Abstract
The biosynthesis of C27–29 sterols from their C30 precursor squalene involves C24-alkylation and the removal of three methyl groups, including two at the C4 position. The two C4 demethylation reactions require a bifunctional enzyme known as 3β-hydroxysteroid dehydrogenase/C4-decarboxylase (3βHSD/D), which removes an oxidized methyl (carboxylic) group at C4 while simultaneously catalyzing the 3β-hydroxyl→3-keto oxidation. Its loss-of-function mutations cause ergosterol-dependent growth in yeast and congenital hemidysplasia with ichthyosiform erythroderma and limb defect (CHILD) syndrome in humans. Although plant 3βHSD/D enzymes were well studied enzymatically, their developmental functions remain unknown. Here we employed a CRISPR/Cas9-based genome-editing approach to generate knockout mutants for two Arabidopsis 3βHSD/D genes, HSD1 and HSD2, and discovered the male gametophytic lethality for the hsd1 hsd2 double mutation. Pollen-specific expression of HSD2 in the heterozygous hsd1 hsd2/+ mutant not only rescued the pollen lethality but also revealed the critical roles of the two HSD genes in embryogenesis. Our study thus demonstrated the essential functions of the two Arabidopsis 3βHSD/D genes in male gametogenesis and embryogenesis.
1. Introduction
Sterols are isoprenoid-derived lipids that have important physiological activities for all eukaryotic organisms, especially for plants that synthesize a wide variety of terpenoids including carotenoids, dolichols, tocopherols, chlorophylls, and squalene [1]. Sterols are significant components of eukaryotic cell membranes, which not only regulate the permeability and fluidity of membranes, the activity of membrane binding proteins, and the integrity of various cellular membranes but also participate in many membrane-related metabolic processes and vesicle transport [2,3]. In addition, sterols are biosynthetic precursors of various steroid hormones, including mammalian androgens and estrogens, insect ecdysteroids, fungal antheridiol and oogoniol, and plant brassinosteroids (BRs) [4]. Some sterols and sterol biosynthesis intermediates also function as signaling molecules to regulate transcription and other cellular activities [5].
In the sterol biosynthesis pathways of plants, animals, and fungi, sterols only become functional after the sequential removal of two methyl groups at the C4 position and one methyl group at the C14 position. Therefore, the C4 demethylation of sterol intermediates is a critical step in sterol biosynthesis. This step is catalyzed by the sterol C4 demethylase complex (SC4DM), which is highly conserved from fungi to plants to humans [6,7,8]. SC4DM is composed of sterol C4 methyl oxidase (SMO), 3βHSD/D, and 3-keto steroid reductase (3KSR). These individual enzymes are connected by ergosterol biosynthesis protein 28 (ERG28), which acts as a scaffold to anchor the SC4DM components to the endoplasmic reticulum (ER) membrane [9,10]. In animals and yeast, the two C4 methyl groups are sequentially removed early in the sterol biosynthetic pathway right after the C14-demethylation step. In higher plants, the first C4 methyl group is removed early in the cyclopropyl sterol precursor, and the second C4 methyl group is removed only after the C14 demethylation. Therefore, the demethylation sequence in animals and yeast is C14, C4, and C4, while in plants, it is C4, C14, and C4. The separation of the two C4-demethylation steps by the C14-demethylation might allow plants to produce unique 4-methylated sterols important for plant terrestrialization and plant stress tolerance [7,11]. Despite the difference in the order of the three demethylation reactions, the enzymatic reactions are identical. The C4 demethylation is initiated with SMO, which oxidizes a C4-methyl to a carboxyl group. Then, the 3β-hydroxyl group on the A-ring is oxidized to a ketone group by 3βHSD/D, which also catalyzes the C4 decarboxylation. Finally, the 3-keto group is reduced back to the 3β hydroxyl group by 3KSR [9,12].
Many studies have revealed the importance of the C4 demethylation reactions in various organisms. In animals, defects in the C4 demethylation process may affect the synthesis of all types of steroid hormones and lead to embryonic lethality [13]. Similarly, yeast mutants defective in the C4 demethylation process are lethal and are therefore absolutely relying on exogenously supplied ergosterols for growth [14]. In Arabidopsis, loss-of-function mutations in SMO, which are encoded by two distinct gene families, SMO1 (with three members known as SMO1-1, SMO1-2, and SMO1-3) and SMO2 (containing two members, SMO2-1 and SMO2-2), resulted in stunted growth in shoots and roots (weak alleles) and embryo lethality (strong alleles) [15,16]. However, it remains unknown whether the 3βHSD/Ds are essential for plant development despite the fact that they were the first components of the plant SC4DM complex to be characterized. Rahier et al. [17] identified two Arabidopsis proteins, At3βHSD/D1 (At1g47290) and At3βHSD/D2 (At2g26260), which exhibit high sequence similarity to the yeast ERG26 and mammalian 3βHSDs catalyzing the 3β-OH to 3-oxo conversion. Detailed biochemical studies coupled with molecular modeling not only revealed that these two enzymes only accept sterol substrates with 3β-hydroxyl and C4 carboxyl groups but also identified their key catalytic residues [17,18]. Virus-induced gene silencing of a tobacco (Nicotiana benthamiana) homolog of the two Arabidopsis 3βHSD/D genes resulted in reduced leaf growth [17]. Interestingly, although the simultaneous elimination of the two Arabidopsis genes had little impact on plant growth, their overexpression led to observable growth defects such as shorter inflorescent internodes with clustered siliques and unequal leaf expansion [19]. These relatively weak phenotypes were quite different than what was observed with the Arabidopsis mutants lacking the two SMOs. This could be explained by the presence of two additional homologs of 3βHSD/D in Arabidopsis [19] or by the possibility of the reported T-DNA double insertional mutant of At3βHSD/D1 and At3βHSD/D2 not being a true double knockout mutant due to the intron location of T-DNA in the analyzed mutant of At3βHSD/D2.
To investigate the physiological functions of the two reported Arabidopsis At3βHSD/D enzymes (renamed here HSD1 (At1g47290) and HSD2 (At2g26260) for simplicity), we employed a CRISPR/Cas9-based genome editing approach to create a true double knockout mutant. We discovered that while single mutants of HSD1 and HSD2 had no detectable effect on plant growth, we were unable to obtain an hsd1 hsd2 double mutant. Reciprocal genetic crosses of the two heterozygous double mutants, hsd1 hsd2/+ or hsd1/+ hsd2, with the wild-type control revealed lethality of the double mutant pollens, which could be fully rescued via pollen-specific expression of the HSD2 gene driven by a widely used pollen-specific promoter pLAT52 [20]. Our phenotypic analyses of the resulting pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic mutants also revealed the essential function of the two HSDs in embryogenesis. Taken together, our study concluded that HSD1 and HSD2 are essential for the plant reproductive development in Arabidopsis.
2. Results
2.1. Generation of hsd1 and hsd2 Mutants via CRISPR/Cas9-Based Genome Editing
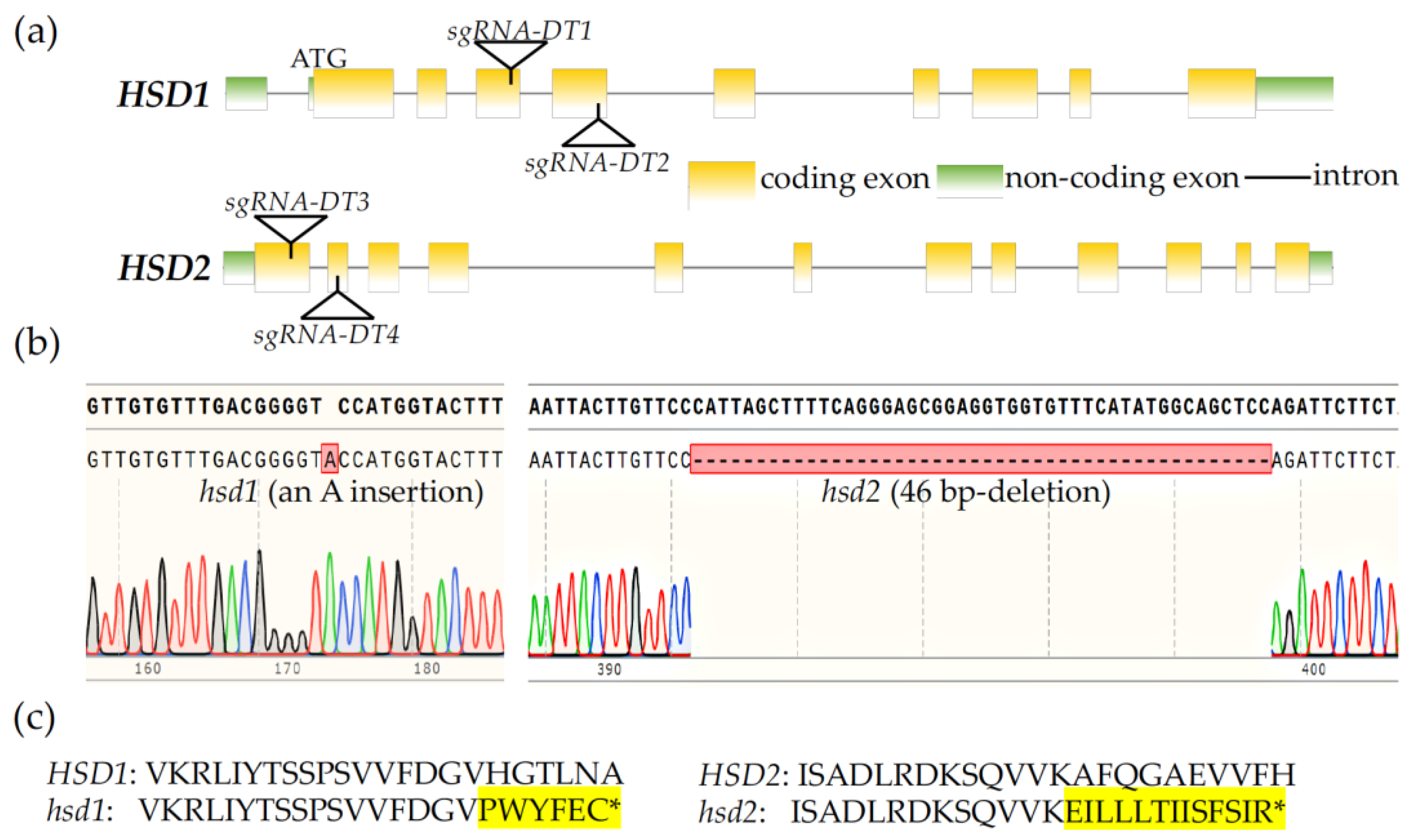
An earlier study that investigated the physiological functions of HSD1 and HSD2 used an hsd1 hsd2 double mutant carrying a T-DNA insertion in each gene but observed no phenotypic change compared to its corresponding wild-type control [19]. However, we suspected that the reported hsd1 hsd2 double mutant might not be a true double knockout mutant because one of the T-DNAs was actually inserted into the seventh intron of the HSD2 gene [19]. In order to analyze the biological importance of these two HSD genes, we decided to create a true hsd1 hsd2 double knockout mutant using the CRISPR/Cas9-mediated genome editing approach. We first generated a CRISPR/Cas9-HSD1/2 transgene carrying four target DNA segments, designated as sgRNA-DT1, sgRNA-DT2, sgRNA-DT3, and sgRNA-DT4, with the first two targeting HSD1 and the last two for HSD2 (Figure 1a) and transformed this CRISPR/Cas9-HSD1/2 transgene into the wild-type Arabidopsis plants. The T1 transgenic lines were screened for the presence of an antibiotic-resistant gene and their self-pollinated T2 offspring were examined by PCR for CRISPR/Cas9 transgene-free plants carrying mutations of the two target HSD genes. The candidate hsd1/hsd2 mutants were subsequently verified by sequencing of PCR-amplified HSD1/HSD2 genomic fragments. Sequencing analysis revealed a single adenine nucleotide insertion at position 423 bp downstream of the initiation codon of the HSD1 mRNA in the hsd1 mutant line. This insertion is in the third exon, likely causing a frameshift and premature translational termination of the HSD1 mRNA (Figure 1b,c). Our DNA sequence analysis found that the hsd2 mutant carries a 46-bp deletion in the HSD2 gene, with the missing nucleotides located in the second exon. Such a deletion also causes a frameshift mutation and premature translational termination of the HSD2 transcript (Figure 1b,c).
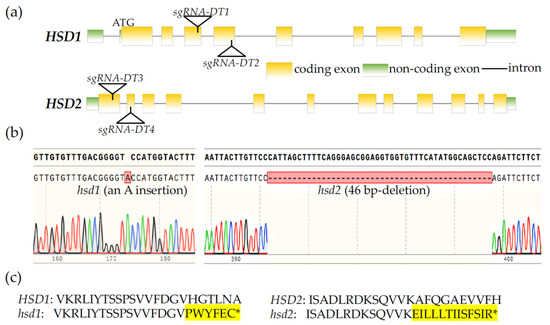
Figure 1.
Generation of hsd1 hsd2 mutants by the CRISPR/Cas9 genome editing approach. (a) Genomic location of guide DNA sequences (sgRNA-DT1, sgRNA-DT2, sgRNA-DT3, and sgRNA-DT4) targeting HSD1 and HSD2. (b) Sequencing chromatograms of the CRISPR/Cas9-mediated genome editing of the HSD1 and HSD2 genes. (c) Amino acid sequences of the hsd1 and hsd2 mutants near the detected hsd1 and hsd2 mutation sites, respectively. * denotes the premature stop codon due to frameshift of the predicted mutant transcripts of HSD1 and HSD2, while the highlighted amino acids are predicted extra amino acids from the mutant HSD1/HSD2 transcripts.
PCR-based genotyping of the T3 generation identified hsd1 and hsd2 single mutants, transheterozygous double mutants (hsd1/+ hsd2/+), and two heterozygous double mutants (hsd1 hsd2/+ and hsd1/+ hsd2). However, no homozygous hsd1 hsd2 mutant was obtained. Phenotypic analyses of these mutants revealed no growth defects in hsd1, hsd2, hsd1 hsd2/+, or hsd1/+ hsd2 mutant at the seedling and maturation stages, most likely due to their functional redundancy (Figure 2a,b). These results were consistent with the earlier report that mutations in the Arabidopsis HSD1 and/or HSD2 genes had little impact on vegetative growth [19]. However, our failure to obtain an hsd1 hsd2 double homozygous mutant suggested that the two HSD genes have essential redundant functions in gametogenesis and/or embryogenesis.

Figure 2.
Phenotypic comparison of the single and heterozygous mutants of HSD1 and HSD2 genes and their wild-type control. (a) Photographs of 14-day-old soil-grown Arabidopsis plants. (b) Photographs of 35-day-old soil-grown mature Arabidopsis plants. In (a,b), scale bar = 1 cm.
2.2. The hsd1 hsd2 Double Mutation Impairs Development of Male Gametophytes
In order to determine the genetic basis for our failed attempts to obtain a single hsd1 hsd2 double mutant, we analyzed the segregation ratio of the self-fertilized progeny of hsd1/+ hsd2 and hsd1 hsd2/+ heterozygous double mutants. As shown in Table 1, both mutants exhibited a segregation ratio of ~1:1 of single mutant:heterozygous double mutant (93:85 for hsd1/+ hsd2 and 103:97 for hsd1 hsd2/+), which deviated from the normal Mendelian segregation ratio of 1:2:1 of single:heterozygous double:homozygous double mutants. These results suggest that the hsd1 hsd2 double mutation likely causes gametophytic defect(s). To determine whether the observed gametophytic defect of the hsd1 hsd2 double mutation was associated with the male or female gametes, or both, we made reciprocal genetic crosses of the hsd1/+ hsd2 and hsd1 hsd2/+ mutants with the wild-type plants. When the two mutants were used as the female recipients, the resulting F1 offspring had a ratio of double heterozygous vs. single heterozygous mutants very close to 1 (1.04 for the WT ♂ × hsd1/+ hsd2 ♀ cross and 0.96 for the WT ♂ × hsd1 hsd2/+ ♀ cross) (Table 2), indicating that the female gametes of the two mutants were transmitted normally. By contrast, when the wild-type plants were pollinated with the pollens of the two heterozygous double mutants, the F1 offspring produced only single heterozygous mutants but no double heterozygous mutant, revealing the male gametophytic defect of the hsd1 hsd2 pollens. In comparison, the genetic crosses using pollens of the single hsd1 or hsd2 mutant to pollinate the wild-type flowers produced all single heterozygous plants, indicating neither hsd1 nor hsd2 male gametes had a gametophytic defect. Together, these results revealed a serious male gametophytic defect of the hsd1 hsd2 double mutation.

Table 1.
Genetic analysis of hsd1/+ hsd2 and hsd1 hsd2/+ mutants.

Table 2.
Reciprocal cross-pollination of hsd1/+ hsd2 and hsd1 hsd2/+ mutants.
2.3. The hsd1 hsd2 Double Mutation in Heterozygosity Does Not Affect Flower Formation
The reciprocal cross experiments of the two heterozygous double mutants, hsd1/+ hsd2 and hsd1 hsd2/+, with the wild-type plants revealed that the hsd1 hsd2 double mutation had severe male gametophyte defect(s) (Table 2). Therefore, we were interested in determining whether or not the hsd1 or/and hsd2 mutation had any detectable impact on the flower development. As shown in Figure 3, the flowers of all of the analyzed mutants were similar to those of wild-type plants in terms of the overall flower size, the number of individual floral organs, and the number of flowers per plant. Careful inspection of the flowers of the mutant plants detected no obvious abnormality of various floral organs. Furthermore, their anthers were able to naturally release pollens, and the stamens extended normally to allow the released pollens to come into contact with the stigma, leading to normal silique and seed development.

Figure 3.
Phenotypes of flowers in the flattening phase of wild-type and mutants. Scale bar = 1 mm.
2.4. The hsd1 hsd2 Double Mutation Causes the Male Pollen Lethality
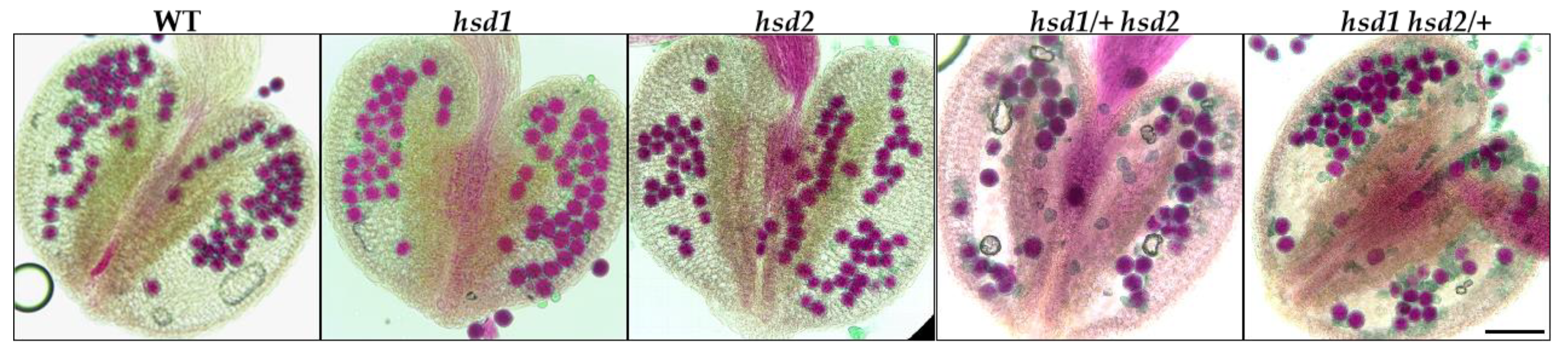
The male gametophytic dysfunction is often caused by defective pollen development. To examine whether or not the pollen viability of the hsd1/+ hsd2 and hsd1 hsd2/+ mutants was affected, we stained mature anthers from the hsd1, hsd2, hsd1/+ hsd2, and hsd1 hsd2/+ mutants and their wild-type control with Alexander’s stain, which colors aborted pollen grains “blue-green” while staining viable pollen grains “magenta-red” [21]. As shown in Figure 4, the pollens from the wild-type and the two single mutants were stained purple-red, whereas the anthers of the hsd1/+ hsd2 and hsd1 hsd2/+ mutants contained both normal purple-red-colored and deformed blue-green stained pollen grains (Figure 4). This dye-based pollen viability test strongly suggested that the hsd1 hsd2 double mutation likely caused pollen lethality, explaining the failure to obtain the hsd2-containing F1 offspring and the hsd1-carrying F1 offspring from the hsd1 hsd2/+ ♂ × wild-type ♀ and hsd1/+ hsd2 ♂ × wild-type ♀ crosses, respectively.
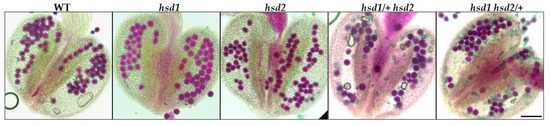
Figure 4.
The hsd1 hsd2 double mutation results in abnormal pollen development. Alexander’s staining of pollen grains in the anthers of wild-type Arabidopsis plants and indicated hsd1/hsd2 mutants. Scale bar = 90 μm.
2.5. The Male Gametophytic Defect of the hsd1 hsd2/+ Mutant Was Complemented by the Pollen-Specific Expression of the HSD2 Gene
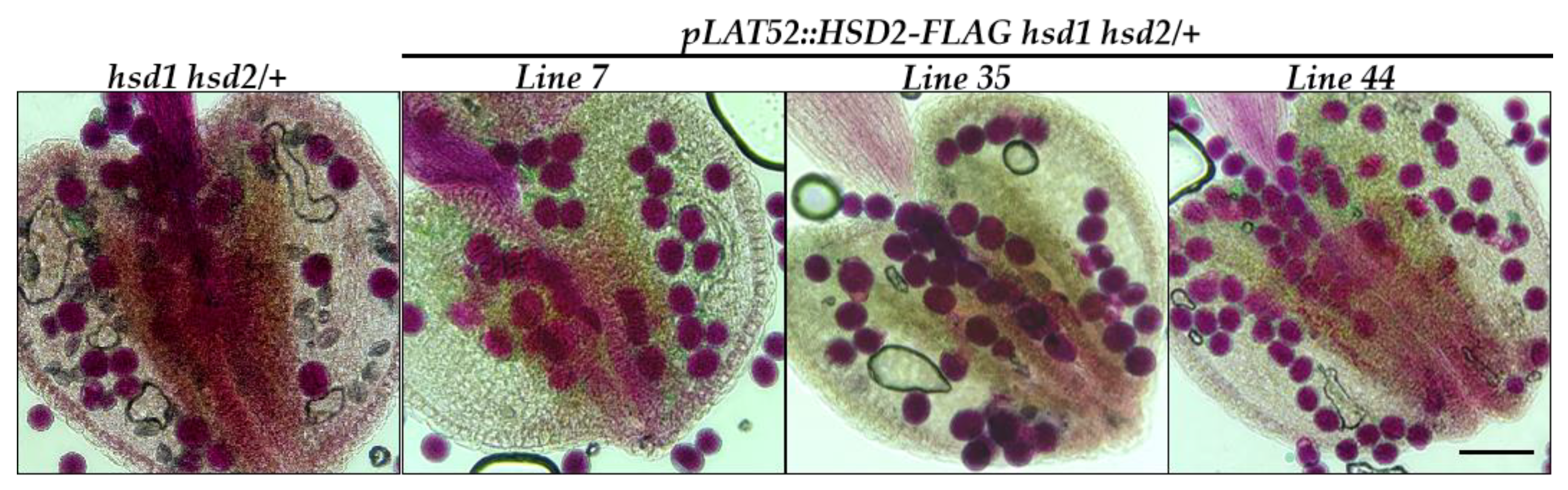
To verify that the observed pollen defect in the hsd1 hsd2/+ mutant was indeed caused by the hsd1 hsd2 double mutation rather than by an unknown CRISPR/Cas9-created off-target mutation, we created a pLAT52::HSD2-FLAG transgene, containing the pLAT52 promoter, one of the best studied pollen-specific promoter from the tomato gene LAT52 [20], the entire coding sequence of the Arabidopsis HSD2 gene, and the coding sequence of the widely used FLAG epitope tag, and transformed the resulting transgene into the hsd1 hsd2/+ mutant. PCR-based genotyping of the resulting T1 transgenic plants identified several pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic mutants. Their anthers were collected and subsequently stained with Alexander’s dye. As shown in Figure 5, almost all pollens of the analyzed transgenic lines were stained “magenta-red”, indicating that the pollen lethality was fully rescued by the pLAT52::HSD2-FLAG transgene.
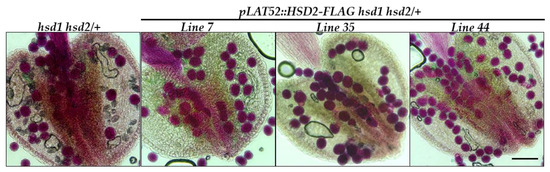
Figure 5.
The pLAT52::HSD2-FLAG transgene rescued the pollen defect of the hsd1 hsd2/+ mutant. Shown above are Alexander’s staining of pollen grains from several independent pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic plants and their parental hsd1 hsd2/+ mutant. Scale bar = 90 μm.
2.6. The hsd1 hsd2 Double Mutation Likely Caused the Embryo Lethality
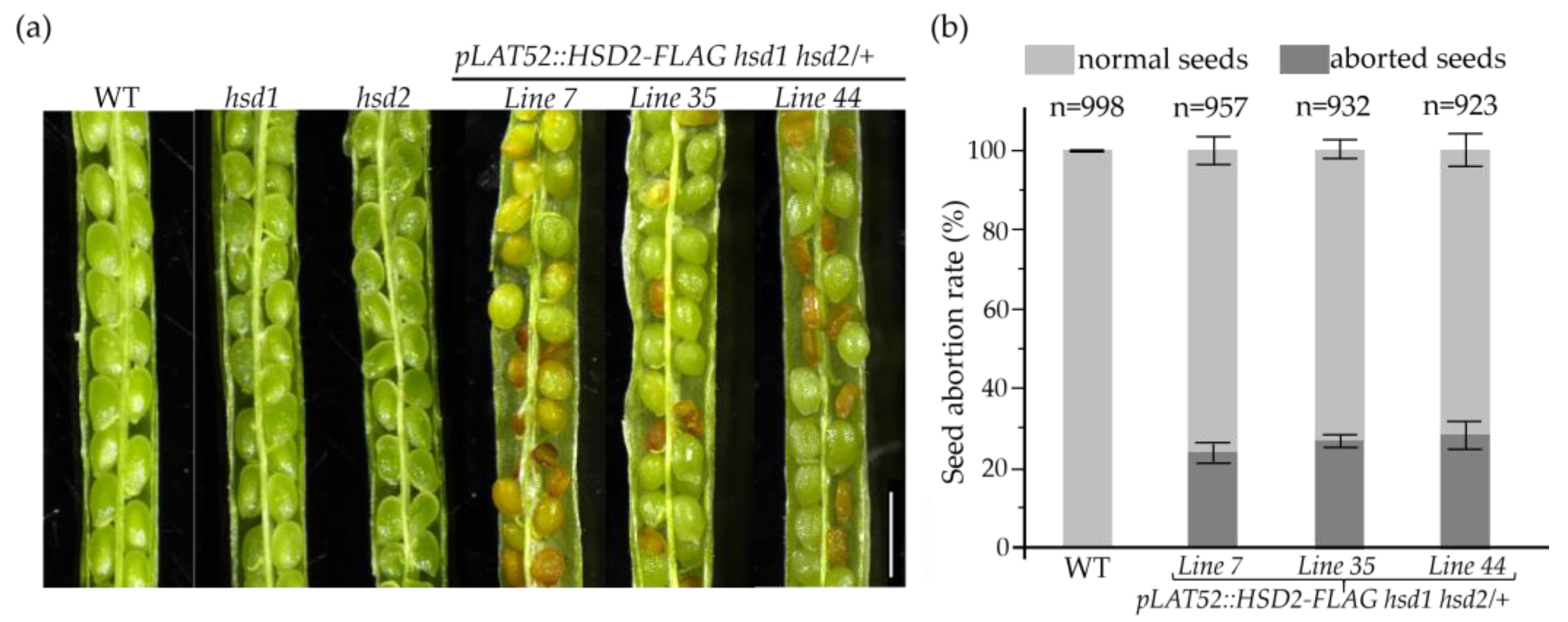
Our PCR-based screening of the pLAT52::HSD2-FLAG transgenic lines failed to identify a single pLAT52::HSD2-FLAG hsd1 hsd2 transgenic double mutant, despite that the introduced transgene was able to successfully rescue the pollen lethality phenotype (Figure 5). We suspected that the hsd1 hsd2 double mutation might also cause embryo lethality, a phenotype that was previously reported in Arabidopsis mutants lacking the two SMOs [15,16], which act upstream of the 3βHSD/D enzyme in demethylating the two C4 methyl groups. Indeed, when we examined green mature siliques of several independent pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic lines, and discovered that while most seeds were healthy and green, approximately 25% of the seeds were small, shrunken, and brown-colored (Figure 6a,b). After maturation, almost all of the normal-looking seeds were germinated, whereas those small/shrunken seeds were not. Together, these experiments strongly suggested that the hsd1 hsd2 double mutation likely caused embryo lethality, and the pollen-specific pLAT52::HSD2-FLAG transgene was not able to complement the seed/embryo defects.
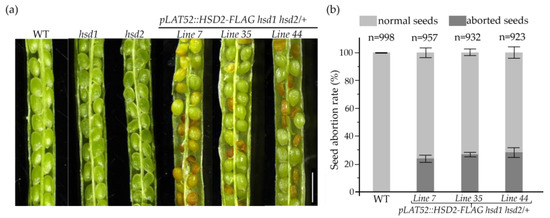
Figure 6.
The hsd1 hsd2 double mutation causes embryo lethality. (a) Photographs of opened mature siliques from the wild-type, two single hsd mutants, and three independent pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic plants. Scale bar = 1 mm. (b) A bar graph presentation of the seed abortion rates of the three pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic plants and their wild-type control. The numbers of seeds analyzed with standard errors are also shown on the graph.
3. Discussion
3.1. The CRISPR/Cas9-Genome Editing Failed to Create a True hsd1 hsd2 Double Mutant
The Arabidopsis genome contains at least two genes encoding bifunctional 3βHSD/D enzymes, HSD1(At1g47290) and HSD2(At2g26260), which catalyze two separate C4 demethylation reactions in phytosterol biosynthesis [17]. It was claimed that they were the first plant hydroxysteroid dehydrogenases to be molecularly characterized and biochemically investigated [17], yet their physiological functions remain unknown. An earlier study using a presumed Arabidopsis double mutant carrying T-DNA insertions in these two genes did not detect any development or growth defect [19]. This could be caused by the functional redundancy between the two 3βHSD/D enzymes and their homologs. An earlier sequence analysis revealed two additional Arabidopsis proteins exhibiting sequence similarity with the two Arabidopsis HSD enzymes: At2g43420 and At2g33630 [19]. At2g43420 is also known as RETICULON 20 (RTN20), a member of the Arabidopsis RTN family that was thought to be involved in the formation of the ER tubules [22] and was recently implicated in sterol/lipid biosynthesis [23]. At2g33630 is also known as the Arabidopsis member of the short-chain alcohol dehydrogenase/reductase (SRD) subfamily 42E [24]. Importantly, a recent study revealed the role of the human SDR42E1 in cholesterol biosynthesis [25], thus suggesting that At2g33630 could also be involved in plant sterol biosynthesis.
Alternatively, the lack of growth defects of the reported hsd1 hsd2 double mutant could be caused by a weak impact of the T-DNA insertion on the HSD2 gene. The reported hsd2 mutant carried a T-DNA insertion in the seventh intron of the annotated HSD2 gene and no data were shown in that study on the impact of the intron-localized T-DNA on the HSD2 transcript [19]. It is quite possible that such an intron-inserted T-DNA could be spliced out by the Arabidopsis splicing machinery, which was known to occur in approximately 4% of analyzed intronic T-DNA inserts [26]. In this study, we used the CRISPR/Cas9-based genome editing technology to generate mutations in the two HSD genes with an A nucleotide insertion in the HSD1 gene and a 46-nucleotide deletion in the HSD2 gene. Both mutations caused a frameshift in the open-reading frame of the resulting hsd1/hsd2 transcripts and consequential early translational termination (Figure 1b,c), thus likely being null mutations. Surprisingly, despite screening hundreds of T3/T4 offspring and identifying many hsd1/+ hsd2 and hsd1 hsd2/+ heterozygous double mutants, we were unable to obtain a single hsd1 hsd2 double homozygous mutant. As expected, no detectable phenotypic change was seen in the hsd1 and hsd2 single mutant or the two heterozygous double mutants (Figure 2).
In recent years, the analysis of sterol biosynthesis defective mutants has revealed the functional significance of sterols in many plant developmental processes. The loss-of-function mutants of Arabidopsis sterol methyltransferase 1 (SMT1), which catalyzes the first S-adenosylmethionine-dependent C24 alkylation [27], exhibited pleiotropic defects, including shorter petioles, smaller and rounder leaves, delayed silique development, as well as abnormal embryonic development [27]. The Arabidopsis mutant defective in the cyclopropylsterol isomerase (CPI1), a plant-specific sterol biosynthetic enzyme converting the pentacyclic cyclopropyl sterols to tetracyclic sterols, was a dwarf mutant with small rounder and dark-green leaves, short hypocotyl, stunted roots, and defective gravity response [28]. Other sterol mutants, such as cyp51a2 (sterol C14-demethylase), fackel/hydra2 (sterol C14 reductase), and hydra1 (sterol Δ8-Δ7 isomerase), all had defects in post-embryogenesis growth, exhibiting poorly developed hypocotyls, fused cotyledons, shortened roots, and seedling lethality [29,30,31,32]. Mutations in the enzymes involved in the downstream steps of the plant sterol biosynthetic pathway often cause phenotypes similar to those observed in mutants defective in BR biosynthesis, including dwarfism and reduced fertility, which could be rescued by exogenous BR applications. Our failure to obtain a homozygous hsd1 hsd2 double mutant impeded our exploration of the significance of the two Arabidopsis 3βHSD/D enzymes in post-embryogenesis, as well as the functional redundancy of the two HSDs and other 3βHSD-like enzymes in sterol biosynthesis in vegetative tissues.
3.2. HSD1 and HSD2 Function Redundantly in Pollen Development
Our unsuccessful attempts to obtain the hsd1 hsd2 double homozygous mutant, results of the reciprocal genetic crosses of the hsd1/+ hsd2 and hsd1 hsd2/+ heterozygous double mutants with their wild-type control, and our findings of both viable and non-viable pollen grains in the pollen sacs of both mutants suggest that HSD1 and HSD2 are critical for normal pollen development. Previous studies suggested that only those mutants defective in the initial steps of sterol biosynthesis showed an abnormal male gametophyte phenotype. For example, the simultaneous elimination of the two Arabidopsis genes encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR), which catalyzes the rate-limiting reaction in the mevalonate pathway and produces precursors for sterol biosynthesis, was shown to cause defective transmission of male gametes [33,34]. Similarly, loss-of-function mutations in the Arabidopsis cycloartenol synthase 1 (CAS1) that initiate the sterol biosynthesis by catalyzing the conversion of the linear 2,3-oxidosqualene molecule to the pentacyclic cycloartenol also cause defective transmission of male gametes [35]. The Arabidopsis genome encodes a single active squalene synthase (SQS) [36] and six homologs of the yeast squalene epoxidase (SQE) with three of them capable of rescuing a yeast SQE mutant [37]. Although there is no report on a loss-of-function mutant of a plant SQS, treatment of Arabidopsis plants with the well-studied SQS inhibitor squalestatin, which could also inhibit plant SQS [38], greatly reduced the plant fertility [33]. Interestingly, despite the presence of six SQE homologs in Arabidopsis, loss-of-function mutations in the Arabidopsis SQE1 gave rise to pleiotropic defects of plant growth and development, including infertility [37,39]. Despite all these reports suggesting the importance of sterol biosynthetic enzymes upstream of the cyclization reaction in male fertility, little is known about the underlying molecular mechanism(s) that link sterol biosynthesis to male gametophytic functions in plants. It is important to note that our study is the first that implicates the role of a post-cyclization sterol biosynthetic enzyme in male gametogenesis. Earlier studies on SMO1, which acts immediately upstream of the 3βHSD/D enzyme to remove the first C4 methyl group, did not detect any defect in male fertility but observed severe embryogenesis and post-embryogenesis defects [16]. However, this could be caused by incomplete elimination of all three SMO1-encoding Arabidopsis genes (SMO1-1, SMO1-2, and SMO1-3) because the reported study only examined the growth and developmental phenotypes of smo1-1 smo1-2 and smo1-1 smo1-3 double mutants [16]. It is also interesting to mention that mutations of the Arabidopsis SMT1, which catalyzes the initial C24 methylation before the first C4 demethylation, also lead to an embryogenesis defect with no apparent impact on male fertility [27]. This could also be caused by functional redundancy between SMT1 and its two homologs, SMT2 and SMT3, as all three enzymes were capable of catalyzing the first and second methyl addition at C24 when expressed in yeast and/or bacterial cells [27,40,41]. It will be interesting to determine if the complete elimination of SMTs or SMO1s could also cause male gametophytic lethality. More importantly, further studies are needed to know the biochemical and cellular causes of the pollen lethality of the hsd1 hsd2 double mutation.
3.3. HSD1 and HSD2 Are Essential for Embryonic Development
To overcome the male fertility problem of the hsd1 hsd2 double mutation and to determine the role of the two HSDs in vegetative growth, we constructed the pLAT52::HSD2-FLAG transgene with pollen-specific promoter pLAT52. Although such a transgene was able to fully rescue the pollen defect of the hsd1 hsd2 double mutation, we did not obtain a transgenic plant containing hsd1 hsd2 double mutation, suggesting the hsd1 hsd2 double mutation could lead to embryo lethality. Thus, these two 3βHSD/D enzymes join other sterol biosynthetic enzymes in regulating embryogenesis, which include SMT1, SMO1/2, CYP51 (C14 demethylase), Fackel/Hydra1 (C14 reductase), and Hydra2 (Δ8-Δ7 isomerase) [15,16,27,29,30,31]. Together, these studies suggest a common mechanism by which mutations of these sterol biosynthetic enzymes affect embryonic development. One potential mechanism could be due to the significant reduction in the abundance of downstream sterols, such as campesterol and sitosterol. However, in Arabidopsis mutants defective in the downstream enzymes of the phytosterol biosynthesis pathway, such as dwarf7 (dwf7, defective in Δ7-sterol C5 desaturase), dwarf5 (dwf5, defective in Δ5,7-sterol C7 reductase), and dwarf1 (dwf1, defective in Δ24-sterol Δ24 reductase), the embryogenesis process seems normal in these mutants despite the similar abundance reduction in the downstream sterols [42,43,44]. It is generally believed that the growth and developmental defects of the late-stage sterol-deficient mutants are mainly caused by the decreased abundance of BRs because most of their growth defects could be rescued by BR treatment [42,43,44]. By contrast, the exogenous application of BR was not able to rescue the embryonic developmental phenotypes of mutants defective in the early stage of the sterol biosynthetic pathway, such as smt1, smo1-1 smo1-2, cyp51a2-3, and fackel [16,27,29,30]. Our finding, coupled with those published studies, suggested that the reduced production of downstream sterols is unlikely the main cause of embryonic defects. We think that the embryogenesis defect might be attributed to the accumulation of certain sterol biosynthetic intermediates, which could be incorporated into the cellular membranes or function as signaling molecules, thereby affecting embryogenesis [30,32,45]. For example, the previously reported smo1-1 smo1-2 and smo2-1 smo2-2 mutants accumulate large amounts of 4,4-dimethylsterols and 4α-methylsterols, respectively [15,16], while the cyp51a2-3 mutant accumulates 14α-methylsterols [29]. These sterol intermediates could somehow affect the biosynthesis or transport of auxin [28,31,46,47,48], which is known to be essential for normal embryogenesis [15,16]. Further experiments are needed to determine the abundance of various sterol intermediates in the relevant tissues of pLAT52::HSD2-FLAG hsd1 hsd2/+ transgenic plants and to examine if treatment with auxin or other plant hormones could rescue some of the observed embryogenesis defects of the transgenic hsd1 hsd2/+ mutants. It is also necessary to generate an HSD2 transgene capable of rescuing both the male fertility defect and the embryo lethality of the hsd1 hsd2 double mutation to determine whether or not the two HSDs also play important roles in the plant vegetative growth and development. Obtaining such a transgenic hsd1 hsd2 double mutant will also indicate whether these two HSDs might function redundantly with the other two Arabidopsis homologs of the mammalian 3βHSDs, At2g43420 (RTN20) and At2g33630, in regulating certain aspects of plant vegetative development and stress tolerance.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
All Arabidopsis mutants and transgenic lines used in this research were in the Columbia-0 (Col-0) ecotype. The hsd1, hsd2, hsd1/+ hsd2, and hsd1 hsd2/+ mutants were generated by the CRISPR/Cas9-based gene editing approach [49]. Stable transgenic plants were selected on half-strength Murashige and Skoog (½ MS) medium supplemented with 50 mg/mL hygromycin. Methods for seed sterilization and growth schemes for young seedlings and mature plants were described previously [50].
4.2. CRISPR/Cas9-Mediated Genome Editing to Create HSD1 and HSD2 Mutant
The CRISPR/Cas9 vectors used to create hsd1/hsd2 mutants were provided by Dr. Qi-Jun Chen [49]. The target sites for introducing mutations into the HSD1/2 genes while minimizing off-target mutations were selected using the web program CRISPR-GE (http://skl.scau.edu.cn/ (accessed on 16 November 2020)) [51] and the oligonucleotides for generating corresponding guide RNAs are listed in Supplementary Table S1. The CRISPR/Cas9 transgenes for introducing mutations in HSD1/2 genes were constructed following a previously reported procedure [52]. The single-guide RNA (sgRNA) expression cassettes containing each target sequence were generated by overlapping PCR and cloned into the pHEE401E binary vector (https://www.addgene.org/71287/ (accessed on 16 November 2020)) using the Golden Gate cloning method [53]. The resulting construct was verified by PCR amplification, restriction enzyme digestion, and DNA sequencing. The CRISPR/Cas9-HSD1/2 construct generated was introduced into the Agrobacterium strain GV3101 by electroporation, which was subsequently used to transform Arabidopsis wild-type plants by the floral-dipping method [54]. T0 seeds were harvested, sterilized, and screened on ½ MS agar medium containing 50 mg/mL hygromycin for T1 lines carrying the CRISPR/Cas9-HSD1/2 constructs. The resulting transgenic T1 lines were subsequently screened for intended mutations of HSD1/2 genes by PCR amplification and subsequent DNA sequencing of the short genomic DNA fragments containing the intended mutation sites using gene-specific primers HSD1-For/Rev and HSD2-For/Rev (Supplementary Table S2). The DSDecodeM program (https://skl.scau.edu.cn/dsdecode/ (accessed on 18 February 2021)) of the CRISPR-GE tool kit was used for decoding the edited genomic sequence of targeted sites [51,55]. Segregated T2/T3 plants carrying no CRISPR/Cas9 transgene but homozygous/heterozygous for the confirmed mutations were identified by PCR analysis using the gene-specific primers and the Cas9-For/Rev primers (see Supplementary Table S2 for their DNA sequences). The HSD1-For/HSD1-Rev primer set-amplified HSD1 genomic fragment was digested by the restriction enzyme AvaII (New England Biolabs, Ipswich, MA, USA) that cuts the wild-type HSD1 fragment but not the corresponding PCR fragment from the hsd1 mutant carrying the A insertion. The HSD2-For/HSD2-Rev primers were used to amplify the HSD2 genomic fragment. Because the hsd2 mutation is caused by a 46-bp deletion, the HSD2 PCR fragment from the wild-type allele is larger than the corresponding PCR fragment from the hsd2 mutant allele, and simple electrophoresis of the HSD2 PCR fragments could easily genotype hsd2/hsd2, hsd2/HSD2, and HSD2/HSD2 plants.
4.3. Total RNA Extraction and cDNA Synthesis
Fifty milligrams of wild-type (Col-0) Arabidopsis seedlings were ground into a fine powder with liquid nitrogen. Total RNAs were extracted using the FastPure® Plant Total RNA Isolation Kit (Vazyme, Nanjing, China) by following the manufacturer’s recommended protocol. After removing residual genomic DNAs, the extracted total RNAs were converted into the first-strand cDNA preparation using the HiScript® III 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme, Nanjing, China) and the manufacturer’s provided protocol. The resulting cDNA products were used immediately for PCR reactions or aliquoted and stored at −80 °C freezer.
4.4. Plasmid Construction and Plant Transformation
When constructing the pC1305-pLAT52::HSD2-FLAG vector, a 20-bp homologous arm sequence was added to the primer to match the vector, and the HSD2 sequence was amplified using the wild-type Arabidopsis cDNA preparation as the template. A target DNA fragment was homologously recombined with the enzyme-linearized vector using homologous recombination enzymes. The pC1305-pLAT52::MCS-FLAG vector for generating the pLAT52::HSD2-FLAG transgene was kindly provided by Professor Jirong Huang [56]. To transform the plants, the plasmids were first introduced into the Agrobacterium strain GV3101, which was used to transform the plants via the floral dip method. All primers used for generating the transgene are listed in Supplementary Table S2.
4.5. Mutant Self-Inbred Offspring Separation Ratio and Forward and Reverse Cross Experiments
After harvesting individual heterozygous mutant seeds, they were germinated, and the resulting seedlings were used for genomic DNA extraction. The genotypes were verified by PCR, and the segregation ratio of the mutant in the self-pollinated progeny was calculated. Heterozygous mutants identified by PCR were used as pollen donors to pollinate wild-type stigma, and vice versa, to ensure maximum pollen transfer. The hybrid seeds were collected when mature and evenly sown onto ½ MS medium. The resulting young seedlings were harvested to extract genomic DNAs for PCR-based genotyping, which were subsequently used to calculate the transmission efficiency (TE) of male and female gametes. The TE was calculated by dividing the numbers of the heterozygous mutant with the total number of wild-type plants × 100% [57].
4.6. Flower Organ Phenotypic Observation and Pollen Alexander Staining
Mutant and wild-type plants of the same growth stage were selected. Flowers at the flat stage were sampled, and their anther morphology was observed and photographed by a volumetric microscope (Nikon: SMZ18; Nikon, Tokyo, Japan). Alexander staining is a simple method for assessing pollen viability, where viable pollen stains purple-red, while non-viable pollen remains green or pale grey due to the inability to take up the stain [21]. For flowers at the anthesis stage, Alexander’s staining solution was dropped onto a microscope slide, and the anther was placed directly into the staining solution to stain the pollen. Then, the Alexander staining solution was added to one side of the anther, covered with a coverslip, and gently pressed until the staining solution permeated the anther evenly. The sample was left at room temperature in the dark for ten minutes, and then observed and pictures were taken under a light microscope.
4.7. Phenotypic Observation of Pods and Statistical Analysis of Seed Abortion Rate
Mature green siliques were dissected under a dissecting microscope to reveal the developing seeds inside. The seeds inside the siliques were observed and photographed using a Nikon stereo microscope SMZ18 [58]. Approximately ten siliques at similar positions on the main inflorescent stem of mature Arabidopsis plants of different genotypes were collected. The siliques were opened and the number of normal and aborted seeds in each silique were recorded. The experiment was repeated for at least three times. The seed abortion rate of both wild-type and mutants was calculated and analyzed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242115565/s1.
Author Contributions
Conceptualization, J.L.; funding acquisition, J.L., J.Z. and L.L.; investigation, J.P.; methodology, J.P., W.L. and B.C.; project administration, L.L. and J.L.; writing—original draft, J.P.; writing—review and editing, J.L., J.Z. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by grants from the National Natural Science Foundation (No. 31970187 to L.L. and No. 31870253 to J.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rieseberg, T.P.; Dadras, A.; Fürst-Jansen, J.M.R.; Dhabalia Ashok, A.; Darienko, T.; de Vries, S.; Irisarri, I.; de Vries, J. Crossroads in the evolution of plant specialized metabolism. Semin. Cell Dev. Biol. 2023, 134, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, K.; Pullen, M.L.; Topping, J.F. Importance of plant sterols in pattern formation and hormone signalling. Trends Plant Sci. 2003, 8, 521–525. [Google Scholar] [CrossRef]
- Hartmann, M.-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Edwards, P.A.; Ericsson, J. Sterols and isoprenoids: Signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 1999, 68, 157–185. [Google Scholar] [CrossRef] [PubMed]
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, F.; Rahier, A.; Camara, B. Biogenesis, molecular regulation and function of plant isoprenoids. Prog. Lipid Res. 2005, 44, 357–429. [Google Scholar] [CrossRef]
- Rahier, A. Dissecting the sterol C-4 demethylation process in higher plants. From structures and genes to catalytic mechanism. Steroids 2011, 76, 340–352. [Google Scholar] [CrossRef]
- Hu, D.; Gao, Y.H.; Yao, X.S.; Gao, H. Recent advances in dissecting the demethylation reactions in natural product biosynthesis. Curr. Opin. Chem. Biol. 2020, 59, 47–53. [Google Scholar] [CrossRef]
- Mialoundama, A.S.; Jadid, N.; Brunel, J.; Di Pascoli, T.; Heintz, D.; Erhardt, M.; Mutterer, J.; Bergdoll, M.; Ayoub, D.; Van Dorsselaer, A. Arabidopsis ERG28 tethers the sterol C4-demethylation complex to prevent accumulation of a biosynthetic intermediate that interferes with polar auxin transport. Plant Cell 2013, 25, 4879–4893. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, P.D.; Pollier, J.; Panda, S.; Szymanski, J.; Massalha, H.; Yona, M.; Unger, T.; Malitsky, S.; Arendt, P.; Pauwels, L. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 2016, 3, 16205. [Google Scholar] [CrossRef]
- Dadras, A.; Rieseberg, T.P.; Zegers, J.M.S.; Fürst-Jansen, J.M.R.; Irisarri, I.; de Vries, J.; de Vries, S. Accessible versatility underpins the deep evolution of plant specialized metabolism. Phytochem. Rev. 2023. [Google Scholar] [CrossRef]
- Acimovic, J.; Rozman, D. Steroidal triterpenes of cholesterol synthesis. Molecules 2013, 18, 4002–4017. [Google Scholar] [CrossRef] [PubMed]
- Konig, A.; Happle, R.; Bornholdt, D.; Engel, H.; Grzeschik, K.H. Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am. J. Med. Genet. 2000, 90, 339–346. [Google Scholar] [CrossRef]
- Bard, M.; Bruner, D.A.; Pierson, C.A.; Lees, N.D.; Biermann, B.; Frye, L.; Koegel, C.; Barbuch, R. Cloning and characterization of ERG25, the Saccharomyces cerevisiae gene encoding C-4 sterol methyl oxidase. Proc. Natl. Acad. Sci. USA 1996, 93, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, S.; Nie, X.; Boutte, Y.; Grison, M.; Li, P.; Kuang, S.; Men, S. Sterol Methyl Oxidases Affect Embryo Development via Auxin-Associated Mechanisms. Plant Physiol. 2016, 171, 468–482. [Google Scholar] [CrossRef]
- Song, J.; Sun, S.; Ren, H.; Grison, M.; Boutté, Y.; Bai, W.; Men, S. The SMO1 Family of Sterol 4α-Methyl Oxidases Is Essential for Auxin- and Cytokinin-Regulated Embryogenesis. Plant Physiol. 2019, 181, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Rahier, A.; Darnet, S.; Bouvier, F.; Camara, B.; Bard, M. Molecular and enzymatic characterizations of novel bifunctional 3beta-hydroxysteroid dehydrogenases/C-4 decarboxylases from Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 27264–27277. [Google Scholar] [CrossRef]
- Rahier, A.; Bergdoll, M.; Genot, G.; Bouvier, F.; Camara, B. Homology modeling and site-directed mutagenesis reveal catalytic key amino acids of 3beta-hydroxysteroid-dehydrogenase/C4-decarboxylase from Arabidopsis. Plant Physiol. 2009, 149, 1872–1886. [Google Scholar] [CrossRef]
- Kim, B.; Kim, G.; Fujioka, S.; Takatsuto, S.; Choe, S.J.M. Cells, Overexpression of 3β-hydroxysteroid dehydrogenases/C-4 decarboxylases causes growth defects possibly due to abnormal auxin transport in arabidopsis. Mol. Cells 2012, 34, 77–84. [Google Scholar] [CrossRef]
- Twell, D.; Yamaguchi, J.; McCormick, S. Pollen-specific gene expression in transgenic plants: Coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 1990, 109, 705–713. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Nziengui, H.; Bouhidel, K.; Pillon, D.; Der, C.; Marty, F.; Schoefs, B. Reticulon-like proteins in Arabidopsis thaliana: Structural organization and ER localization. FEBS Lett. 2007, 581, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaumer, V.; Maneta-Peyret, L.; Fouillen, L.; Botchway, S.W.; Upson, J.; Hughes, L.; Richardson, J.; Kittelmann, M.; Moreau, P.; Hawes, C. The odd one out: Arabidopsis reticulon 20 does not bend ER membranes but has a role in lipid regulation. Sci. Rep. 2018, 8, 2310. [Google Scholar] [CrossRef] [PubMed]
- Moummou, H.; Kallberg, Y.; Tonfack, L.B.; Persson, B.; van Der Rest, B. The plant short-chain dehydrogenase (SDR) superfamily: Genome-wide inventory and diversification patterns. BMC Plant Biol. 2012, 12, 219. [Google Scholar] [CrossRef]
- Bouhouche, A.; Albaroudi, N.; El Alaoui, M.A.; Askander, O.; Habbadi, Z.; El Hassani, A.; Iraqi, H.; El Fahime, E.; Belmekki, M. Identification of the novel SDR42E1 gene that affects steroid biosynthesis associated with the oculocutaneous genital syndrome. Exp. Eye Res. 2021, 209, 108671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H. How effective is T-DNA insertional mutagenesis in Arabidopsis? J. Biol. Chem. 2008, 1, 11–20. [Google Scholar]
- Diener, A.C.; Li, H.; Zhou, W.; Whoriskey, W.J.; Nes, W.D.; Fink, G.R. Sterol methyltransferase 1 controls the level of cholesterol in plants. Plant Cell 2000, 12, 853–870. [Google Scholar] [CrossRef]
- Men, S.; Boutte, Y.; Ikeda, Y.; Li, X.; Palme, K.; Stierhof, Y.D.; Hartmann, M.A.; Moritz, T.; Grebe, M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 2008, 10, 237–244. [Google Scholar] [CrossRef]
- Kim, T.W.; Hwang, J.Y.; Kim, Y.S.; Joo, S.H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Schrick, K.; Mayer, U.; Horrichs, A.; Kuhnt, C.; Bellini, C.; Dangl, J.; Schmidt, J.; Jürgens, G. FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev. 2000, 14, 1471–1484. [Google Scholar] [CrossRef]
- Souter, M.; Topping, J.; Pullen, M.; Friml, J.; Palme, K.; Hackett, R.; Grierson, D.; Lindsey, K. hydra Mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 2002, 14, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.C.; Fujioka, S.; Tasaka, M.; Seto, H.; Takatsuto, S.; Ishii, A.; Aida, M.; Yoshida, S.; Sheen, J. A critical role of sterols in embryonic patterning and meristem programming revealed by the fackel mutants of Arabidopsis thaliana. Genes. Dev. 2000, 14, 1485–1497. [Google Scholar] [CrossRef]
- Suzuki, M.; Kamide, Y.; Nagata, N.; Seki, H.; Ohyama, K.; Kato, H.; Masuda, K.; Sato, S.; Kato, T.; Tabata, S.; et al. Loss of function of 3-hydroxy-3-methylglutaryl coenzyme A reductase 1 (HMG1) in Arabidopsis leads to dwarfing, early senescence and male sterility, and reduced sterol levels. Plant J. 2004, 37, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakagawa, S.; Kamide, Y.; Kobayashi, K.; Ohyama, K.; Hashinokuchi, H.; Kiuchi, R.; Saito, K.; Muranaka, T.; Nagata, N. Complete blockage of the mevalonate pathway results in male gametophyte lethality. J. Exp. Bot. 2009, 60, 2055–2064. [Google Scholar] [CrossRef]
- Babiychuk, E.; Bouvier-Nave, P.; Compagnon, V.; Suzuki, M.; Muranaka, T.; Van Montagu, M.; Kushnir, S.; Schaller, H. Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and plastid biogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3163–3168. [Google Scholar] [CrossRef]
- Busquets, A.; Keim, V.; Closa, M.; Arco, A.D.; Boronat, A.; Arró, M.; Ferrer, A. Arabidopsis thaliana contains a single gene encoding squalene synthase. Plant Mol. Biol. 2008, 67, 25–36. [Google Scholar] [CrossRef]
- Rasbery, J.M.; Shan, H.; LeClair, R.J.; Norman, M.; Matsuda, S.P.; Bartel, B. Arabidopsis thaliana squalene epoxidase 1 is essential for root and seed development. J. Biol. Chem. 2007, 282, 17002–17013. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.A.; Wentzinger, L.; Hemmerlin, A.; Bach, T.J. Metabolism of farnesyl diphosphate in tobacco BY-2 cells treated with squalestatin. Biochem. Soc. T 2000, 28, 794–796. [Google Scholar] [CrossRef]
- Posé, D.; Castanedo, I.; Borsani, O.; Nieto, B.; Rosado, A.; Taconnat, L.; Ferrer, A.; Dolan, L.; Valpuesta, V.; Botella, M.A. Identification of the Arabidopsis dry2/sqe1-5 mutant reveals a central role for sterols in drought tolerance and regulation of reactive oxygen species. Plant J. 2009, 59, 63–76. [Google Scholar] [CrossRef]
- Husselstein, T.; Gachotte, D.; Desprez, T.; Bard, M.; Benveniste, P. Transformation of Saccharomyces cerevisiae with a cDNA encoding a sterol C-methyltransferase from Arabidopsis thaliana results in the synthesis of 24-ethyl sterols. FEBS Lett. 1996, 381, 87–92. [Google Scholar] [CrossRef]
- Bouvier-Navé, P.; Husselstein, T.; Desprez, T.; Benveniste, P. Identification of cDNAs encoding sterol methyl-transferases involved in the second methylation step of plant sterol biosynthesis. EUR J. Biochem. 2010, 246, 518–529. [Google Scholar] [CrossRef]
- Choe, S.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tissier, C.P.; Gregory, B.D.; Ross, A.S.; Tanaka, A.; Yoshida, S.; Tax, F.E.; et al. The Arabidopsis dw f 7/ste1 Mutant Is Defective in the Delta7 Sterol C-5 Desaturation Step Leading to Brassinosteroid Biosynthesis. Plant Cell 1999, 11, 207–222. [Google Scholar]
- Choe, S.; Dilkes, B.P.; Gregory, B.D.; Ross, A.S.; Yuan, H.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tanaka, A.; Yoshida, S.; et al. The Arabidopsis dwarf1 Mutant Is Defective in the Conversion of 24-Methylenecholesterol to Campesterol in Brassinosteroid Biosynthesis. Plant Physiol. 1999, 119, 897–907. [Google Scholar] [CrossRef]
- Choe, S.; Tanaka, A.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ross, A.; Tax, F.; Yoshida, S.; Feldmann, K.A. Lesions in the sterol delta reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 2000, 21, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D. Plant development: A role for sterols in embryogenesis. Curr. Biol. 2000, 10, R601–R604. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, V.; Friml, J.; Grebe, M.; Van Den Toorn, A.; Palme, K.; Scheres, B. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 2003, 15, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, P.; Ma, Y.; Nie, X.; Grebe, M.; Men, S. Membrane sterol composition in Arabidopsis thaliana affects root elongation via auxin biosynthesis. Int. J. Mol. Sci. 2021, 22, 437. [Google Scholar] [CrossRef]
- Pullen, M.; Clark, N.; Zarinkamar, F.; Topping, J.; Lindsey, K. Analysis of vascular development in the hydra sterol biosynthetic mutants of Arabidopsis. PLoS ONE 2010, 5, e12227. [Google Scholar] [CrossRef]
- Wang, Z.P.; Xing, H.L.; Dong, L.; Zhang, H.Y.; Han, C.Y.; Wang, X.C.; Chen, Q.J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015, 16, 144. [Google Scholar] [CrossRef]
- Li, J.; Nam, K.H.; Vafeados, D.; Chory, J. BIN2, a New Brassinosteroid-Insensitive Locus in Arabidopsis. Plant Physiol. 2001, 127, 14–22. [Google Scholar] [CrossRef]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.G. CRISPR-GE: A Convenient Software Toolkit for CRISPR-Based Genome Editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Engler, C.; Marillonnet, S. Golden gate cloning. DNA Cloning Assem. Methods 2014, 1116, 119–131. [Google Scholar]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Ma, X.; Chen, L.; Zhu, Q.; Chen, Y.; Liu, Y.G. Rapid Decoding of Sequence-Specific Nuclease-Induced Heterozygous and Biallelic Mutations by Direct Sequencing of PCR Products. Mol. Plant 2015, 8, 1285–1287. [Google Scholar] [CrossRef]
- Suo, Y.; Huang, J. Arabidopsis BIG1 and BIG5 are crucial for male gametophyte transmission. J. Integr. Plant Biol. 2019, 61, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Chantha, S.-C.; Gray-Mitsumune, M.; Houde, J.; Matton, D.P. The MIDASIN and NOTCHLESS genes are essential for female gametophyte development in Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2010, 16, 3–18. [Google Scholar] [CrossRef]
- Liu, C.M.; Meinke, D.W. The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J. 1998, 16, 21–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).