLoS Biol. 2009, 7, e1000067. [Google Scholar] [CrossRef]
- Paller, A.S.; Kabashima, K.; Bieber, T. Therapeutic pipeline for atopic dermatitis: End of the drought? J. Allergy Clin. Immunol. 2017, 140, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, K.-H.; Yook, H.-S. Antioxidative Effects of Campanula takesimana Nakai Extract. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1331–1337. [Google Scholar] [CrossRef]
- Qi, Y.; Choi, S.-I.; Son, S.-R.; Han, H.-S.; Ahn, H.S.; Shin, Y.-K.; Lee, S.H.; Lee, K.-T.; Kwon, H.C.; Jang, D.S. Chemical Constituents of the Leaves of Campanula takesimana (Korean Bellflower) and Their Inhibitory Effects on LPS-induced PGE2 Production. Plants 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Yuki, T.; Tobiishi, M.; Kusaka-Kikushima, A.; Ota, Y.; Tokura, Y. Impaired Tight Junctions in Atopic Dermatitis Skin and in a Skin-Equivalent Model Treated with Interleukin-17. PLoS ONE 2016, 11, e0161759. [Google Scholar] [CrossRef]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef]
- Bae, Y.J.; Park, K.Y.; Han, H.S.; Kim, Y.S.; Hong, J.Y.; Han, T.Y.; Seo, S.J. Effects of Particulate Matter in a Mouse Model of Oxazolone-Induced Atopic Dermatitis. Ann. Dermatol. 2020, 32, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Ho-Kyung, J.; Ji-Hun, J.; Byung-Kwan, A.; Byoung-Man, K.; Jung-Hee, C.; Hyun-Woo, C. Campanula takesimana Nakai water extract attenuates lipopolysaccharide-stimulated proinflammatory responses in RAW 264.7 cells. Korean J. Crop Sci. 2014, 22, 354–355. [Google Scholar]
- Sugawara, T.; Iwamoto, N.; Akashi, M.; Kojima, T.; Hisatsune, J.; Sugai, M.; Furuse, M. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J. Dermatol Sci. 2013, 70, 12–18. [Google Scholar] [CrossRef]
- Katsarou, S.; Makris, M.; Vakirlis, E.; Gregoriou, S. The Role of Tight Junctions in Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2023, 12, 1538. [Google Scholar] [CrossRef]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased Level of Ceramides in Stratum Corneum of Atopic Dermatitis: An Etiologic Factor in Atopic Dry Skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef]
- Choi, M.J.; Maibach, H.I. Role of Ceramides in Barrier Function of Healthy and Diseased Skin. Am. J. Clin. Dermatol. 2005, 6, 215–223. [Google Scholar] [CrossRef]
- Sawada, E.; Yoshida, N.; Sugiura, A.; Imokawa, G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: An implication for the disrupted barrier mechanism in atopic dermatitis. J. Dermatol. Sci. 2012, 68, 25–35. [Google Scholar] [CrossRef]
- Hatano, Y.; Terashi, H.; Arakawa, S.; Katagiri, K. Interleukin-4 Suppresses the Enhancement of Ceramide Synthesis and Cutaneous Permeability Barrier Functions Induced by Tumor Necrosis Factor-α and interferon-γ in Human Epidermis. J. Investig. Dermatol. 2005, 124, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2012, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Mok, B.R.; Kim, A.R.; Baek, S.H.; Ahn, J.H.; Seok, S.H.; Shin, J.U.; Kim, D.H. PFN1 Prevents Psoriasis Pathogenesis through IκBζ Regulation. J. Investig. Dermatol. 2022, 142, 2455–2463.e2459. [Google Scholar] [CrossRef]



| Gene | FDR | FC | Function of the Gene |
|---|---|---|---|
| Upregulated | |||
| ARMC10 | 0 | 1.92075265 | Direct interaction with the DNA-binding domain of p53 may play a role in cell growth and survival |
| ADIPOR1 | 0 | 1.80296869 | Activation of an AMP-activated kinase signaling pathway, which affects levels of fatty acid oxidation and insulin sensitivity |
| VSIG8 | 0 | 1.6961644 | Enables RNA-binding activity |
| AQP9 | 0 | 1.64439391 | Allows the passage of a broad range of non-charged solutes |
| LIPK | 0 | 1.63993719 | Cornification |
| LIPM | 0 | 1.63287672 | Cornification |
| GPR87 | 0 | 1.62555006 | G protein-coupled receptor |
| LIPN | 0 | 1.61028427 | Lipase that is highly expressed in granular keratinocytes |
| GALNT1 | 0 | 1.58661081 | A member of the UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase (GalNAc-T) family of enzymes |
| SLPI | 0 | 1.58600084 | Secreted inhibitor that protects epithelial tissues from serine proteases |
| BPIFC | 0 | 1.5751966 | Lipid-binding activity |
| YIF1A | 0 | 1.57078565 | Endoplasmic reticulum to Golgi vesicle-mediated transport |
| ERLEC1 | 0 | 1.5590954 | Endoplasmic reticulum-associated degradation |
| CNPY2 | 0 | 1.55241235 | Active in the endoplasmic reticulum |
| RHBDL2 | 0 | 1.53925667 | Release soluble growth factors via proteolytic cleavage |
| EI24 | 0 | 1.52976924 | A putative tumor suppressor |
| GJB6 | 0 | 1.50980776 | Transport of ions and metabolites between the adjacent cells |
| SGMS2 | 0 | 1.4972011 | Transfer of phosphocholine from phosphatidylcholine onto ceramide |
| NFE2L3 | 0 | 1.49358595 | Heterodimerizes with small musculoaponeurotic fibrosarcoma factors to bind antioxidant response elements in target genes. |
| PI3 | 0 | 1.48234845 | Elastase-specific inhibitor that functions as an antimicrobial peptide against Gram-positive and Gram-negative bacteria, and fungal pathogens |
| KRTDAP | 0 | 1.48098994 | Regulation of keratinocyte differentiation and maintenance of stratified epithelia |
| ANKRD22 | 0 | 1.4777997 | Unknown |
| AADACL2 | 0 | 1.47697589 | Enable hydrolase activity. |
| RDH12 | 0 | 1.47588748 | NADPH-dependent retinal reductase |
| NLRP10 | 0 | 1.47438664 | Regulatory role in the innate immune system |
| GORASP2 | 0 | 1.47282828 | Stacking of Golgi cisternae and Golgi ribbon formation, as well as Golgi fragmentation during apoptosis or mitosis |
| ADIPOR2 | 0 | 1.47198093 | Mediate increased AMPK and PPAR-alpha ligand activities, as well as fatty acid oxidation and glucose uptake by adiponectin |
| MUC15 | 0 | 1.47072629 | Located in the Golgi lumen and plasma membrane |
| ELOVL4 | 0 | 1.47011945 | Biosynthesis of fatty acids |
| TNFAIP6 | 0 | 1.45982512 | A secretory protein that contains a hyaluronan-binding domain |
| Downregulated | |||
| DAZAP2 | 0 | 0.78833111 | A proline-rich protein which interacts with the deleted in azoospermia (DAZ) and transforming growth factor-beta signaling molecule Smad anchor for receptor activation (SARA) |
| NCOR2 | 0 | 0.78563125 | A member of a family of thyroid hormone- and retinoic acid receptor-associated co-repressors |
| CNBP | 0 | 0.7824024 | Functions in Cap-independent translation of ornithine decarboxylase mRNA and sterol-mediated transcriptional regulation |
| PDLIM1 | 0 | 0.77357901 | Adapter to bring other LIM-interacting proteins to the cytoskeleton |
| PEBP1 | 0 | 0.74116878 | Modulate multiple signaling pathways, including the MAP kinase (MAPK), NF-kappa B, and glycogen synthase kinase-3 (GSK-3) signaling pathways. |
| UBE2L3 | 0 | 0.7370626 | Ubiquitination of p53, c-Fos, and the NF-kB precursor p105 |
| S100A11 | 0 | 0.73682603 | A member of the S100 family of proteins containing two EF-hand calcium-binding motifs; may function in motility, invasion, and tubulin polymerization |
| CFL1 | 0 | 0.73597491 | Widely distributed intracellular actin-modulating protein that binds and depolymerizes filamentous F-actin and inhibits the polymerization of monomeric G-actin in a pH-dependent manner |
| NUDC | 0 | 0.73283037 | Spindle formation during mitosis and in microtubule organization during cytokinesis |
| SPRR1B | 0 | 0.68475142 | Crosslinked to membrane proteins by transglutaminase, forming an insoluble layer under the plasma membrane |
| SH3BGRL3 | 0 | 0.65155543 | Located in nuclear bodies |
| NUCKS1 | 7.7716 × 10−16 | 0.74810539 | Phosphorylated in vivo by Cdk1 during mitosis of the cell cycle |
| LITAF | 1.3878 × 10−14 | 0.79073672 | A DNA-binding protein; mediates the expression of TNF-alpha by directly binding to the promoter region of the TNF-alpha gene |
| MRFAP1 | 2.5513 × 10−13 | 0.77753748 | An intracellular protein that interacts with members of the MORF4/MRG (mortality factor on chromosome 4/MORF4-related gene) family and the tumor suppressor Rb (retinoblastoma protein.) |
| SAP18 | 4.3332 × 10−13 | 0.6983495 | A component of the histone deacetylase complex |
| LCE3D | 9.0949 × 10−13 | 0.79201422 | Keratinization |
| MTPN | 1.2578 × 10−12 | 0.78751906 | Encode both myotrophin and leucine zipper protein 6 |
| SPRR2E | 2.0592 × 10−12 | 0.78798856 | A family of small proline-rich proteins clustered in the epidermal differentiation complex on chromosome 1q21 |
| SUMO1 | 2.5585 × 10−12 | 0.6757033 | Nuclear transport, transcriptional regulation, apoptosis, and protein stability |
| ARPC5 | 2.8555 × 10−12 | 0.79739882 | One of seven subunits of the human Arp2/3 protein complex |
| DBI | 8.7995 × 10−12 | 0.75534482 | Lipid metabolism and the displacement of beta-carbolines and benzodiazepines |
| SH3BP4 | 3.2123 × 10−11 | 0.7996902 | Cargo-specific control of clathrin-mediated endocytosis, specifically controlling the internalization of a specific protein receptor |
| IMPACT | 1.1463 × 10−10 | 0.7043169 | Actin-binding activity and ribosome-binding activity |
| PPARA | 2.1865 × 10−10 | 0.72814971 | DNA-binding transcription factor activity; RNA polymerase II cis-regulatory region sequence-specific DNA-binding activity; and lipid-binding activity |
| CNN2 | 7.2918 × 10−10 | 0.7927439 | Structural organization of actin filaments |
| MYG1 | 1.3996 × 10−9 | 0.35205611 | Nuclease activity |
| ZNF592 | 1.6664 × 10−9 | 0.78021028 | Developmental pathway, and the regulation of genes involved in cerebellar development |
| CDKN1A | 2.7186 × 10−9 | 0.79306698 | Inhibits the activity of cyclin/cyclin-dependent kinase 2 or /cyclin-dependent kinase 4 complexes |
| TBC1D16 | 1.4699 × 10−8 | 0.78832422 | Regulation of receptor recycling |
| PLAGL2 | 3.0912 × 10−8 | 0.78914576 | A zinc-finger protein that recognizes DNA and/or RNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mok, B.; Jang, Y.S.; Moon, J.H.; Moon, S.; Jang, Y.K.; Kim, S.Y.; Jang, S.J.; Moh, S.H.; Kim, D.H.; Shin, J.U. The Potential of Campanula takesimana Callus Extract to Enhance Skin Barrier Function. Int. J. Mol. Sci. 2023, 24, 17333. https://doi.org/10.3390/ijms242417333
Mok B, Jang YS, Moon JH, Moon S, Jang YK, Kim SY, Jang SJ, Moh SH, Kim DH, Shin JU. The Potential of Campanula takesimana Callus Extract to Enhance Skin Barrier Function. International Journal of Molecular Sciences. 2023; 24(24):17333. https://doi.org/10.3390/ijms242417333
Chicago/Turabian StyleMok, Boram, Young Su Jang, Ji Hwan Moon, Sujin Moon, Yun Kyung Jang, Soo Yun Kim, Sung Joo Jang, Sang Hyun Moh, Dong Hyun Kim, and Jung U Shin. 2023. "The Potential of Campanula takesimana Callus Extract to Enhance Skin Barrier Function" International Journal of Molecular Sciences 24, no. 24: 17333. https://doi.org/10.3390/ijms242417333
APA StyleMok, B., Jang, Y. S., Moon, J. H., Moon, S., Jang, Y. K., Kim, S. Y., Jang, S. J., Moh, S. H., Kim, D. H., & Shin, J. U. (2023). The Potential of Campanula takesimana Callus Extract to Enhance Skin Barrier Function. International Journal of Molecular Sciences, 24(24), 17333. https://doi.org/10.3390/ijms242417333





