Structural and Functional Properties of Kappa Tropomyosin
Abstract
1. Introduction
2. Results
2.1. Properties of Coiled-Coil Tropomyosin Dimers
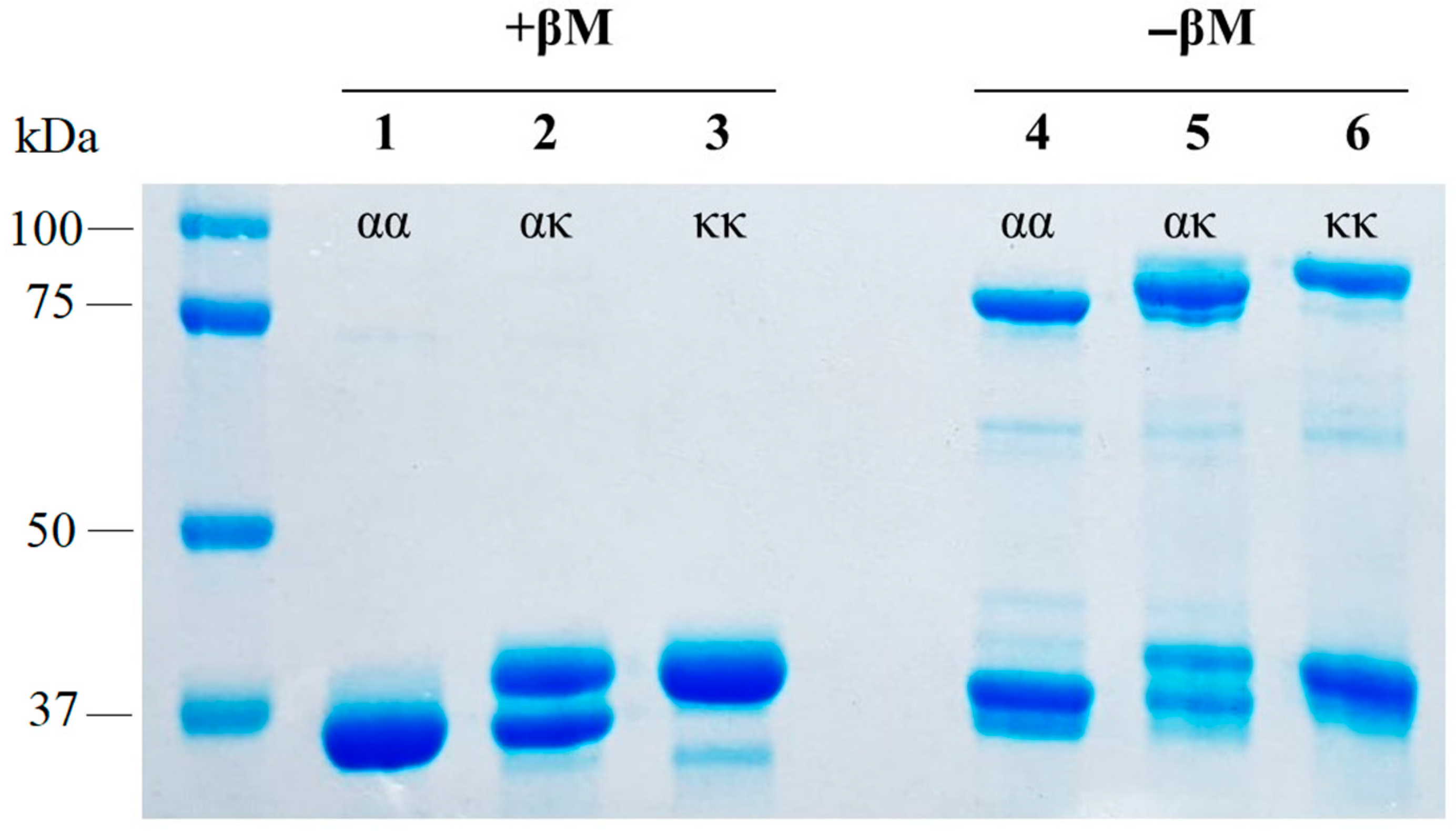
2.1.1. Formation of ακ-Heterodimers of Tpm
2.1.2. Formation of ακ-Heterodimers of Tpm
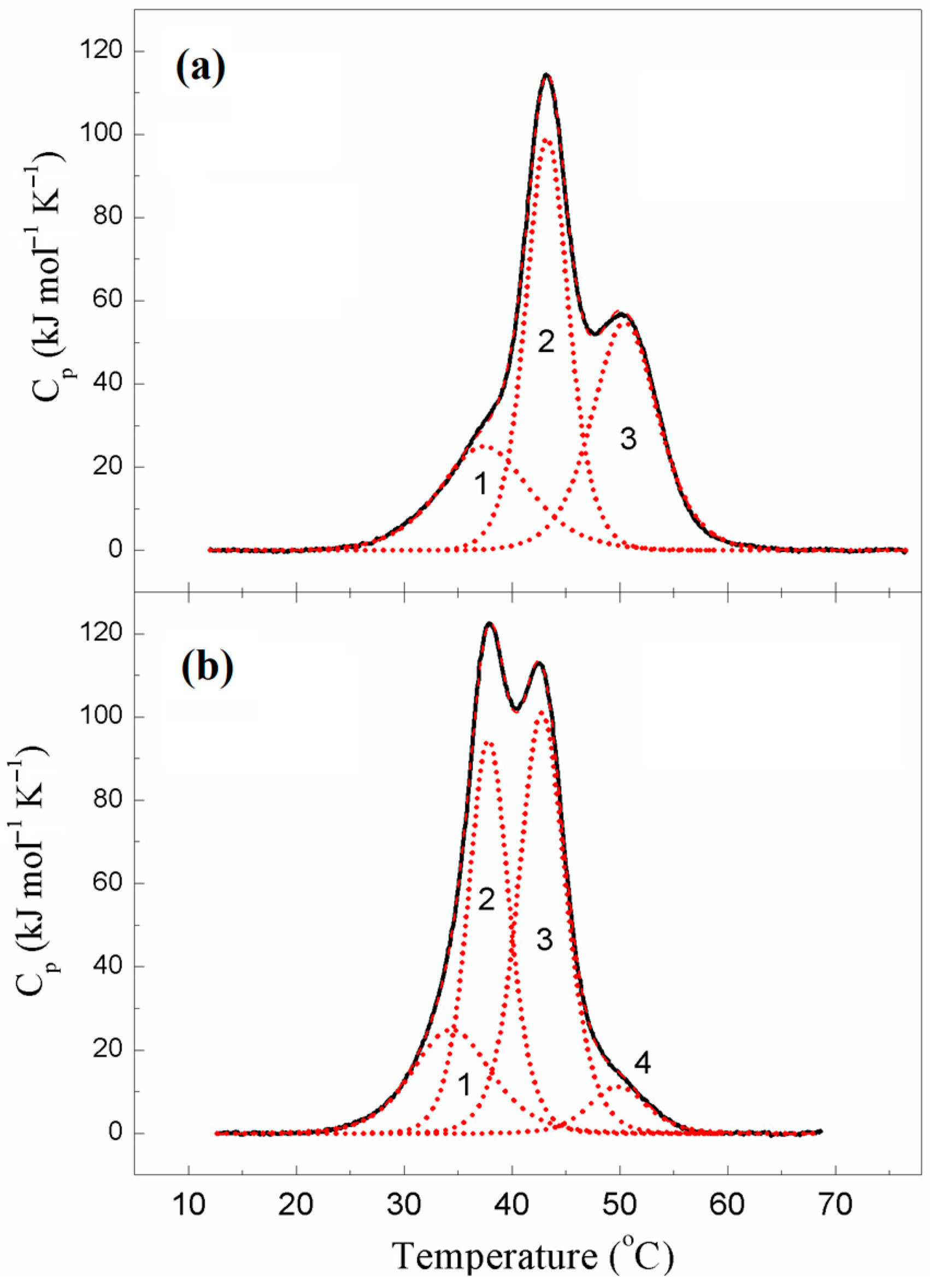
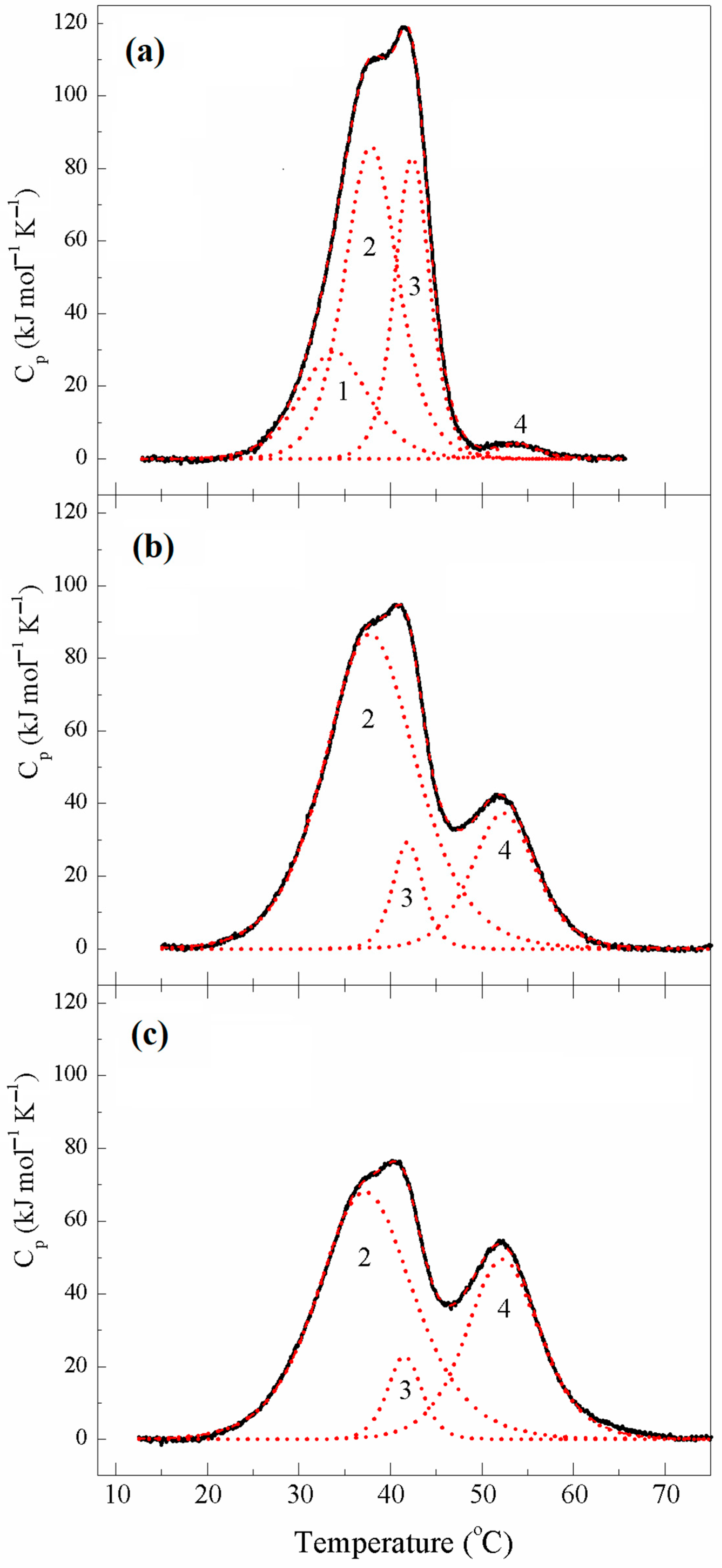
2.1.3. Thermal Unfolding and Domain Structure of κκTpm Studied with Differential Scanning Calorimetry
2.1.4. Interaction of N- and C-Ends of ααTpm and κκTpm
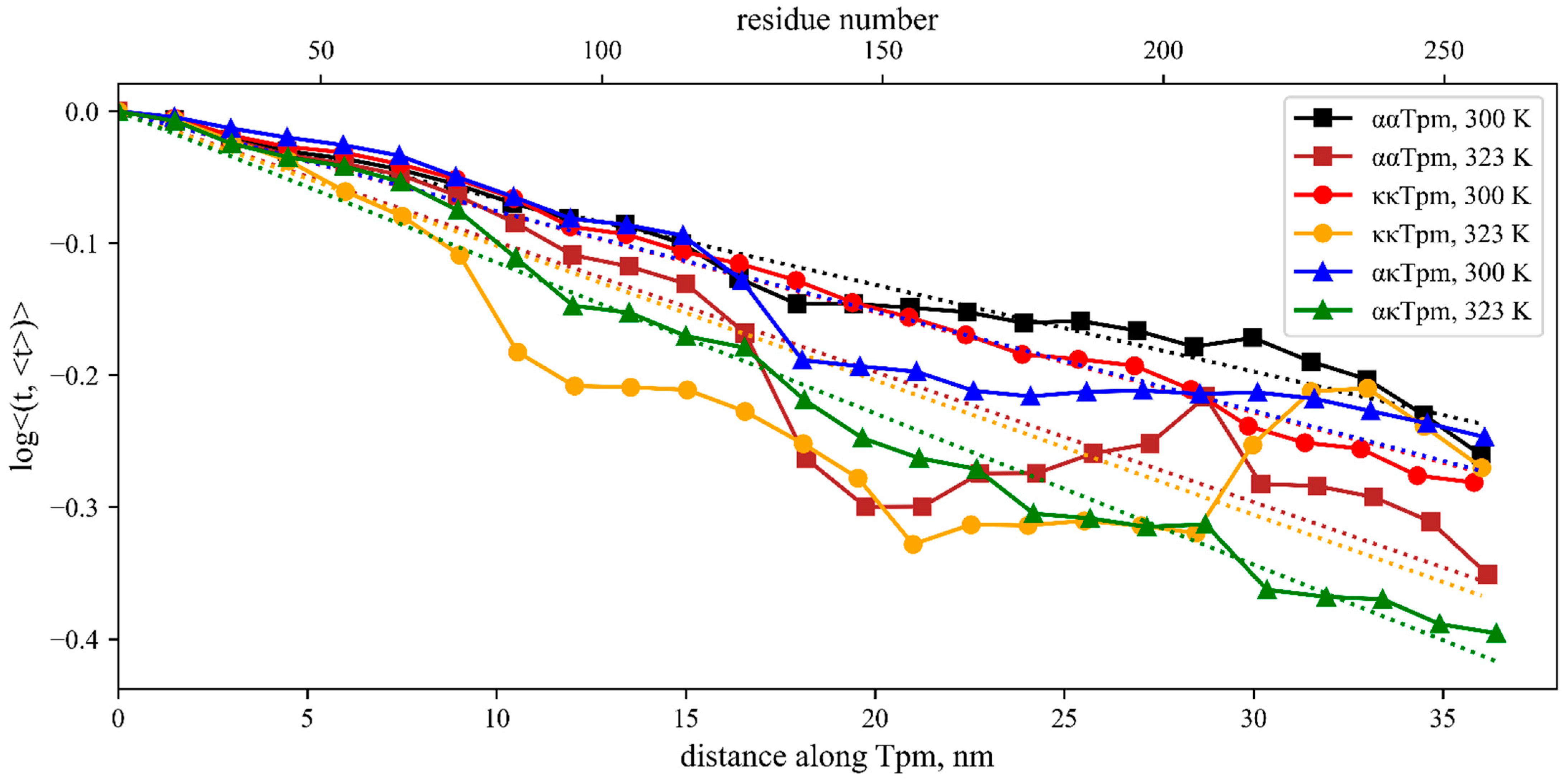
2.1.5. Molecular Dynamics (MD) Simulation
2.2. Interaction of the Tpm Isoforms with F-Actin
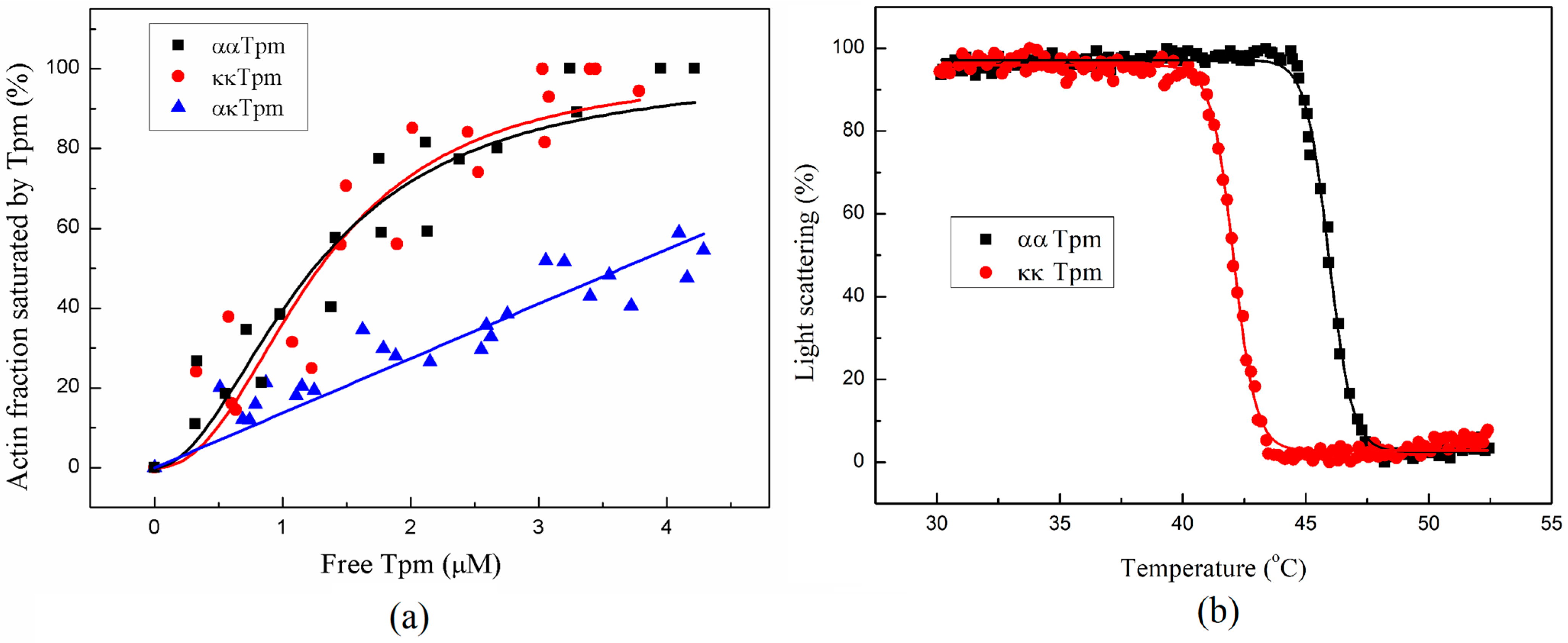
2.2.1. Affinity of ααTpm and κκTpm to F-Actin
2.2.2. The Thermal Stability of the Tpm–F-Actin Complex
2.3. Compering Regulatory Properties of ααTpm, κκTpm, and ακTpm
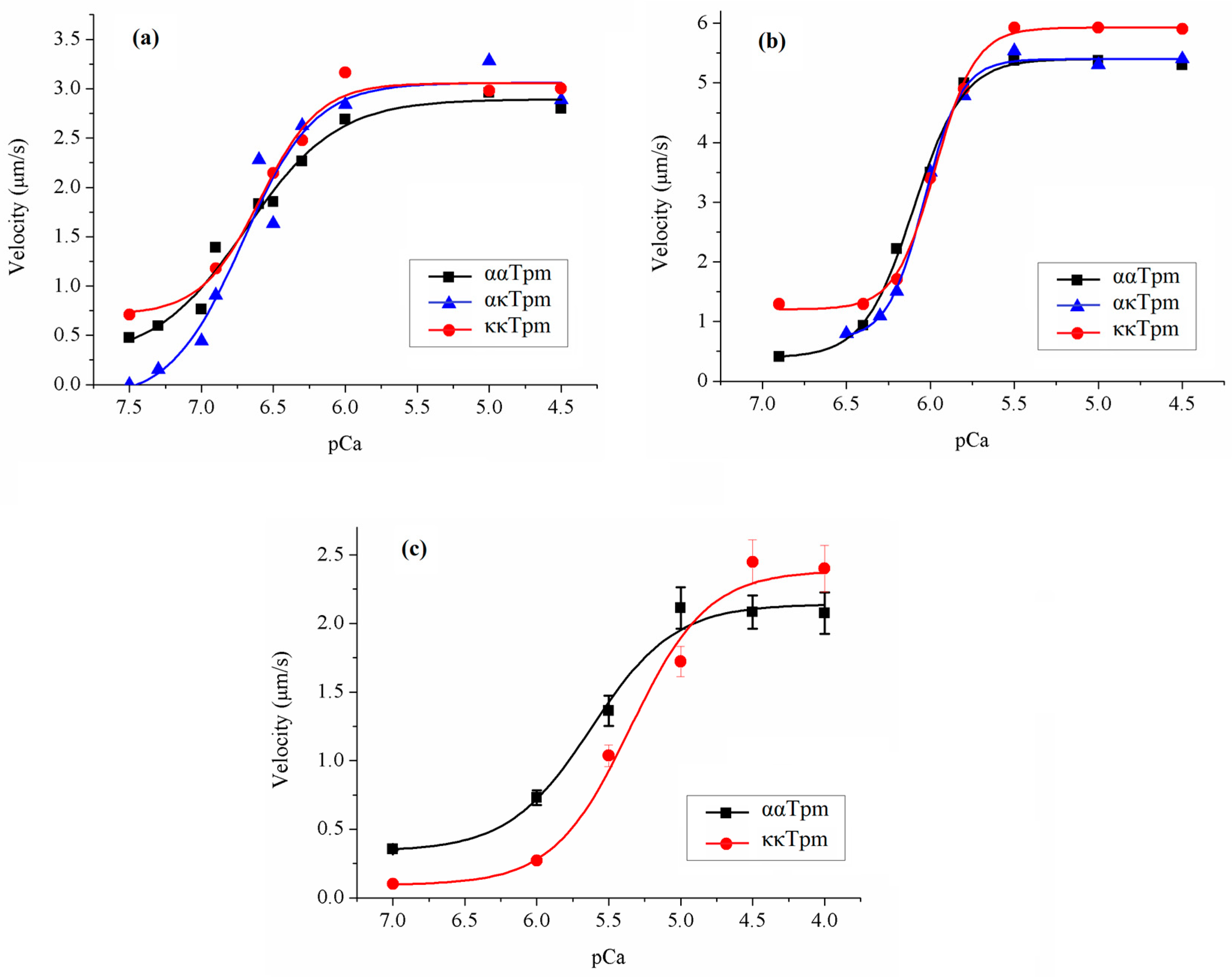
2.3.1. Effects of ααTpm, κκTpm, and ακTpm on Calcium Regulation of Actin–Myosin Interaction
2.3.2. Activation of Thin Filaments Containing ααTpm, κκTpm, and ακTpm by Myosin Cross-Bridges
2.4. Effect of Tn on the Interaction of ααTpm, κκTpm, and ακTpm with F-Actin
3. Discussion
4. Materials and Methods
4.1. Proteins
4.2. Differential Scanning Calorimetry (DSC)
4.3. Circular Dichroism
4.4. The Affinity of Tpm to F-Actin
4.5. Thermal Stability of the Tpm Complex with F-Actin
4.6. Viscosity Measurement
4.7. In Vitro Motility Assay
4.8. MD Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKeown, C.R.; Nowak, R.B.; Gokhin, D.S.; Fowler, V.M. Tropomyosin is required for cardiac morphogenesis, myofibril assembly, and formation of adherens junctions in the developing mouse embryo. Dev. Dyn. 2014, 243, 800–817. [Google Scholar] [CrossRef] [PubMed]
- England, J.; Granados-Riveron, J.; Polo-Parada, L.; Kuriakose, D.; Moore, C.; Brook, J.D.; Rutland, C.S.; Setchfield, K.; Gell, C.; Ghosh, T.K.; et al. Tropomyosin 1: Multiple roles in the developing heart and in the formation of congenital heart defects. J. Mol. Cell. Cardiol. 2017, 106, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.V. Vertebrate tropomyosin: Distribution, properties and function. J. Muscle Res. Cell. Motil. 2001, 22, 5–49. [Google Scholar] [CrossRef] [PubMed]
- Denz, C.R.; Narshi, A.; Zajdel, R.W.; Dube, D.K. Expression of a novel cardiac-specific tropomyosin isoform in humans. Biochem. Biophys. Res. Commun. 2004, 320, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Geeves, M.A.; Hitchcock-DeGregori, S.E.; Gunning, P.W. A systematic nomenclature for mammalian tropomyosin isoforms. J. Muscle Res. Cell. Motil. 2015, 36, 147–153. [Google Scholar] [CrossRef]
- Muthuchamy, M.; Boivin, G.P.; Grupp, I.L.; Wieczorek, D.F. Beta-tropomyosin overexpression induces severe cardiac abnormalities. J. Mol. Cell. Cardiol. 1998, 30, 1545–1557. [Google Scholar] [CrossRef]
- Rajan, S.; Jagatheesan, C.N.; Karam, M.L.; Alves, M.L.; Bodi, I.; Schwartz, A.; Bulcao, C.F.; D’Souza, K.M.; Akhter, S.A.; Boivin, G.P.; et al. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation 2010, 121, 410–418. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, D.; Gregorich, Z.; Chen, X.; Beyer, A.M.; Gutterman, D.D.; Ge, Y. In-depth proteomic analysis of human tropomyosin by top-down mass spectrometry. J. Muscle Res. Cell. Motil. 2013, 34, 199–210. [Google Scholar] [CrossRef]
- Karam, C.N.; Warren, C.M.; Rajan, S.; de Tombe, P.P.; Wieczorek, D.F.; Solaro, R.J. Expression of tropomyosin-κ induces dilated cardiomyopathy and depresses cardiac myofilament tension by mechanisms involving cross-bridge dependent activation and altered tropomyosin phosphorylation. J. Muscle Res. Cell. Motil. 2011, 31, 315–322. [Google Scholar] [CrossRef]
- Zajdel, R.W.; McLean, M.D.; Dube, S.; Dube, D.K. Expression of tropomyosin in relation to myofibrillogenesis in axolotl hearts. Regen. Med. Res. 2013, 1, 8. [Google Scholar] [CrossRef]
- Lehrer, S.S. Intramolecular crosslinking of tropomyosin via disulfide bond formation: Evidence for chain register. Proc. Natl. Acad. Sci. USA 1975, 72, 3377–3381. [Google Scholar] [CrossRef]
- Freire, E.; Biltonen, R.L. Statistical mechanical deconvolution of thermal transitions in macromolecules. I. Theory and application to homogeneous systems. Biopolymers 1978, 17, 463–479. [Google Scholar] [CrossRef]
- Kremneva, E.; Boussouf, S.; Nikolaeva, O.; Maytum, R.; Geeves, M.A.; Levitsky, D.I. Effects of two familial hypertrophic cardiomyopathy mutations in a-tropomyosin, Asp175Asn and Glu180Gly, on the thermal unfolding of actin-bound tropomyosin. Biophys. J. 2004, 87, 3922–3933. [Google Scholar] [CrossRef]
- Matyushenko, A.M.; Koubassova, N.A.; Shchepkin, D.V.; Kopylova, G.V.; Nabiev, S.R.; Nikitina, L.V.; Bershitsky, S.Y.; Levitsky, D.I.; Tsaturyan, A.K. The effects of cardiomyopathy-associated mutations in the head-to-tail overlap junction of α-tropomyosin on its properties and interaction with actin. Int. J. Biol. Macromol. 2019, 125, 1266–1274. [Google Scholar] [CrossRef]
- Matyushenko, A.M.; Artemova, N.V.; Sluchanko, N.N.; Levitsky, D.I. Effects of two stabilizing substitutions, D137L and G126R, in the middle part of α-tropomyosin on the domain structure of its molecule. Biophys. Chem. 2015, 196, 77–85. [Google Scholar] [CrossRef]
- Matyushenko, A.M.; Shchepkin, D.V.; Kopylova, G.V.; Bershitsky, S.Y.; Koubassova, N.A.; Tsaturyan, A.K.; Levitsky, D.I. Functional role of the core gap in the middle part of tropomyosin. FEBS J. 2018, 285, 871–886. [Google Scholar] [CrossRef]
- Minakata, S.; Maeda, K.; Oda, N.; Wakabayashi, K.; Nitanai, Y.; Maéda, Y. Two-crystal structures of tropomyosin C-terminal fragment 176-273: Exposure of the hydrophobic core to the solvent destabilizes the tropomyosin molecule. Biophys. J. 2008, 95, 710–719. [Google Scholar] [CrossRef]
- Sumida, J.P.; Wu, E.; Lehrer, S.S. Conserved Asp-137 imparts flexibility to tropomyosin and affects function. J. Biol. Chem. 2008, 283, 6728–6734. [Google Scholar] [CrossRef]
- Ohtsuki, I. Troponin: Structure, function and dysfunction. Adv. Exp. Med. Biol. 2007, 592, 21–36. [Google Scholar] [CrossRef]
- Katrukha, I.A. Human cardiac troponin complex. Structure and function. Biochemistry 2013, 78, 1447–1465. [Google Scholar] [CrossRef]
- Jin, J.-P. Evolution, regulation, and function of N-terminal variable region of troponin T: Modulation of muscle contractility and beyond international review of cell and molecular biology. Int. Rev. Cell. Mol. Biol. 2016, 321, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, V.V.; Kopylova, G.V.; Shchepkin, D.V.; Kochurova, A.M.; Kechko, O.I.; Borzova, V.A.; Ryabkova, N.S.; Katrukha, I.A.; Mitkevich, V.A.; Bershitsky, S.Y.; et al. Impact of Troponin in Cardiomyopathy Development Caused by Mutations in Tropomyosin. Int. J. Mol. Sci. 2022, 23, 15723. [Google Scholar] [CrossRef]
- Palm, T.; Graboski, S.; Hitchcock-DeGregori, S.E.; Greenfield, N.J. Disease-causing mutations in cardiac troponin T: Identification of a critical tropomyosin-binding region. Biophys. J. 2001, 81, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J.; Huang, Y.J.; Swapna, G.V.; Bhattacharya, A.; Rapp, B.; Singh, A.; Montelione, G.T.; Hitch-cock-DeGregori, S.E. Solution NMR structure of the junction between tropomyosin molecules: Implications for actin binding and regulation. J. Mol. Biol. 2006, 364, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.; Viswanathan, M.C.; Woulfe, K.C.; Schmidt, W.; Sidor, A.; Liu, T.; Nguyen, T.H.; Trinh, B.; Wilson, C.; Madathil, S.; et al. TNNT2 mutations in the tropomyosin binding region of TNT1 disrupt its role in contractile inhibition and stimulate cardiac dysfunction. Proc. Natl. Acad. Sci. USA 2020, 117, 18822–18831. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, B.; Sunitha, M.S.; Mukherjee, S.; Chowdhury, R.R.; Haque, F.; Sekar, N.; Sowdhamini, R.; Spudich, J.A.; Mercer, J.A. Molecular mechanisms and structural features of cardiomyopathy-causing troponin T mutants in the tropomyosin overlap region. Proc. Natl. Acad. Sci. USA 2017, 114, 11115–11120. [Google Scholar] [CrossRef]
- Colpan, M.; Ly, T.; Grover, S.; Tolkatchev, D.; Kostyukova, A.S. The cardiomyopathy-associated K15N mutation in tro-pomyosin alters actin filament pointed end dynamics. Arch. Biochem. Biophys. 2017, 630, 18–26. [Google Scholar] [CrossRef]
- Sundar, S.; Rynkiewicz, M.J.; Ghosh, A.; Lehman, W.; Moore, J.R. Cardiomyopathy mutation alters end-to-end junction of tropomyosin and reduces calcium sensitivity. Biophys. J. 2020, 118, 303–312. [Google Scholar] [CrossRef]
- Racca, W.; Rynkiewicz, M.J.; LaFave, N.; Ghosh, A.; Lehman, W.; Moore, J.R. M8R tropomyosin mutation disrupts actin binding and filament regulation: The beginning affects the middle and end. J. Biol. Chem. 2020, 295, 17128–17137. [Google Scholar] [CrossRef]
- Lehman, W.; Hatch, V.; Korman, V.; Rosol, M.; Thomas, L.; Maytum, R.; Geeves, M.A.; Van Eyk, J.E.; Tobacman, L.S.; Craig, R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J. Mol. Biol. 2000, 302, 593–606. [Google Scholar] [CrossRef]
- Sliwińska, M.; Zukowska, M.; Borys, D.; Moraczewska, J. Different positions of tropomyosin isoforms on actin filament are determined by specific sequences of end-to-end overlaps. Cytoskeleton 2011, 68, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, U.P.; Aronshtam, A.; Lowey, S. Cardiac myosin isoforms from different species have unique enzymatic and mechanical properties. Biochemistry 2004, 43, 15058–15065. [Google Scholar] [CrossRef] [PubMed]
- Prodanovic, M.; Geeves, M.A.; Poggesi, C.; Regnier, M.; Mijailovich, S.M. Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans. Int. J. Mol. Sci. 2022, 23, 1135. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; McGreig, J.E.; Jeanfavre, S.T.; Walklate, J.; Vera, C.D.; Farré, M.; Mulvihill, D.P.; Baines, A.J.; Ridout, M.; Leinwand, L.A.; et al. Identification of sequence changes in myosin II that adjust muscle contraction velocity. PLoS Biol. 2021, 19, e3001248. [Google Scholar] [CrossRef]
- Doran, M.H.; Pavadai, E.; Rynkiewicz, M.J.; Walklate, J.; Bullitt, E.; Moore, J.R.; Regnier, M.; Geeves, M.A.; Lehman, W. Cryo-EM and Molecular Docking Shows Myosin Loop 4 Contacts Actin and Tropomyosin on Thin Filaments. Biophys. J. 2020, 119, 821–830. [Google Scholar] [CrossRef]
- Doran, M.H.; Rynkiewicz, M.J.; Pavadai, E.; Bodt, S.M.L.; Rasicci, D.; Moore, J.R.; Yengo, C.M.; Bullitt, E.; Lehman, W. Myosin loop-4 is critical for optimal tropomyosin repositioning on actin during muscle activation and relaxation. J. Gen. Physiol. 2023, 155, e202213274. [Google Scholar] [CrossRef]
- Walklate, J.; Ferrantini, C.; Johnson, C.A.; Tesi, C.; Poggesi, C.; Geeves, M.A. Alpha and beta myosin isoforms and human atrial and ventricular contraction. Cell. Mol. Life Sci. 2021, 78, 7309–7337. [Google Scholar] [CrossRef]
- Yamashita, H.; Sugiura, S.; Fujita, H.; Yasuda, S.; Nagai, R.; Saeki, Y.; Sunagawa, K.; Sugi, H. Myosin light chain isoforms modify force-generating ability of cardiac myosin by changing the kinetics of actin-myosin interaction. Cardiovasc. Res. 2003, 60, 580–588. [Google Scholar] [CrossRef]
- Shchepkin, D.V.; Nikitina, L.V.; Bershitsky, S.Y.; Kopylova, G.V. The isoforms of α-actin and myosin affect the Ca2+ regulation of the actin-myosin interaction in the heart. Biochem. Biophys. Res. Commun. 2017, 490, 324–329. [Google Scholar] [CrossRef]
- Kopylova, G.V.; Berg, V.Y.; Kochurova, A.M.; Matyushenko, A.M.; Bershitsky, S.Y.; Shchepkin, D.V. The effects of the tropomyosin cardiomyopathy mutations on the calcium regulation of actin-myosin interaction in the atrium and ventricle differ. Biochem. Biophys. Res. Commun. 2022, 588, 29–33. [Google Scholar] [CrossRef]
- Monteiro, P.B.; Lataro, R.C.; Ferro, J.A.; Reinach Fde, C. Functional alpha-tropomyosin produced in Escherichia coli. A dipeptide extension can substitute the amino-terminal acetyl group. J. Biol. Chem. 1994, 269, 10461–10466. [Google Scholar] [CrossRef] [PubMed]
- Kalyva, A.; Schmidtmann, A.; Geeves, M.A. In vitro formation and characterization of the skeletal muscle alpha.beta tropomyosin heterodimers. Biochemistry 2012, 51, 6388–6399. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.D. Preparation of troponin and its subunits. Methods Enzymol. 1982, 85, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Margossian, S.S.; Lowey, S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982, 85, 55–71. [Google Scholar] [CrossRef]
- Pardee, J.D.; Spudich, J.A. Purification of muscle actin. Methods Enzymol. 1982, 85 Pt B, 164–179. [Google Scholar] [CrossRef]
- Mashanov, G.I.; Molloy, J.E. Automatic detection of single fluorophores in live cells. Biophys. J. 2007, 92, 2199–2211. [Google Scholar] [CrossRef]
- Brunet, N.M.; Chase, P.B.; Mihajlović, G.; Schoffstall, B. Ca2+-regulatory function of the inhibitory peptide region of cardiac troponin I is aided by the C-terminus of cardiac troponin T: Effects of familial hypertrophic cardiomyopathy mutations cTnI R145G and cTnT R278C, alone and in combination, on filament sliding. Arch. Biochem. Biophys. 2014, 552–553, 11–20. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Whitby, F.G.; Phillips, G.N., Jr. Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins 2000, 38, 49–59. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Shapovalov, M.V.; Dunbrack Jr, R.L. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 2011, 19, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Statistical Mechanics of Chain Molecules; Interscience Publishers: Hoboken, NJ, USA, 1969. [Google Scholar]




| Tpm | Tm (°C) | ||
|---|---|---|---|
| First Transition | Second Transition | Third Transition | |
| ααTpm | 36.6 | 45.2 | 52.2 |
| ακTpm | 36.8 | 44.6 | 55.8 |
| κκTpm | 41.4 | 45.6 | |
| Tpm | Tm # (°C) | ∆Hcal (kJ mol−1) | ∆Hcal (% of Total) | Total ∆Hcal § (kJ mol−1) |
|---|---|---|---|---|
| ααTpm | 1300 | |||
| Domain 1 | 36.5 | 200 | 15 | |
| Domain 2 | 43.2 | 580 | 45 | |
| Domain 3 | 50.8 | 520 | 40 | |
| κκTpm (reduced) | 1400 | |||
| Domain 1 | 34.4 | 250 | 18 | |
| Domain 2 | 37.8 | 480 | 34 | |
| Domain 3 | 42.8 | 590 | 42 | |
| Domain 4 | 49.8 | 80 | 6 |
| κκTpm | Tm # (°C) | ∆Hcal (kJ mol−1) | ∆Hcal (% of Total) | Total ∆Hcal § (kJ mol−1) | Cross-Linking (%) |
|---|---|---|---|---|---|
| 1st heating | 1405 | ~5% | |||
| Domain 1 | 33.9 | 290 | 20 | ||
| Domain 2 | 37.9 | 670 | 48 | ||
| Domain 3 | 42.4 | 420 | 30 | ||
| Domain 4 | 53.9 | 25 | 2 | ||
| 2nd heating | 1625 | ~73% | |||
| Domain 2 | 37.9 | 1150 | 70 | ||
| Domain 3 | 41.9 | 130 | 8 | ||
| Domain 4 | 52.4 | 345 | 22 | ||
| 5th heating | 1580 | ~82% | |||
| Domain 2 | 37.4 | 945 | 60 | ||
| Domain 3 | 41.6 | 115 | 7 | ||
| Domain 4 | 52.3 | 525 | 33 |
| Tpm | 300 K (27 °C) | 315 K (42 °C) | 323 K (50 °C) | |||
|---|---|---|---|---|---|---|
| ξ (nm) | Kb (pN·nm2) | ξ (nm) | Kb (pN·nm2) | ξ (nm) | Kb (pN·nm2) | |
| ααTpm | 147 (157) 152 (144) | 607 (650) 630 (597) | 116 (118) 106 (85) | 503 (512) 460 (369) | 115 (148) 101 (159) | 512 (662) 452(708) |
| κκTpm | 148 (171) 132 (129) | 614 (710) 545 (533) | 105 (107) 128 (129) | 458 (463) 559 (561) | 70 (93) 98 (118) | 312 (417) 437 (528) |
| ακTpm | 155 (162) 132 (184) | 640 (673) 547 (761) | 149 (161) 131 (169) | 648 (702) 569 (733) | 94 (121) 87 (110) | 421 (540) 389 (492) |
| Myosin | Tpm | Vmax (µm/s) | V0 (µm/s) | pCa50 |
|---|---|---|---|---|
| pig LV | ααTpm | 2.9 ± 0.1 | 0.3 ± 0.2 | 6.69 ± 0.09 |
| κκTpm | 3.1 ± 0.2 | 0.7 ± 0.2 | 6.59 ± 0.08 | |
| ακTpm | 3.1 ± 0.1 | 0 * | 6.71 ± 0.09 | |
| pig LA | ααTpm | 5.4 ± 0.1 | 0.4 ± 0.2 | 6.10 ± 0.02 |
| κκTpm | 5.5 ± 0.1 | 1.2 ± 0.1 | 5.97 ± 0.01 | |
| ακTpm | 5.4 ± 0.1 | 0.7 ± 0.1 | 6.03 ± 0.01 | |
| rat LV | ααTpm | 2.1 ± 0.1 | 0.3 ± 0.1 | 5.62 ± 0.08 |
| κκTpm | 2.4 ± 0.1 * | 0.2 ± 0.1 | 5.35 ± 0.09 * |
| Myosin | Tpm | Vmax (µm/s) | C50 (µg/mL) |
|---|---|---|---|
| LA | ααTpm | 5.2 ± 0.1 | 76.5 ± 2.1 |
| κκTpm | 5.8 ± 0.01 | 62.5 ± 0.4 * | |
| αĸTpm | 5.4 ± 0.2 | 55.1 ± 1.0 * | |
| LV | ααTpm | 2.8 ± 0.2 | 30.1 ± 1.0 |
| κκTpm | 3.0 ± 0.1 | 23.4 ± 5.2 | |
| αĸTpm | 2.9 ± 0.3 | 30.2 ± 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopylova, G.V.; Kochurova, A.M.; Yampolskaya, D.S.; Nefedova, V.V.; Tsaturyan, A.K.; Koubassova, N.A.; Kleymenov, S.Y.; Levitsky, D.I.; Bershitsky, S.Y.; Matyushenko, A.M.; et al. Structural and Functional Properties of Kappa Tropomyosin. Int. J. Mol. Sci. 2023, 24, 8340. https://doi.org/10.3390/ijms24098340
Kopylova GV, Kochurova AM, Yampolskaya DS, Nefedova VV, Tsaturyan AK, Koubassova NA, Kleymenov SY, Levitsky DI, Bershitsky SY, Matyushenko AM, et al. Structural and Functional Properties of Kappa Tropomyosin. International Journal of Molecular Sciences. 2023; 24(9):8340. https://doi.org/10.3390/ijms24098340
Chicago/Turabian StyleKopylova, Galina V., Anastasia M. Kochurova, Daria S. Yampolskaya, Victoria V. Nefedova, Andrey K. Tsaturyan, Natalia A. Koubassova, Sergey Y. Kleymenov, Dmitrii I. Levitsky, Sergey Y. Bershitsky, Alexander M. Matyushenko, and et al. 2023. "Structural and Functional Properties of Kappa Tropomyosin" International Journal of Molecular Sciences 24, no. 9: 8340. https://doi.org/10.3390/ijms24098340
APA StyleKopylova, G. V., Kochurova, A. M., Yampolskaya, D. S., Nefedova, V. V., Tsaturyan, A. K., Koubassova, N. A., Kleymenov, S. Y., Levitsky, D. I., Bershitsky, S. Y., Matyushenko, A. M., & Shchepkin, D. V. (2023). Structural and Functional Properties of Kappa Tropomyosin. International Journal of Molecular Sciences, 24(9), 8340. https://doi.org/10.3390/ijms24098340






