The Functional Characterization of DzCYP72A12-4 Related to Diosgenin Biosynthesis and Drought Adaptability in Dioscorea zingiberensis
Abstract
1. Introduction
2. Results
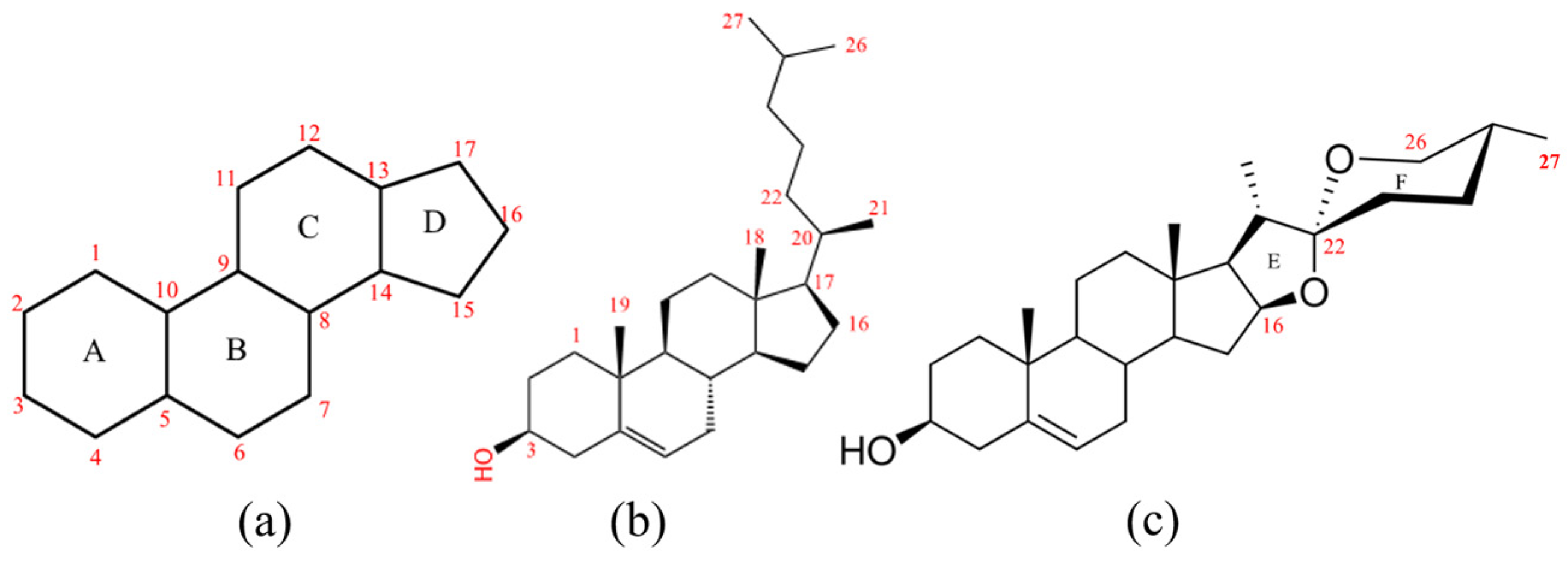
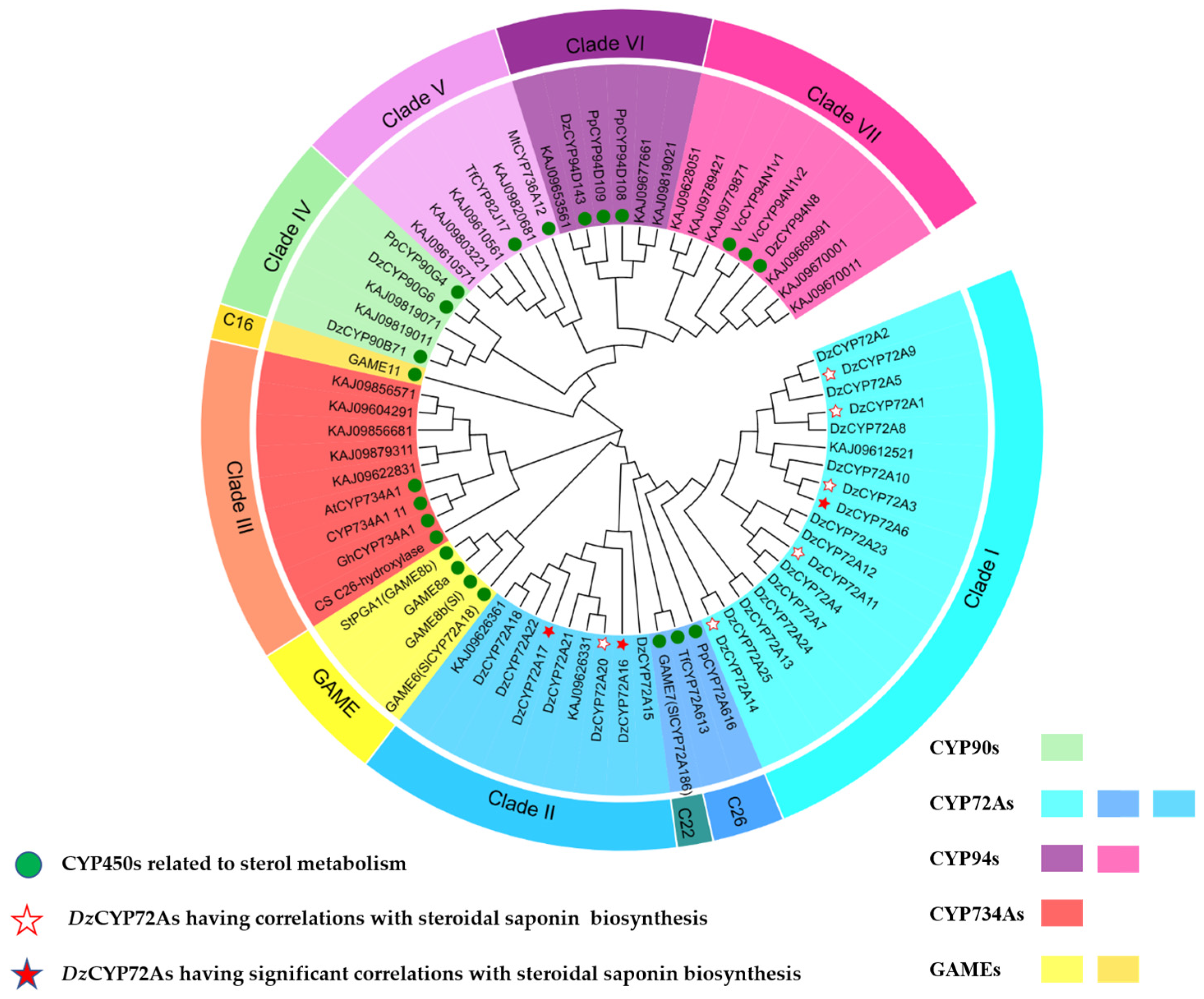
2.1. Sequence Analysis of the CYP72As in D. zingiberensis
2.2. Gene Clone and Sequence Analysis of DzCYP72A from D. zingiberensis
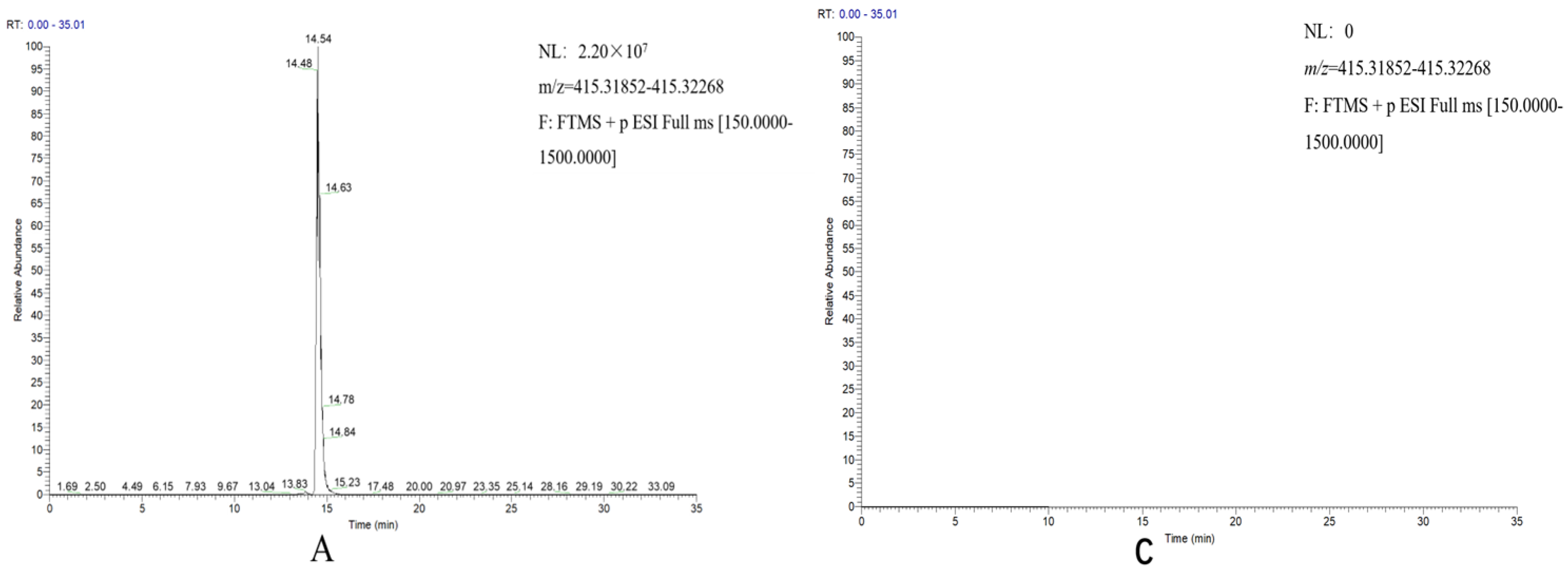
2.3. Reconstitution of the Diosgenin Heterogenous Biosynthesis Pathway and the Function Characterization of DzCYP72A12-4 in Tobacco
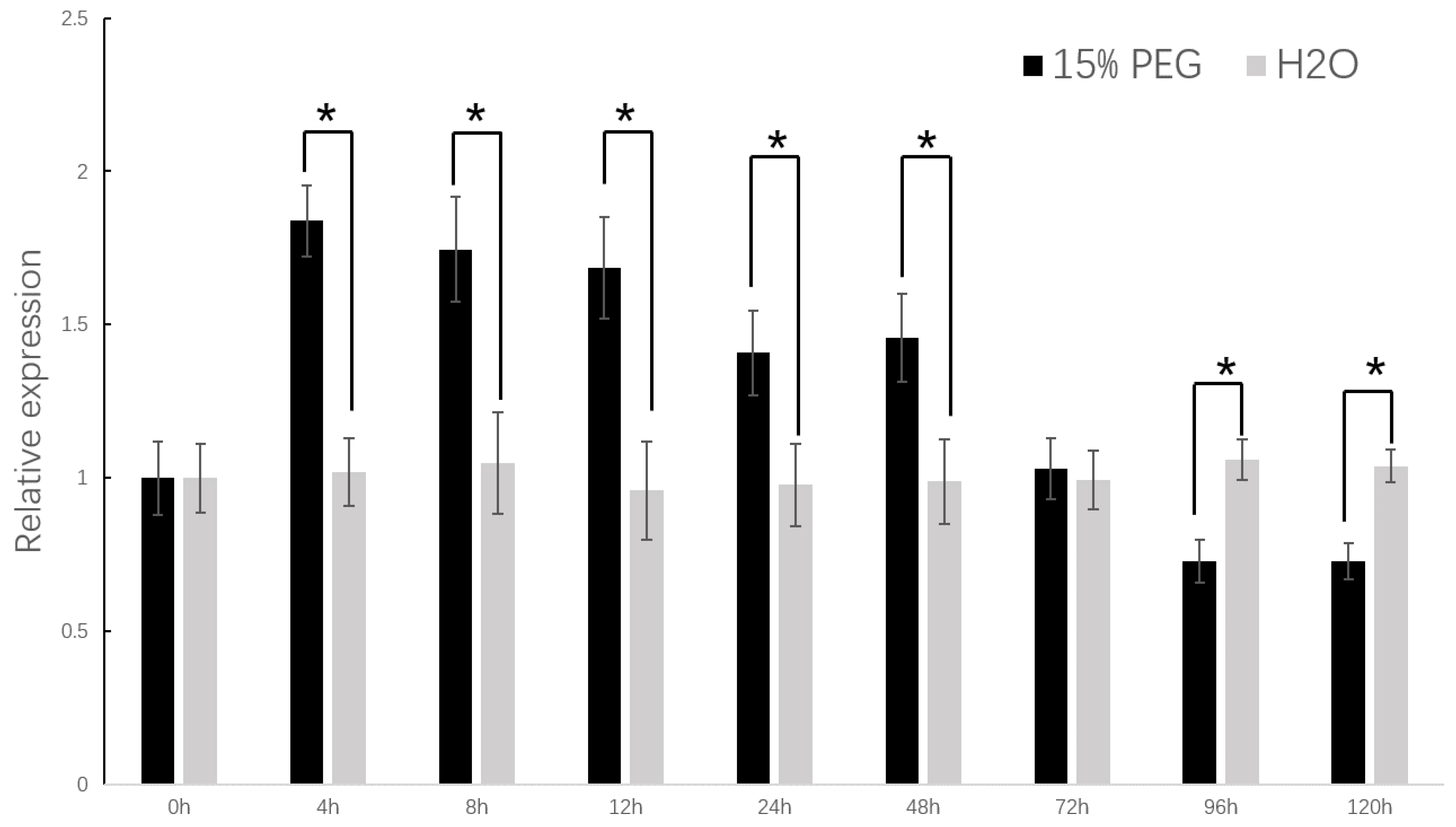
2.4. Drought Resistance Increased in the Transgenic Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Standards and Chemical Reagents
4.3. Bioinformatics Analysis of CYP72A Genes
- InterPro (http://www.ebi.ac.uk/interpro/search/sequence accessed on 15 November 2022) [37];
- SMART (http://smart.embl-heidelberg.de accessed on 15 November 2022) [38];
- NCBI Conserved Domain Database (https://www.ncbi.nlm.nih.gov/Structure/cdd accessed on 18 November 2022) [39].
4.4. Vector Construction and Agrobacterium Tumefaciens-Mediated Transformation
4.5. RNA Extraction and Gene Expression Analysis
4.6. Analysis of Diosgenin
4.7. Drought Stress Treatment in Transgenic Tobacco
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Zhang, Y.; Guo, Y.; Xue, P.; Xue, Z.; Zhang, Y.; Zhang, H.; Ito, Y.; Dou, J.; Guo, Z. Research progress of diosgenin extraction from Dioscorea zingiberensis C. H. Wright: Inspiration of novel method with environmental protection and efficient characteristics. Steroids 2023, 192, 109181. [Google Scholar] [CrossRef]
- Jesus, M.; Martins, A.P.J.; Gallardo, E.; Silvestre, S. Diosgenin: Recent Highlights on Pharmacology and Analytical Methodology. J. Anal. Methods Chem. 2016, 2016, 4156293. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Da¸stan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An Updated Pharmacological Review and Therapeutic Perspectives. Oxid. Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Warrier, S.; Merarchi, M.; Arfuso, F.; Kumar, A.P.; Bishayee, A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients 2018, 10, 645. [Google Scholar] [CrossRef]
- Arya, P.; Kumar, P. Diosgenin: An ingress towards solving puzzle for diabetes treatment. J. Food Biochem. 2022, 46, e14390. [Google Scholar] [CrossRef] [PubMed]
- Adomeniene, A.; Venskutonis, P.R. Dioscorea spp.: Comprehensive Review of Antioxidant Properties and Their Relation to Phytochemicals and Health Benefits. Molecules 2022, 27, 2530. [Google Scholar] [CrossRef] [PubMed]
- Zhizun, D. Review and prospect of exploitation and utilization of raw materials of natural steroid drugs. Chin. Tradit. Herb. Drugs 1985, 16, 28–36. (In Chinese) [Google Scholar]
- Raymond, D.; Bennett, E.H. Biosynthesis of Dioscorea sapogenins from cholesterol. Phytochemistry 1965, 4, 577–586. [Google Scholar]
- Waldemar, E. Incorporation of [4-(14)C] cholesterol into steryl derivatives and saponins of oat (Avena sativa L.) plants. Plant Cell Rep. 1982, 6, 253–256. [Google Scholar]
- Christ, B.; Xu, C.; Xu, M.; Li, F.S.; Wada, N.; Mitchell, A.J.; Han, X.L.; Wen, M.L.; Fujita, M.; Weng, J.K. Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants. Nat. Commun. 2019, 10, 3206. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Tian, J.; Wu, Y.; An, F.; Li, C.; Zhang, Y. 22R- but not 22S-hydroxycholesterol is recruited for diosgenin biosynthesis. Plant J. 2022, 109, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Sonawane, P.D.; Pollier, J.; Panda, S.; Szymanski, J.; Massalha, H.; Yona, M.; Unger, T.; Malitsky, S.; Arendt, P.; Pauwels, L.; et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 2016, 3, 16205. [Google Scholar] [CrossRef] [PubMed]
- Sawai, S.; Ohyama, K.; Yasumoto, S.; Seki, H.; Sakuma, T.; Yamamoto, T.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Aoki, T.; et al. Sterol Side Chain Reductase 2 Is a Key Enzyme in the Biosynthesis of Cholesterol, the Common Precursor of Toxic Steroidal Glycoalkaloids in Potato. Plant Cell 2014, 26, 3763–3774. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Suzuki, M.; Kikuchi, J.; Saito, K.; Muranaka, T. Dual biosynthetic pathways to phytosterol via cycloartenol and lanosterol in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 725–730. [Google Scholar] [CrossRef]
- Itkin, M.; Heinig, U.; Tzfadia, O.; Bhide, A.J.; Shinde, B.; Cardenas, P.D.; Bocobza, S.E.; Unger, T.; Malitsky, S.; Finkers, R.; et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 2013, 341, 175–179. [Google Scholar] [CrossRef]
- Cardenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism. Phytochemistry 2015, 113, 24–32. [Google Scholar] [CrossRef]
- Ye, T.; Song, W.; Zhang, J.J.; An, M.; Feng, S.; Yan, S.; Li, J. Identification and functional characterization of DzS3GT, a cytoplasmic glycosyltransferase catalyzing biosynthesis of diosgenin 3-O-glucoside in Dioscorea zingiberensis. Plant Cell Tissue Organ Cult. 2017, 129, 399–410. [Google Scholar] [CrossRef]
- Louveau, T.; Osbourn, A. The Sweet Side of Plant-Specialized Metabolism. Cold Spring Harb. Perspect. Biol. 2019, 11, a034744. [Google Scholar] [CrossRef]
- Xu, L.; Wang, D.; Chen, J.; Li, B.; Li, Q.; Liu, P.; Qin, Y.; Dai, Z.; Fan, F.; Zhang, X. Metabolic engineering of Saccharomyces cerevisiae for gram-scale diosgenin production. Metab. Eng. 2022, 70, 115–128. [Google Scholar] [CrossRef]
- Yin, X.; Liu, J.; Kou, C.; Lu, J.; Zhang, H.; Song, W.; Li, Y.; Xue, Z.; Hua, X. Deciphering the network of cholesterol biosynthesis in Paris polyphylla laid a base for efficient diosgenin production in plant chassis. Metab. Eng. 2023, 76, 232–246. [Google Scholar] [CrossRef]
- Tal, B.; Tamir, I.; Rokem, J.S.; Goldberg, I. Isolation and characterization of an intermediate steroid metabolite in diosgenin biosynthesis in suspension cultures of Dioscorea deltoidea cells. Biochem. J. 1984, 219, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Dilkes, B.P.; Fujioka, S.; Takatsuto, S.; Sakurai, A.; Feldmann, K.A. The DWF4 Gene of Arabidopsis Encodes a Cytochrome P450 That Mediates Multiple 22-Hydroxylation Steps in Brassinosteroid Biosynthesis. Plant Cell 1998, 10, 231–243. [Google Scholar] [PubMed]
- Ohnishi, T.; Yokota, T.; Mizutani, M. Insights into the function and evolution of P450s in plant steroid metabolism. Phytochemistry 2009, 70, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, C.; Li, Z.; Guo, J.; Li, S.; Chen, X.; Wang, C.; Dai, X.; Yang, H.; Song, W.; et al. The genome of Dioscorea zingiberensis sheds light on the biosynthesis, origin and evolution of the medicinally important diosgenin saponins. Hortic. Res. 2022, 9, uhac165. [Google Scholar] [CrossRef]
- Hou, L.; Yuan, X.; Li, S.; Li, Y.; Li, Z.; Li, J. Genome-Wide Identification of CYP72A Gene Family and Expression Patterns Related to Jasmonic Acid Treatment and Steroidal Saponin Accumulation in Dioscorea zingiberensis. Int. J. Mol. Sci. 2021, 22, 10953. [Google Scholar] [CrossRef]
- Turk, E.M.; Fujioka, S.; Seto, H.; Shimada, Y.; Takatsuto, S.; Yoshida, S.; Wang, H.; Torres, Q.I.; Ward, J.M.; Murthy, G.; et al. BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. Plant J. 2005, 42, 23–34. [Google Scholar] [CrossRef]
- Seeman, J.I. Russell Earl Marker and the Beginning of the Steroidal Pharmaceutical Industry. Chem. Rec. 2023, 23, e202300048. [Google Scholar] [CrossRef]
- Ren, Q.L.; Wang, Q.; Zhang, X.Q.; Wang, M.; Hu, H.; Tang, J.J.; Yang, X.T.; Ran, Y.H.; Liu, H.H.; Song, Z.X.; et al. Anticancer Activity of Diosgenin and Its Molecular Mechanism. Chin. J. Integr. Med. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, S.; Tao, S.; Hou, G.; Zhao, F.; Tan, S.; Meng, Q. Dioscorea spp.: Bioactive Compounds and Potential for the Treatment of Inflammatory and Metabolic Diseases. Molecules 2023, 28, 2878. [Google Scholar] [CrossRef]
- Sun, F.; Yang, X.; Ma, C.; Zhang, S.; Yu, L.; Lu, H.; Yin, G.; Liang, P.; Feng, Y.; Zhang, F. The Effects of Diosgenin on Hypolipidemia and Its Underlying Mechanism: A Review. Diabetes Metab. Syndr. Obes. 2021, 14, 4015–4030. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Jiang, J.G. Effects of diosgenin and its derivatives on atherosclerosis. Food Funct. 2019, 10, 7022–7036. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Qu, L.; Chen, Y.; Yuan, C.; Qin, A.; Liang, J.; Huang, Q.; Jiang, M.; Zou, W. Dioscin and diosgenin: Insights into their potential protective effects in cardiac diseases. J. Ethnopharmacol. 2021, 274, 114018. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook, 1st ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Pagès, H.; Aboyoun, P.; Gentleman, R.; DebRoy, S. Biostrings: Efficient Manipulation of Biological Strings, R Package, Version 2.66.0; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.; Bileschi, M.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2022, 51, D418–D427. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hou, L.; Li, S.; Li, J. The Functional Characterization of DzCYP72A12-4 Related to Diosgenin Biosynthesis and Drought Adaptability in Dioscorea zingiberensis. Int. J. Mol. Sci. 2023, 24, 8430. https://doi.org/10.3390/ijms24098430
Wang W, Hou L, Li S, Li J. The Functional Characterization of DzCYP72A12-4 Related to Diosgenin Biosynthesis and Drought Adaptability in Dioscorea zingiberensis. International Journal of Molecular Sciences. 2023; 24(9):8430. https://doi.org/10.3390/ijms24098430
Chicago/Turabian StyleWang, Weipeng, Lixiu Hou, Song Li, and Jiaru Li. 2023. "The Functional Characterization of DzCYP72A12-4 Related to Diosgenin Biosynthesis and Drought Adaptability in Dioscorea zingiberensis" International Journal of Molecular Sciences 24, no. 9: 8430. https://doi.org/10.3390/ijms24098430
APA StyleWang, W., Hou, L., Li, S., & Li, J. (2023). The Functional Characterization of DzCYP72A12-4 Related to Diosgenin Biosynthesis and Drought Adaptability in Dioscorea zingiberensis. International Journal of Molecular Sciences, 24(9), 8430. https://doi.org/10.3390/ijms24098430





