Epi-Brassinolide Regulates ZmC4 NADP-ME Expression through the Transcription Factors ZmbHLH157 and ZmNF-YC2
Abstract
1. Introduction
2. Results
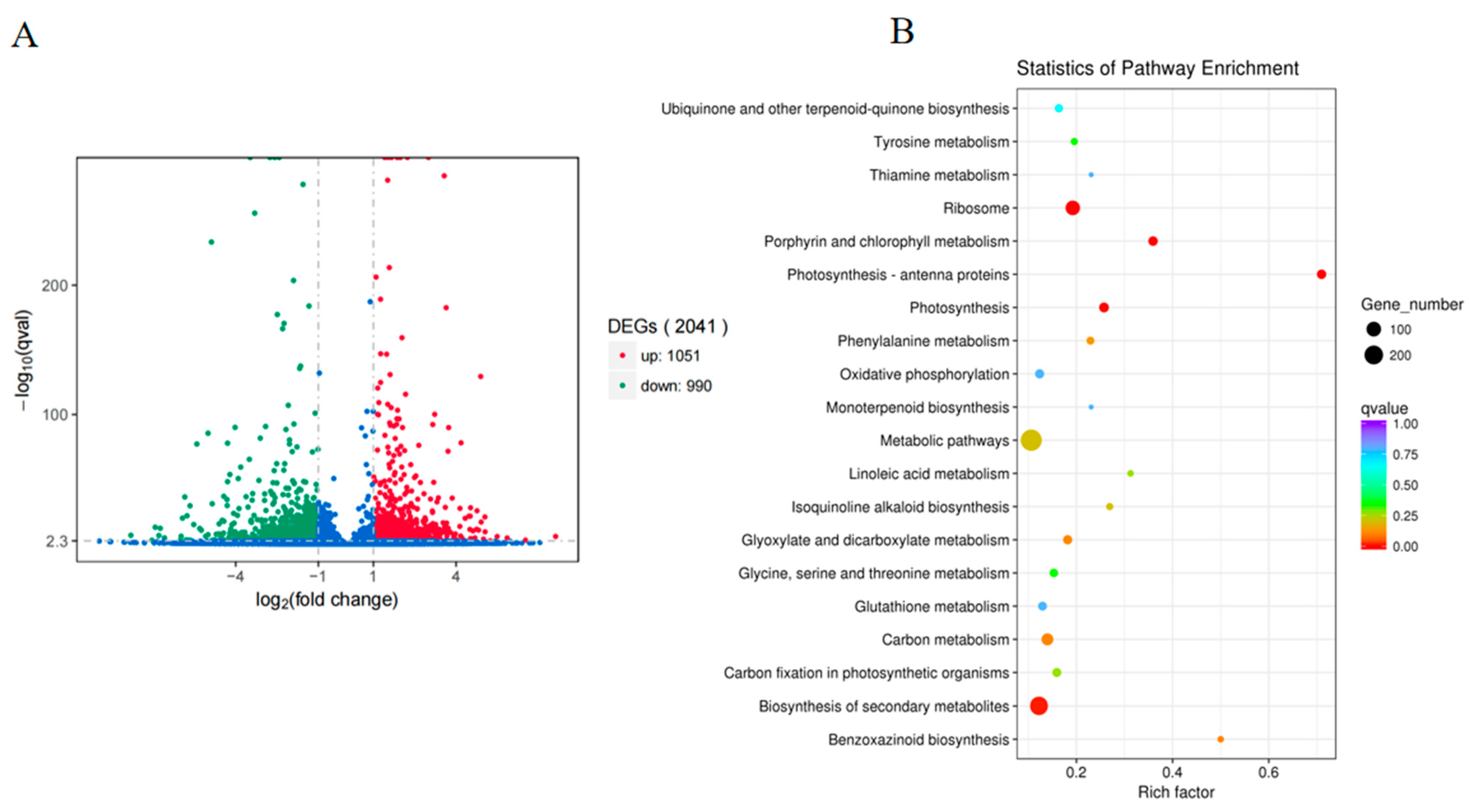
2.1. Transcriptome Data Analysis of EBL-Treated Maize Leaves
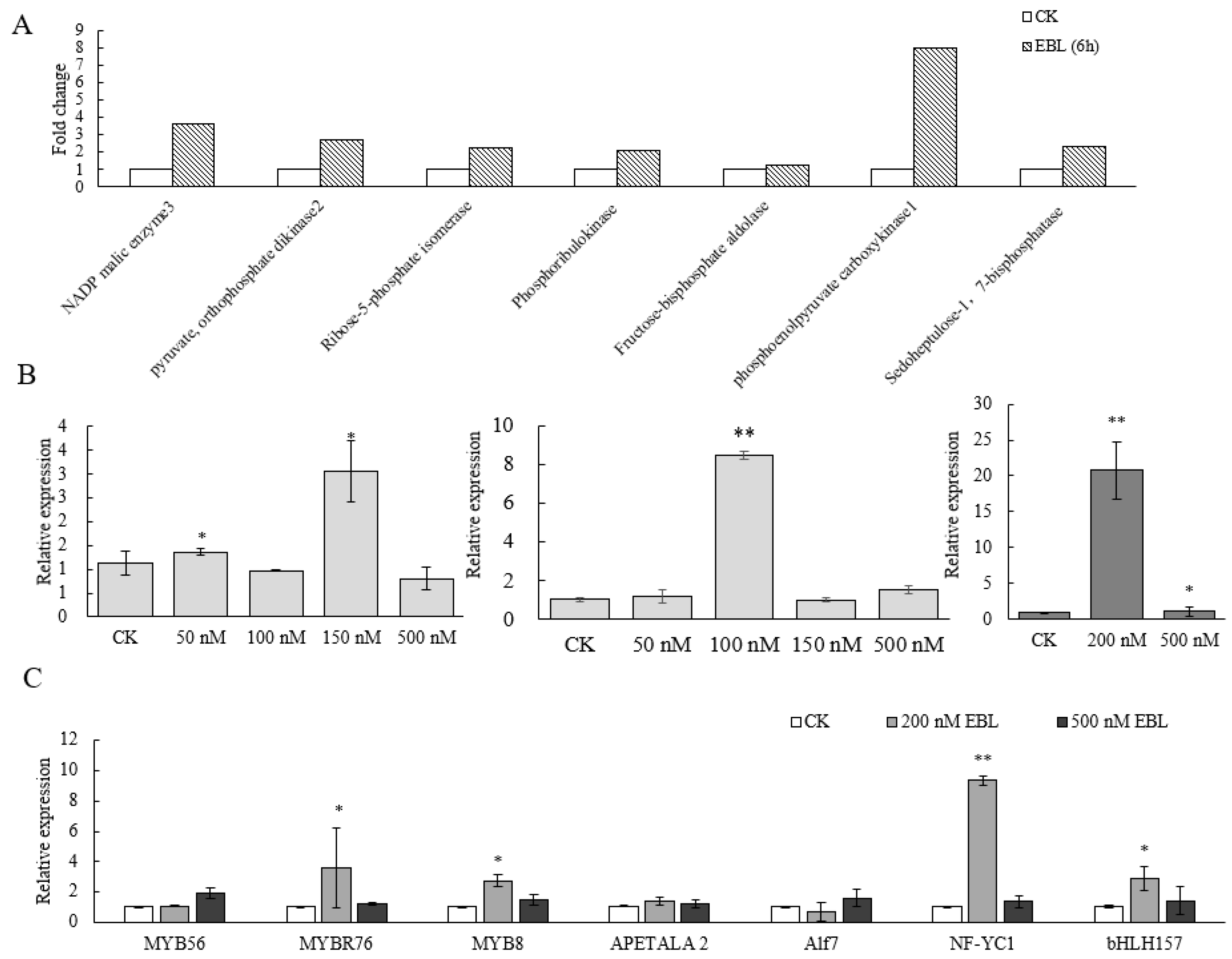
2.2. The Impact of EBL on C4-NADP-ME Expression in Maize Leaves
2.3. bHLH157 and NF-YC2 Are Induced by EBL and Affect ZmC4-NADP-ME Expression
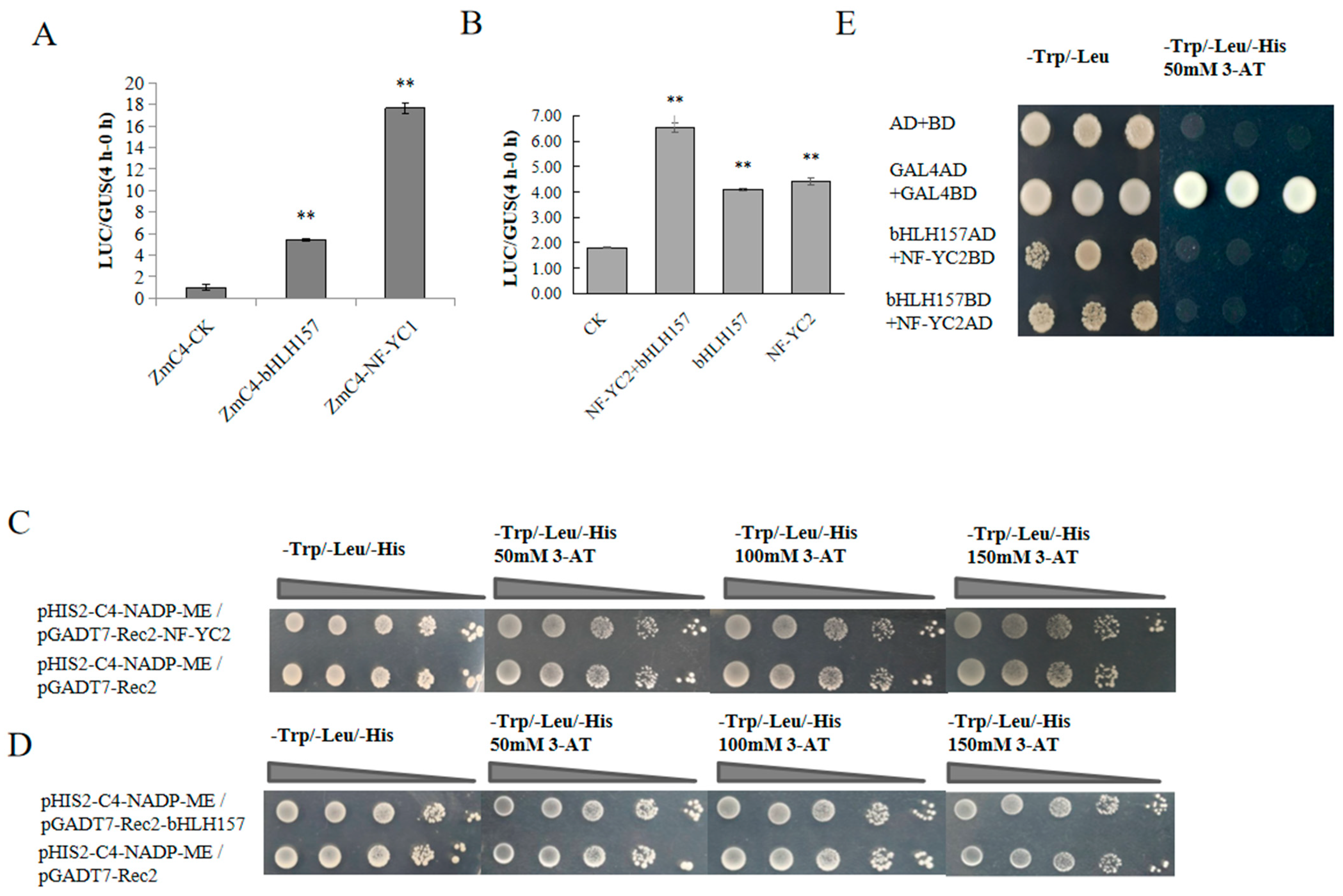
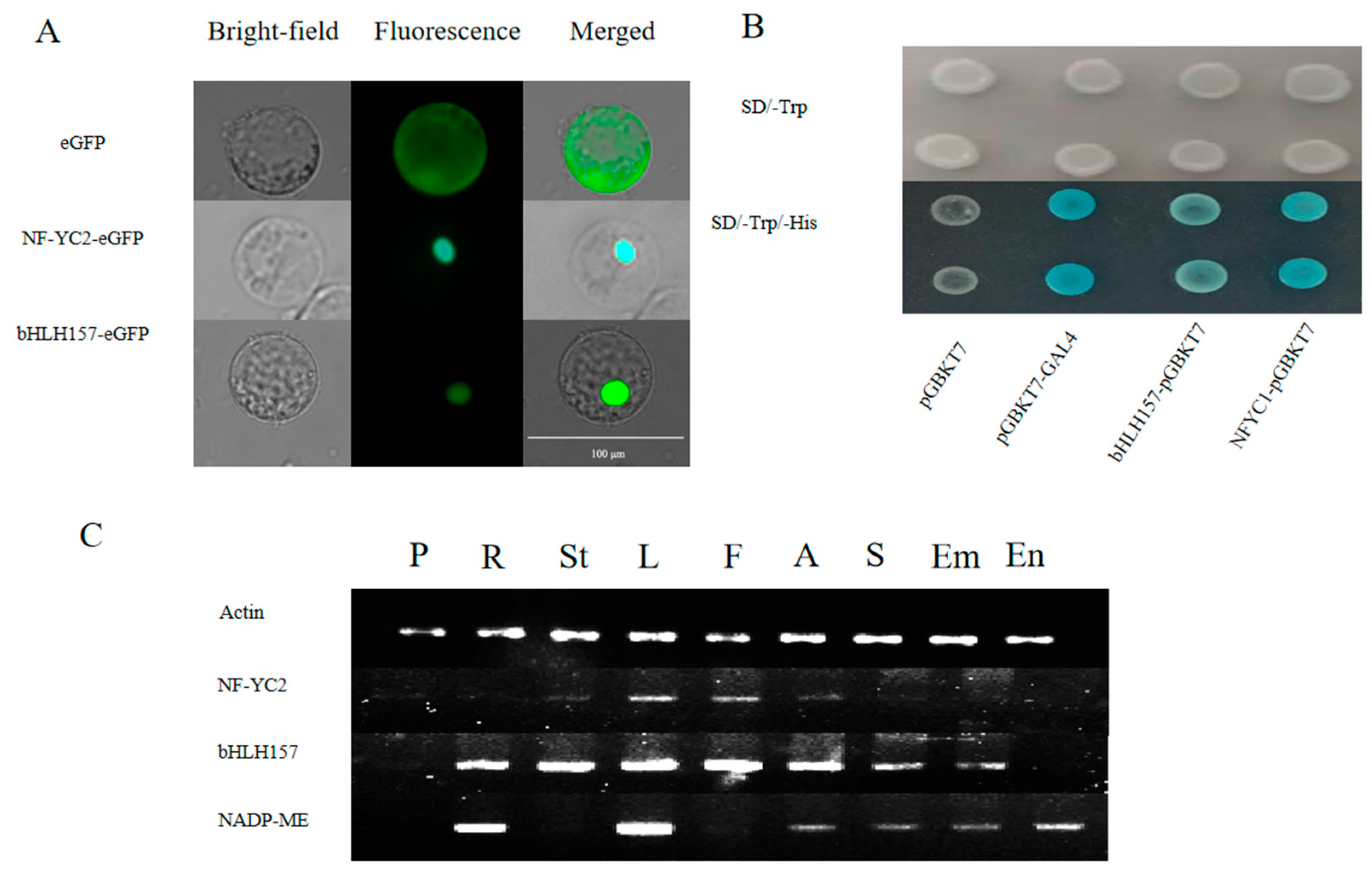
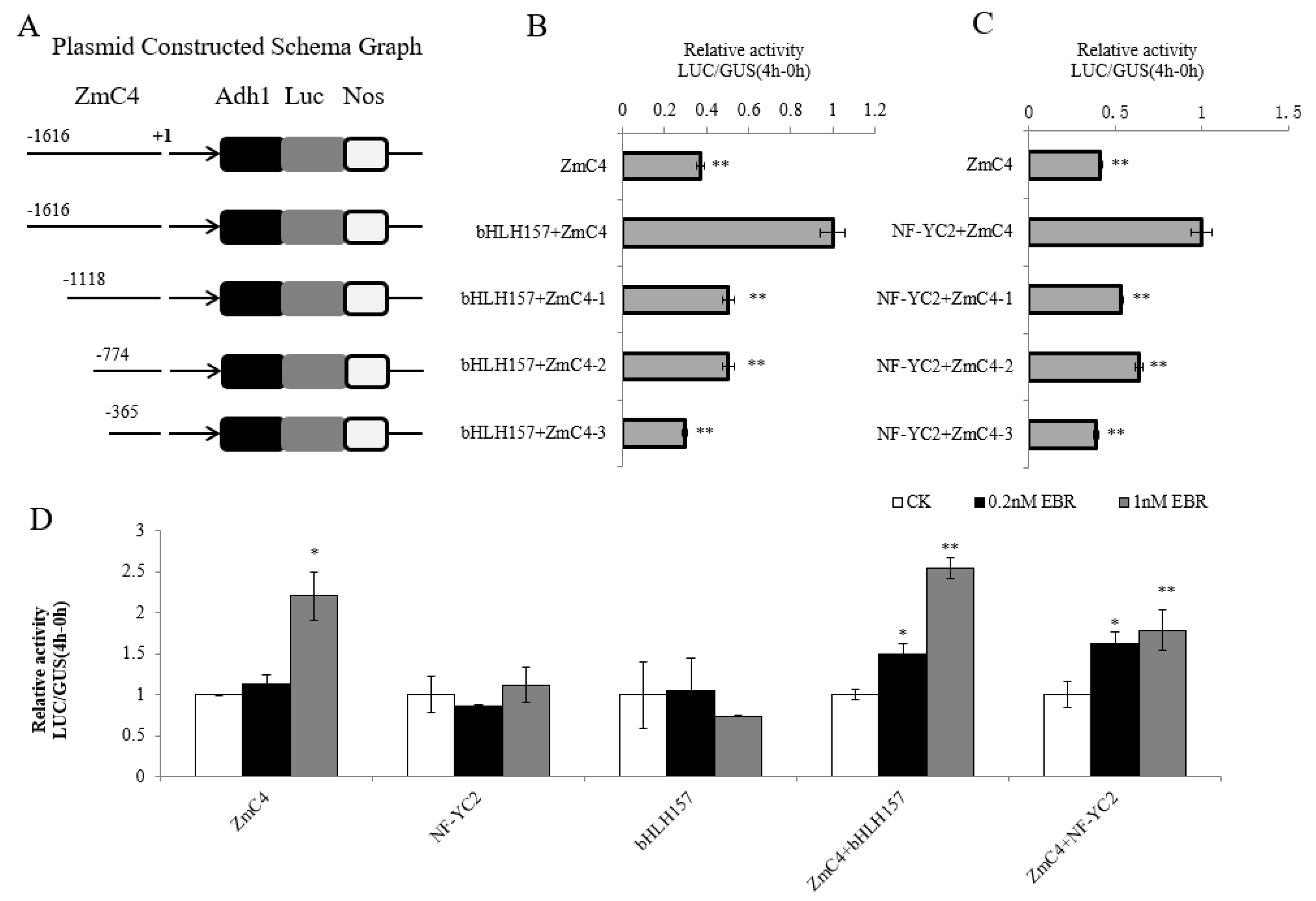
2.4. Functional Verification of bHLH157 and NF-YC2 Transcription Factor
2.5. Preliminary Study on the Binding Region of bHLH157, NF-YC2 and ZmC4-NADP-ME Promoter
2.6. EBL Further Enhances ZmC4 NADP-ME Promoter Activity by increasing bHLH157 and NF-YC2 Expression
3. Discussion
4. Materials and Methods
4.1. Maize Materials, Growth Conditions and EBL Treatment
4.2. High-Throughput RNA-seq
4.3. Transcription Activation and Protein Structure Prediction
4.4. Subcellular Localization
4.5. Yeast One-Hybrid
4.6. Protoplasts Transformation and Dual Luciferase System
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Hou, P.; Duan, F.; Niu, L.; Dai, T.; Wang, K.; Zhao, M.; Li, S.; Zhou, W. Improving photosynthesis to increase grain yield potential: An analysis of maize hybrids released in different years in China. Photosynth. Res. 2021, 150, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Borba, A.R.; Serra, T.S.; Gorska, A.; Gouveia, P.; Cordeiro, A.M.; Reyna-Llorens, I.; Knerova, J.; Barros, P.M.; Abreu, I.A.; Oliveira, M.M.; et al. Synergistic Binding of bHLH Transcription Factors to the Promoter of the Maize NADP-ME Gene Used in C4 Photosynthesis Is Based on an Ancient Code Found in the Ancestral C3 State. Mol. Biol. Evol. 2018, 35, 1690–1705. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, B.V.; Sharwood, R.; Whitney, S.; Ghannoum, O. Shade compromises the photosynthetic efficiency of NADP-ME less than that of PEP-CK and NAD-ME C-4 grasses. J. Exp. Bot. 2018, 69, 3053–3068. [Google Scholar] [CrossRef]
- Pardo, J.; VanBuren, R. Evolutionary innovations driving abiotic stress tolerance in C4 grasses and cereals. Plant Cell 2021, 33, 3391–3401. [Google Scholar] [CrossRef]
- Voll, L.M.; Engelsdorf, T.; Saur, A.; Wheeler, M.G.; Drincovich, M.F.; Weber, A.P.; Maurino, V.G. Loss of cytosolic NADP-malic enzyme 2 in Arabidopsis thaliana is associated with enhanced susceptibility to Colletotrichum higginsianum. New Phytologist. 2012, 195, 189–202. [Google Scholar] [CrossRef]
- Detarsio, E.; Maurino, V.G.; Alvarez, C.E.; Müller, G.L.; Andreo, C.S.; Drincovich, M.F. Maize cytosolic NADP-malic enzyme (ZmCytNADP-ME): A phylogenetically distant isoform specifically expressed in embryo and young root. Plant Mol. Biol. 2008, 68, 355–367. [Google Scholar] [CrossRef]
- Saigo, M.; Alvarez, C.E.; Andreo, C.S.; Drincovich, M.F. Plastidial NADP-malic enzymes from grasses: Unraveling the way to the C4 specific isoforms. Plant Physiol. Biochem. 2013, 63, 39–48. [Google Scholar] [CrossRef]
- Alvarez, C.E.; Saigo, M.; Margarit, E.; Andreo, C.S.; Drincovich, M.F. Kinetics and functional diversity among the five members of the NADP-malic enzyme family from Zea mays, a C-4 species. Photosynth. Res. 2013, 115, 65–80. [Google Scholar] [CrossRef]
- Müller, G.L.; Lara, M.V.; Oitaven, P.; Andreo, C.S.; Maurino, V.G.; Drincovich, M.F. Improved water use efficiency and shorter life cycle of Nicotiana tabacum due to modification of guard and vascular companion cells. Sci. Rep. 2018, 8, 4380. [Google Scholar] [CrossRef]
- Sirhindi, G.; Kumar, S.; Bhardwaj, R.; Kumar, M. Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol. Mol. Biol. Plants 2009, 15, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Fariduddin, Q.; Hussain, A.; Khan, T.A. Brassinosteroid and hydrogen peroxide improve photosynthetic machinery, stomatal movement, root morphology and cell viability and reduce Cu-triggered oxidative burst in tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tian, X. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul. 2008, 56, 257–264. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Hayat, S. Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol. Biochem. 2018, 129, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, P. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017, 81, 377–384. [Google Scholar] [CrossRef]
- Wu, C.Y.; Trieu, A.; Radhakrishnan, P.; Kwok, S.F.; Harris, S.; Zhang, K.; Wang, J.; Wan, J.; Zhai, H.; Takatsuto, S.; et al. Brassinosteroids regulate grain filling in rice. Plant Cell 2008, 20, 2130–2145. [Google Scholar] [CrossRef]
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef]
- Li, X.J.; Guo, X.; Zhou, Y.H.; Shi, K.; Zhou, J.; Yu, J.Q.; Xia, X.J. Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Kim, T.W.; Michniewicz, M.; Bergmann, D.C.; Wang, Z.Y. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 2012, 482, 419–422. [Google Scholar] [CrossRef]
- Gudesblat, G.E.; Joanna, S.P.; Camilla, B.; Juliane, M.; Isabelle, V.; Walter, V.D.; Sjef, B.; Miroslava, Z.; Sacco, D.V.; Claudia, J.; et al. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat. Cell Biol. 2012, 14, 548–554. [Google Scholar] [CrossRef]
- Rojas-Gonzalez, J.A.; Soto-Suarez, M.; Garcia-Diaz, A.; Romero-Puertas, M.C.; Sandalio, L.M.; Merida, A.; Thormahlen, I.; Geigenberger, P.; Serrato, A.J.; Sahrawy, M. Disruption of both chloroplastic and cytosolic FBPase genes results in a dwarf phenotype and important starch and metabolite changes in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 2673–2689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Sun, Q.; Deng, D.; Liu, H.; Chen, S.; Yin, Z. Genetic determinants controlling maize rubisco activase gene expression and a comparison with rice counterparts. BMC Plant Biol. 2019, 19, 351. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, T.; Xiang, Y.; He, X.; Zhang, J. Epi-Brassinolide Positively Affects Chlorophyll Content and Dark-Reaction Enzymes of Maize Seedlings. Phyton-Int. J. Exp. Bot. 2021, 90, 1465–1476. [Google Scholar] [CrossRef]
- Siddiqui, H.; Hayat, S.; Bajguz, A. Regulation of photosynthesis by brassinosteroids in plants. Acta Physiol. Plant. 2018, 40, 40–59. [Google Scholar] [CrossRef]
- Stelpflug, S.C.; Sekhon, R.S.; Vaillancourt, B.; Hirsch, C.N.; Buell, C.R.; de Leon, N.; Kaeppler, S.M. An Expanded Maize Gene Expression Atlas based on RNA Sequencing and its Use to Explore Root Development. Plant Genome-US 2016, 9, 27898762. [Google Scholar] [CrossRef] [PubMed]
- Thijs, G.; Marchal, K.; Lescot, M.; Rombauts, S.; De Moor, B.; Rouze, P.; Moreau, Y. A gibbs sampling method to detect overrepresented motifs in the upstream regions of coexpressed genes. J. Comput. Biol. 2002, 9, 447–464. [Google Scholar] [CrossRef]
- Yu, X.F.; Li, L.; Zola, J.; Aluru, M.; Ye, H.X.; Foudree, A.; Guo, H.Q.; Anderson, S.; Aluru, S.; Liu, P.; et al. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 2011, 65, 634–646. [Google Scholar] [CrossRef]
- Liu, Z.H.; Chen, Y.; Wang, N.N.; Chen, Y.H.; Wei, N.; Lu, R.; Li, Y.; Li, X.B. A basic helix-loop-helix protein (GhFP1) promotes fibre elongation of cotton (Gossypium hirsutum) by modulating brassinosteroid biosynthesis and signalling. New Phytol. 2020, 225, 2439–2452. [Google Scholar] [CrossRef]
- Otani, Y.; Tomonaga, Y.; Tokushige, K.; Kamimura, M.; Sasaki, A.; Nakamura, Y.; Nakamura, T.; Matsuo, T.; Okamoto, S. Expression profiles of four BES1/BZR1 homologous genes encoding bHLH transcription factors in Arabidopsis. J. Pestic. Sci. 2020, 45, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Gorska, A.M.; Gouveia, P.; Borba, A.R.; Zimmermann, A.; Serra, T.S.; Lourenco, T.F.; Margarida-Oliveira, M.; Peterhansel, C.; Saibo, N.J.M. ZmbHLH80 and ZmbHLH90 transcription factors act antagonistically and contribute to regulate PEPC1 cell-specific gene expression in maize. Plant J. 2019, 99, 270–285. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef]
- Miyoshi, K.; Ito, Y.V.; Serizawa, A.; Kurata, N. OsHAP3 genes regulate chloroplast biogenesis in rice. Plant J. 2003, 36, 532–540. [Google Scholar] [CrossRef]
- Bello, B.K.; Hou, Y.; Zhao, J.; Jiao, G.; Wu, Y.; Li, Z.; Wang, Y.; Tong, X.; Wang, W.; Yuan, W.; et al. NF-YB1-YC12-bHLH144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.). Plant Biotechnol. J. 2019, 17, 1222–1235. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, C.; Zhang, C.; Cui, L.; Ai, G.; Wang, X.; Zheng, F.; Zhang, D.; Larkin, R.M.; et al. NF-Y plays essential roles in flavonoid biosynthesis by modulating histone modifications in tomato. New Phytol. 2021, 229, 3237–3252. [Google Scholar] [CrossRef]
- Junker, A.; Monke, G.; Rutten, T.; Keilwagen, J.; Seifert, M.; Thi, T.M.; Renou, J.P.; Balzergue, S.; Viehover, P.; Hahnel, U.; et al. Elongation-related functions of LEAFY COTYLEDON1 during the development of Arabidopsis thaliana. Plant J. 2012, 71, 427–442. [Google Scholar] [CrossRef]
- Su, H.; Chen, Z.; Dong, Y.; Ku, L.; Abou-Elwafa, S.F.; Ren, Z.; Cao, Y.; Dou, D.; Liu, Z.; Liu, H.; et al. Identification of ZmNF-YC2 and its regulatory network for maize flowering time. J. Exp. Bot. 2021, 72, 7792–7807. [Google Scholar] [CrossRef]
- Niu, B.; Deng, H.; Li, T.; Sharma, S.; Yun, Q.; Li, Q.; Chen, C. OsbZIP76 interacts with OsNF-YBs and regulates endosperm cellularization in rice (Oryza sativa). J. Integr. Plant Biol. 2020, 62, 1983–1996. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, G.; Li, B.; Xie, Y.; Wei, Y.; Shang, S.V.; Tian, L.; Shi, H. Functional analysis of the heterotrimeric NF-Y transcription factor complex in cassava disease resistance. Ann. Bot. 2020, 124, 1185–1198, Erratum in 2020, 124, 1243. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, Q.; Zhang, C.; Du, J.; Li, X.; Huang, H.; Wei, B.; Li, Y.; Yu, G.; Liu, H.; et al. Identification and characterization of transcription factor ZmEREB94 involved in starch synthesis in maize. J. Plant Physiol. 2017, 216, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.W.; Zhang, X.G. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.L.; Wang, Y.Y.; Du, J.; Li, H.; Wei, B.; Wang, Y.B.; Li, Y.P.; Yu, G.W.; Liu, H.M.; Zhang, J.J.; et al. ZmMYB14 is an important transcription factor involved in the regulation of the activity of the ZmBT1 promoter in starch biosynthesis in maize. FEBS J. 2017, 284, 3079–3099. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, X.; Li, H.; Hu, Y.; Liu, H.; Wen, S.; Li, Y.; Liu, Y.; Huang, H.; Yu, G.; et al. Gibberellin induced transcription factor bZIP53 regulates CesA1 expression in maize kernels. PLoS ONE 2021, 16, e0244591. [Google Scholar] [CrossRef] [PubMed]
| cis-Element | Position (−) | Sequence | Predicted Function |
|---|---|---|---|
| E-box | 435 | GTGCAC | bHLH transcription factor potential binding site |
| 649 | CACGTG | ||
| 669 | AACGTG | ||
| 1152 | TACGTG | ||
| CCAAT-box | 1495 | CCAAT | CCAAT transcription factor potential binding site |
| CAAT-box | 350 | CAAT | |
| 844 | CAAT | ||
| 603 | CAAAT | ||
| 1572 | |||
| BRRE | 245 | CGTGCG | brassinosteroid responsiveness |
| 239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; He, X.; Lv, H.; Liu, H.; Li, Y.; Hu, Y.; Liu, Y.; Huang, Y.; Zhang, J. Epi-Brassinolide Regulates ZmC4 NADP-ME Expression through the Transcription Factors ZmbHLH157 and ZmNF-YC2. Int. J. Mol. Sci. 2023, 24, 4614. https://doi.org/10.3390/ijms24054614
Gao Y, He X, Lv H, Liu H, Li Y, Hu Y, Liu Y, Huang Y, Zhang J. Epi-Brassinolide Regulates ZmC4 NADP-ME Expression through the Transcription Factors ZmbHLH157 and ZmNF-YC2. International Journal of Molecular Sciences. 2023; 24(5):4614. https://doi.org/10.3390/ijms24054614
Chicago/Turabian StyleGao, Yuanfen, Xuewu He, Huayang Lv, Hanmei Liu, Yangping Li, Yufeng Hu, Yinghong Liu, Yubi Huang, and Junjie Zhang. 2023. "Epi-Brassinolide Regulates ZmC4 NADP-ME Expression through the Transcription Factors ZmbHLH157 and ZmNF-YC2" International Journal of Molecular Sciences 24, no. 5: 4614. https://doi.org/10.3390/ijms24054614
APA StyleGao, Y., He, X., Lv, H., Liu, H., Li, Y., Hu, Y., Liu, Y., Huang, Y., & Zhang, J. (2023). Epi-Brassinolide Regulates ZmC4 NADP-ME Expression through the Transcription Factors ZmbHLH157 and ZmNF-YC2. International Journal of Molecular Sciences, 24(5), 4614. https://doi.org/10.3390/ijms24054614






