Effect of Physiological Fluid on the Photothermal Properties of Gold Nanostructured
Abstract
1. Introduction
2. Results
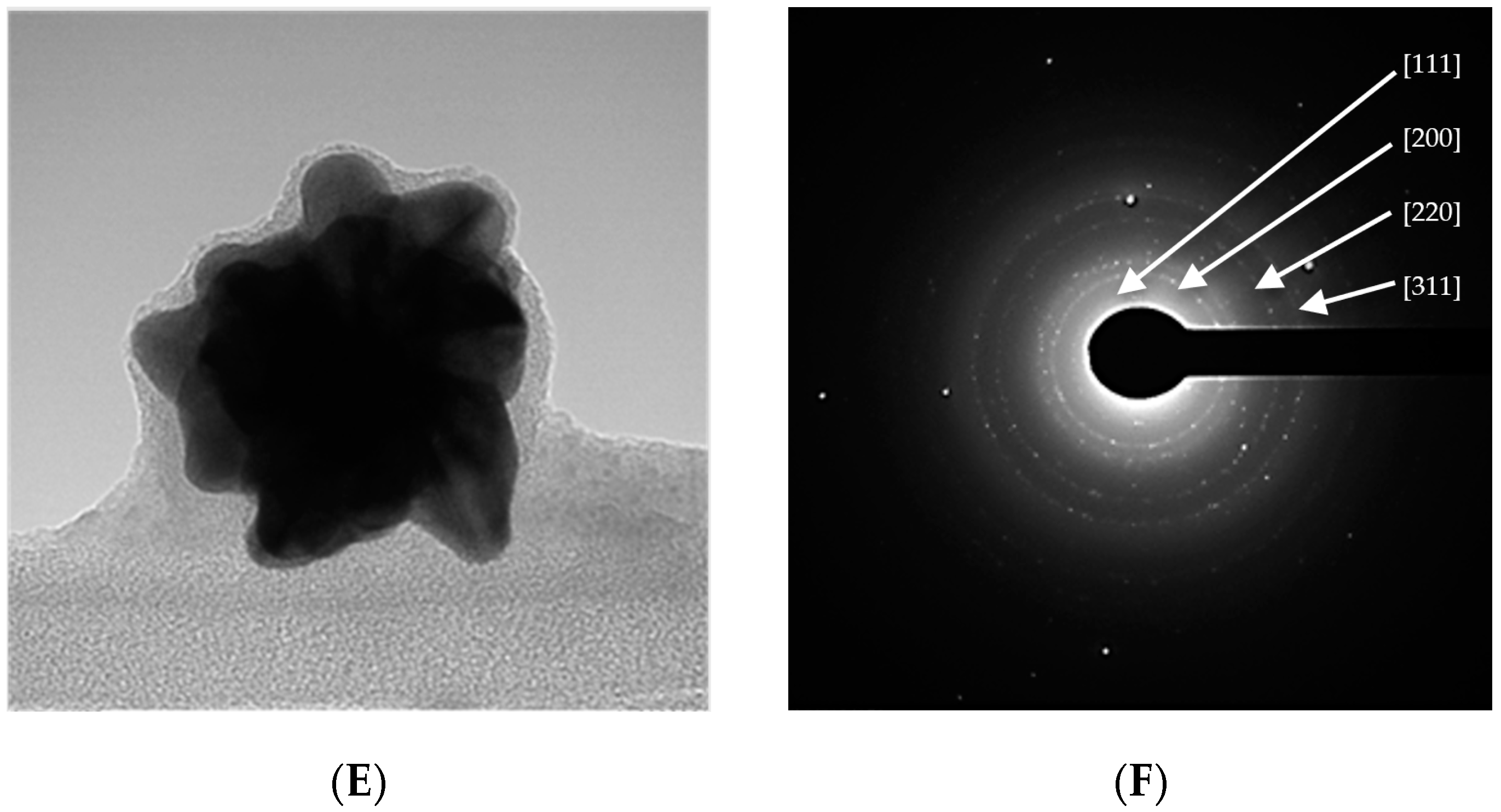
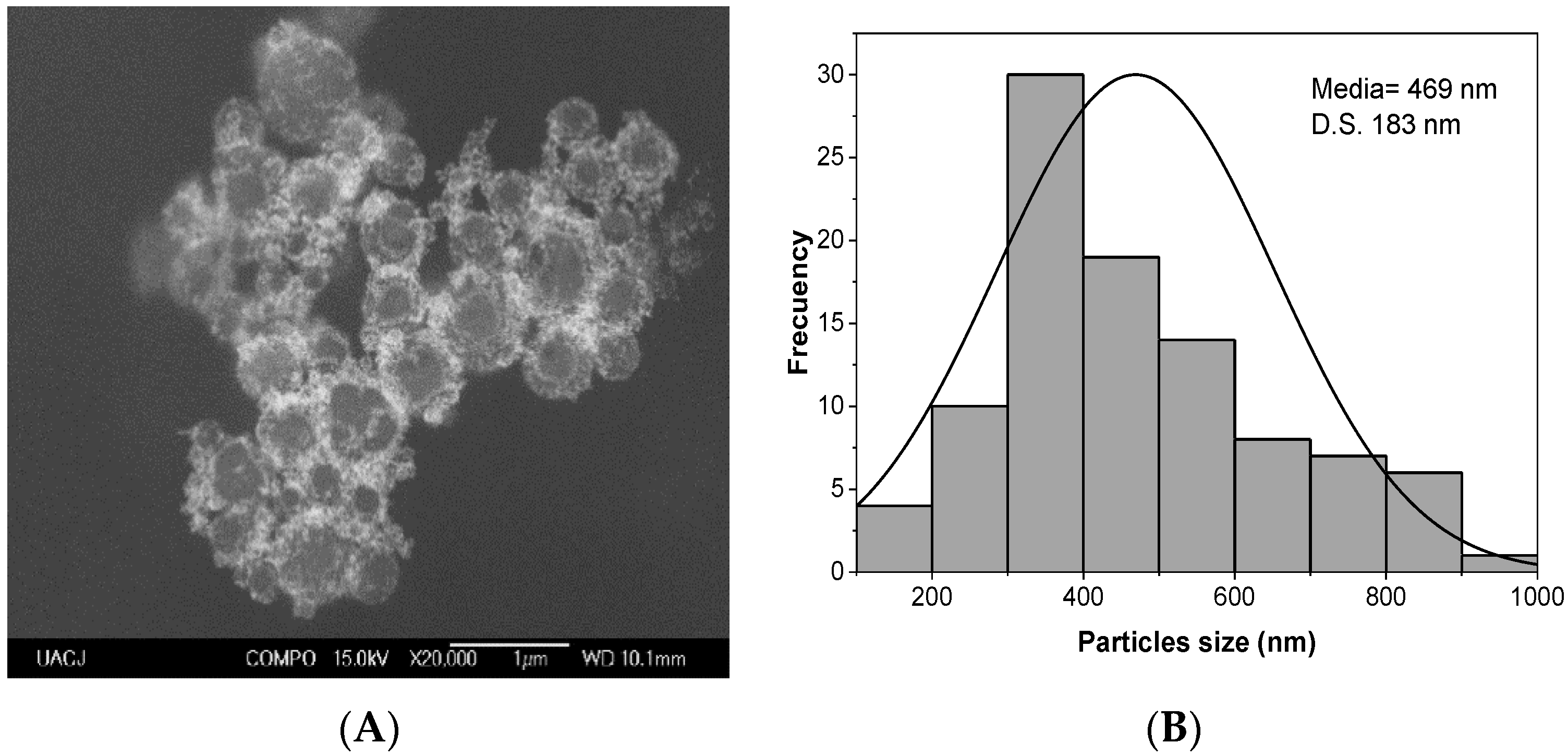
2.1. Raw Gold Colloids
2.2. Photothermal Properties
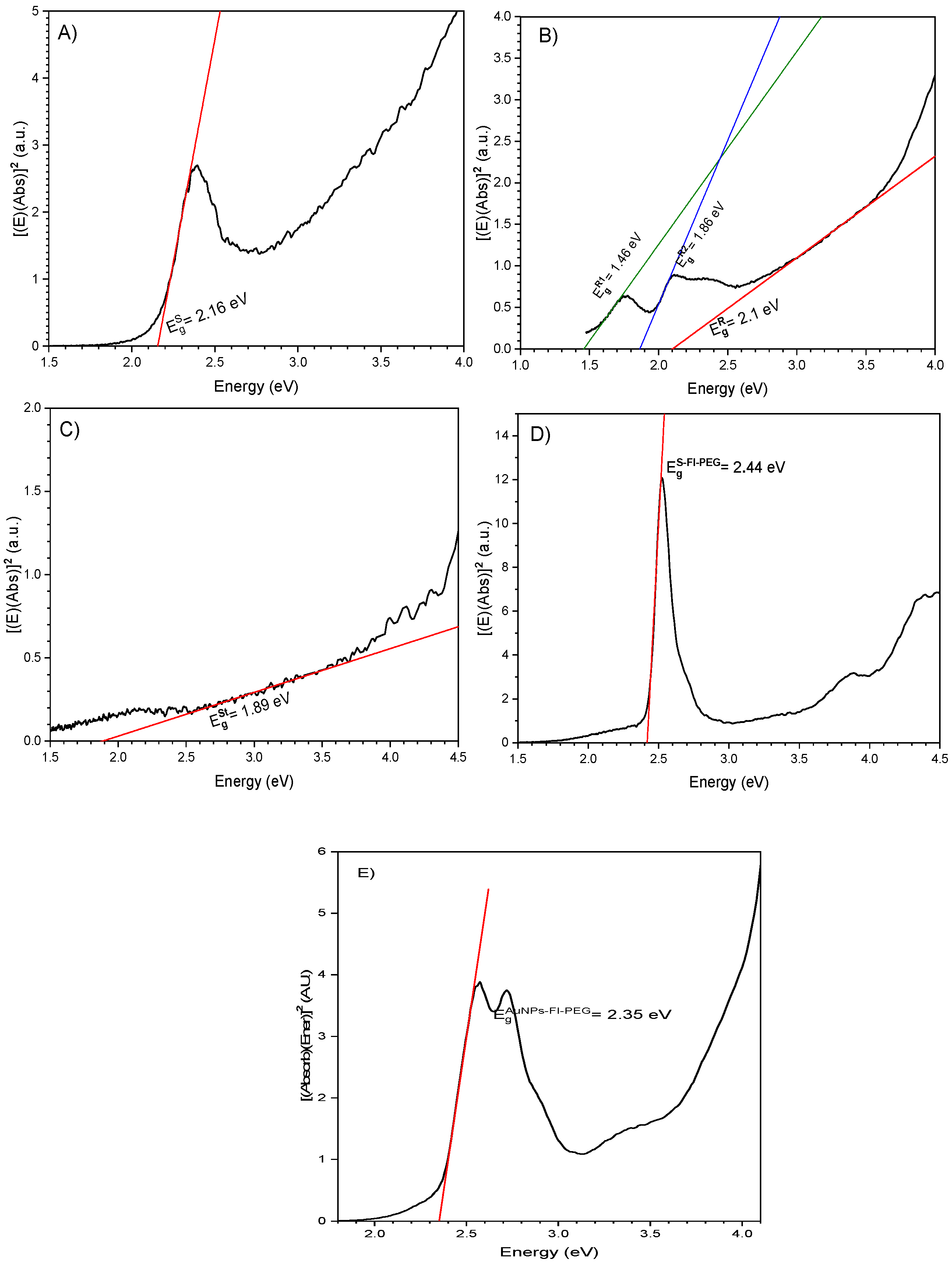
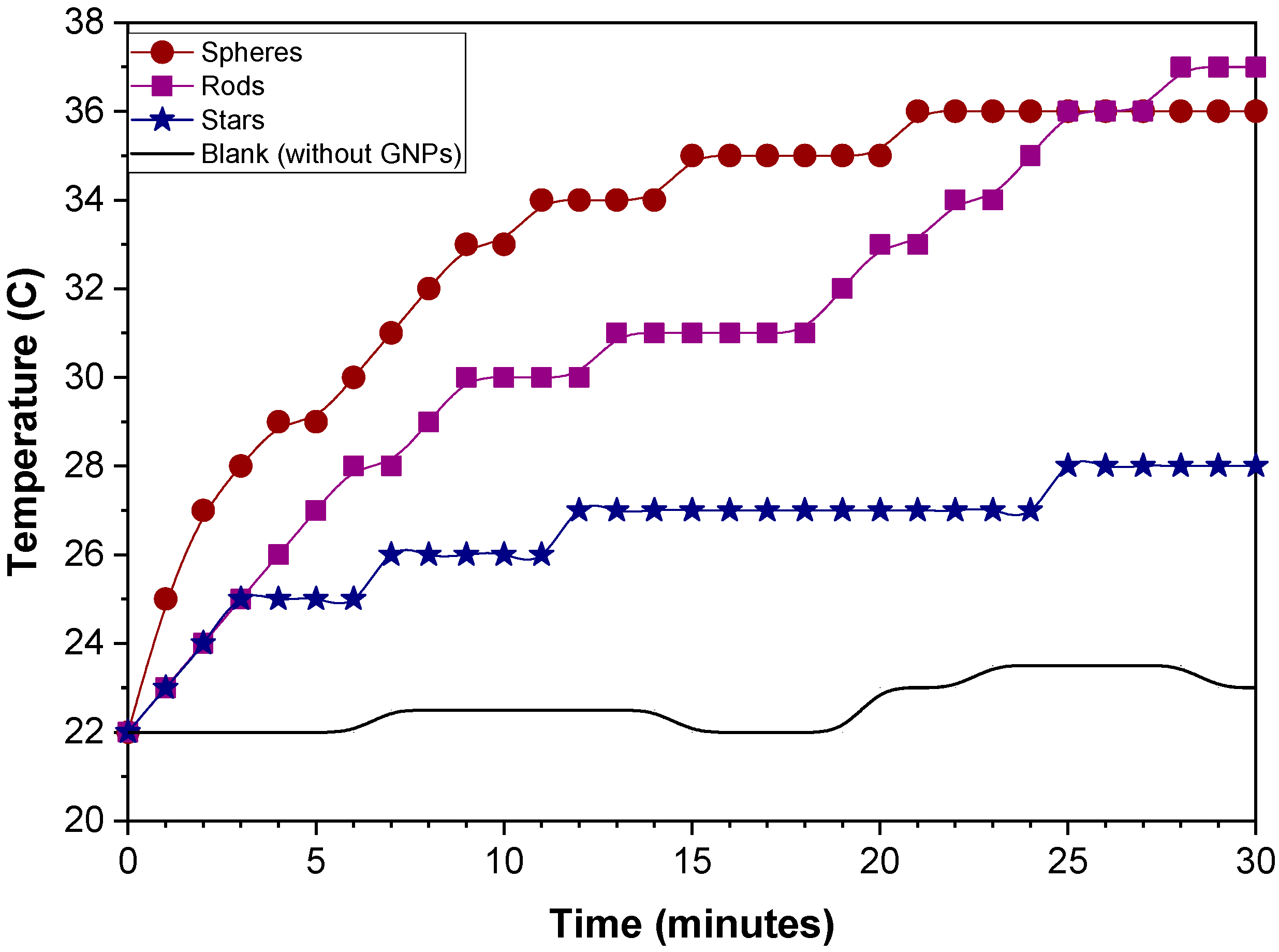
2.2.1. Particle Shape Effect
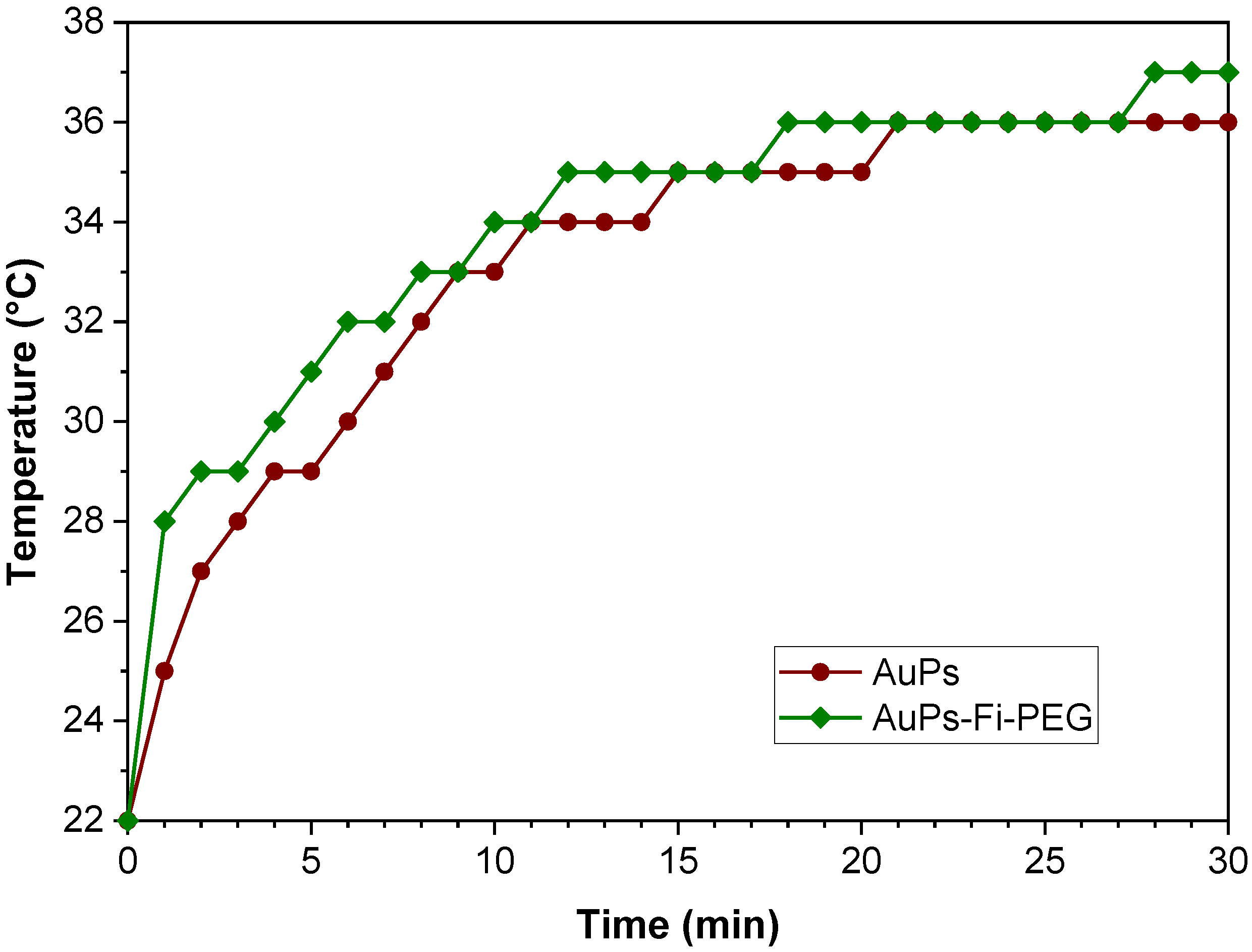
2.2.2. Effect of Attached Organic Molecules
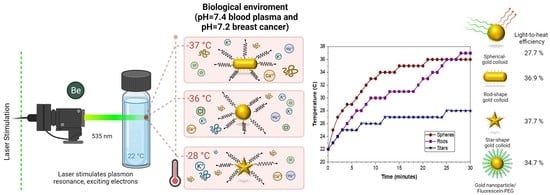
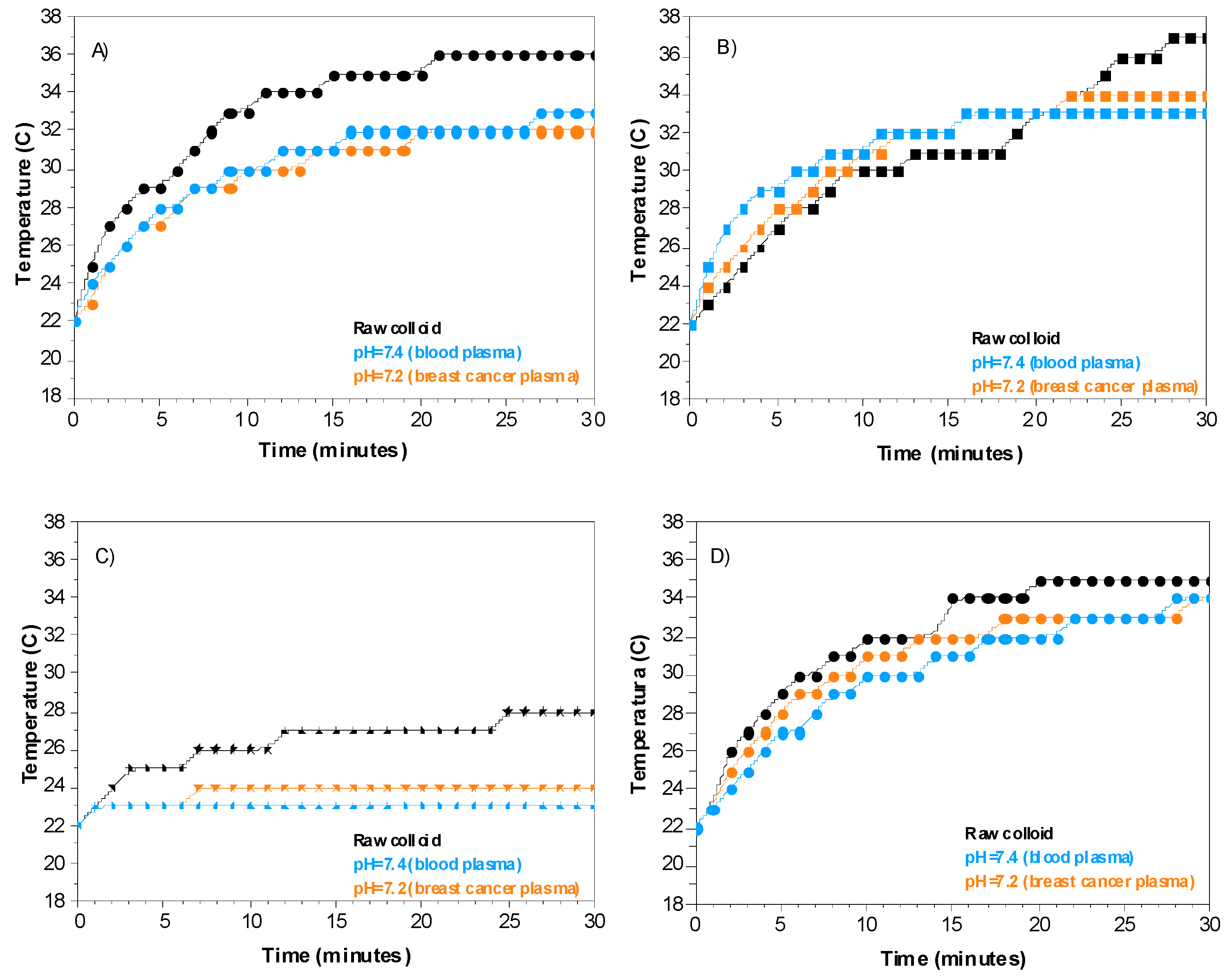
2.2.3. Effect of the Physiological Environment
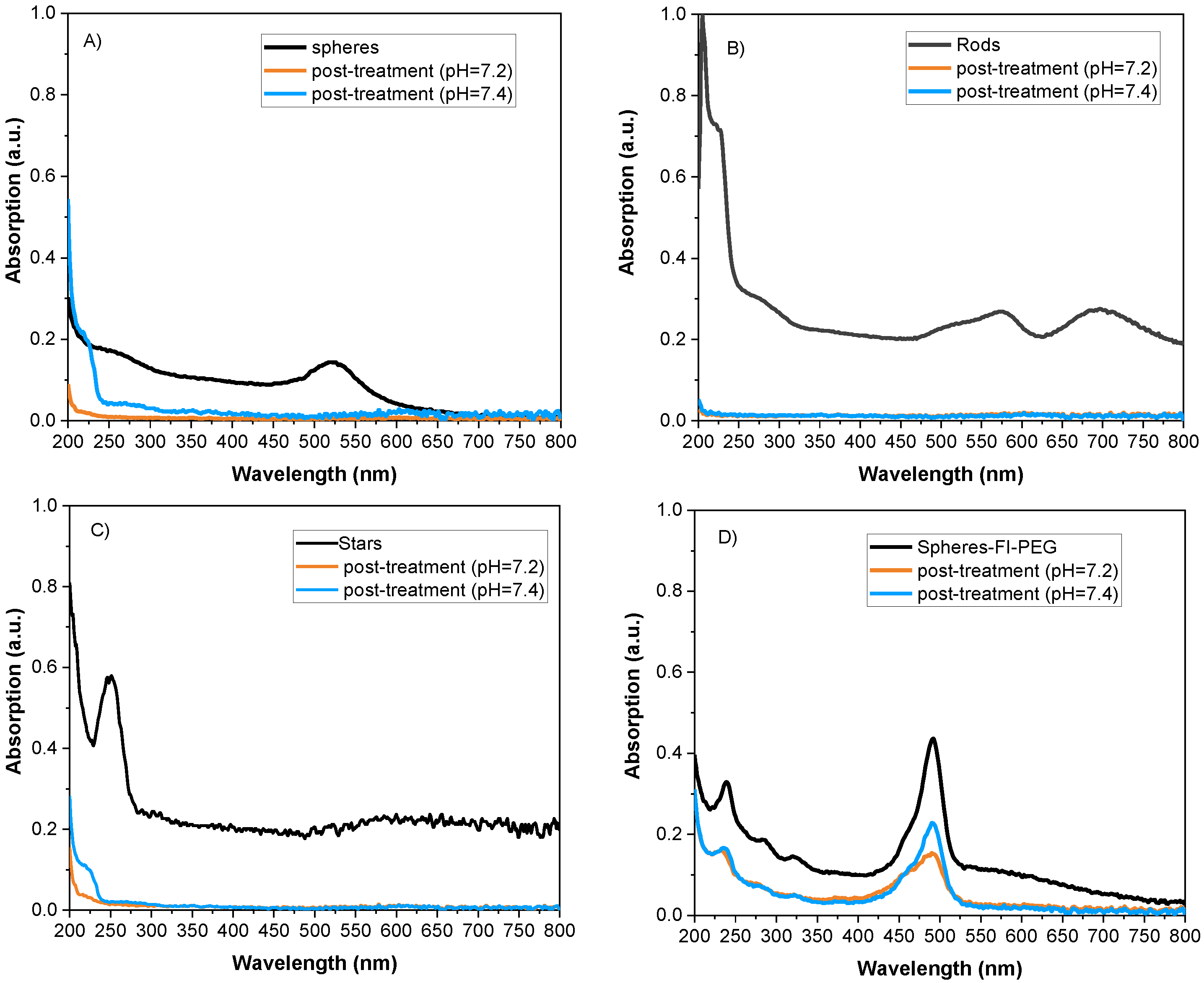
2.3. Photodegradation
3. Discussion
4. Materials and Methods
4.1. Synthesis of Raw Colloid
4.2. Particle Shape Modification
4.3. Gold Nanoparticles with Fluorescein and Polyethylene Glycol Colloid
4.4. Determination of Photothermal Properties
4.5. Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2022, 33, 1–16. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2021, 3, 792–828. [Google Scholar] [CrossRef]
- Mikhailova, E.O.; Thakur, K. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Van Vlerken, L.E.; Amiji, M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Bharathiraja, S.; Bui, N.Q.; Jang, B.; Oh, Y.O.; Lim, I.G.; Oh, J. Doxorubicin-loaded fucoidan capped gold nanoparticles for drug delivery and photoacoustic imaging. Int. J. Biol. Macromol. 2016, 91, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cao, C.; Gao, J.; Cai, W.; Li, J.; Wu, D.; Kong, Y. Gold Nanorods@Mesoporous SiO2@Hyaluronic Acid Core-Shell Nanoparticles for Controlled Drug Delivery. ACS Appl. Nano Mater. 2022, 5, 7440–7448. [Google Scholar] [CrossRef]
- Lemire, C.; Meyer, R.; Shaikhutdinov, S.; Freund, H.J. Do Quantum Size Effects Control CO Adsorption on Gold Nanoparticles? Angew. Chem. Int. Ed. 2004, 43, 118–121. [Google Scholar] [CrossRef]
- Bergen, J.M.; von Recum, H.A.; Goodman, T.T.; Massey, A.P.; Pun, S.H. Gold Nanoparticles as a Versatile Platform for Optimizing Physicochemical Parameters for Targeted Drug Delivery. Macromol. Biosci. 2006, 6, 506–516. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; MacKey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Kingston, D.G.I.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Kouneski, S. Gold Nanoparticles as a Drug Delivery Vehicle System Against ErbB2+ Breast Cancer. FASEB J. 2022, 36, 0892-6638. [Google Scholar] [CrossRef]
- Visaria, R.K.; Griffin, R.J.; Williams, B.W.; Ebbini, E.S.; Paciotti, G.F.; Song, C.W.; Bischof, J.C. Enhancement of tumor thermal therapy using gold nanoparticle–assisted tumor necrosis factor-α delivery. Mol. Cancer Ther. 2006, 5, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Schärtl, W. Current directions in core–shell nanoparticle design. Nanoscale 2010, 2, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Amin, M.; Huang, W.; Seynhaeve, A.L.B.; ten Hagen, T.L.M. Hyperthermia and temperature-sensitive nanomaterials for spatiotemporal drug delivery to solid tumors. Pharmaceutics 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-dependent photothermal conversion efficiencies of plasmonically heated gold nanoparticles. J. Phys. Chem. C 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Mateu Ferrando, R.; Lay, L.; Polito, L. Gold nanoparticle-based platforms for vaccine development. Drug Discov. Today Technol. 2020, 38, 57–67. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2010, 19, 409–453. [Google Scholar] [CrossRef]
- Ma, W.Y.; Yang, H.; Hilton, J.P.; Lin, Q.; Liu, J.Y.; Huang, L.X.; Yao, J.; Huang, H.J.; Yu, C.P.; Chang, H.C.; et al. A numerical investigation of the effect of vertex geometry on localized surface plasmon resonance of nanostructures. Opt. Express 2010, 18, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6446–6465. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Periakaruppan, P.; Thomas, V.; John, J.; Mathe, S.; Thomas, T.; Jose, J.; Rejeena, I.; Mujeeb, A. Morphology dependent nonlinear optical and photocatalytic activity of anisotropic plasmonic silver. RSC Adv. 2018, 8, 41288–41298. [Google Scholar] [CrossRef] [PubMed]
- Ojea-Jiménez, I.; Campanera, J.M. Molecular modeling of the reduction mechanism in the citrate-mediated synthesis of gold nanoparticles. J. Phys. Chem. C 2012, 116, 23682–23691. [Google Scholar] [CrossRef]
- Kereselidze, Z.; Romero, V.H.; Peralta, X.G.; Santamaria, F. Gold Nanostar Synthesis with a Silver Seed Mediated Growth Method. J. Vis. Exp. 2012, 59, 3570. [Google Scholar] [CrossRef]
- Ivaskovic, P. Bottom-Up Fabrication of a Plasmonic Nanodevice for Guiding Light. Ph.D. Thesis, École Doctorale des Sciences Chimiques, Bordeaux, France, 2017. Available online: https://tel.archives-ouvertes.fr/tel-01668475/ (accessed on 7 March 2023).
- Pu, Y.; Zhao, Y.; Zheng, P.; Li, M. Elucidating the Growth Mechanism of Plasmonic Gold Nanostars with Tunable Optical and Photothermal Properties. Inorg. Chem. 2018, 57, 8599–8607. [Google Scholar] [CrossRef]
- Ramsey, J.D.; Zhou, L.; Kyle Almlie, C.; Lange, J.D.; Burrows, S.M. Achieving plasmon reproducibility from surfactant free gold nanostar synthesis. New J. Chem. 2015, 39, 9098–9108. [Google Scholar] [CrossRef]
- Moustaoui, H.; Saber, J.; Djeddi, I.; Liu, Q.; Diallo, A.T.; Spadavecchia, J.; Lamy De La Chapelle, M.; Djaker, N. Shape and Size Effect on Photothermal Heat Elevation of Gold Nanoparticles: Absorption Coefficient Experimental Measurement of Spherical and Urchin-Shaped Gold Nanoparticles. J. Phys. Chem. C 2019, 123, 17548–17554. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Kayanuma, Y. Quantum-size effects of interacting electrons and holes in semiconductor microcrystals with spherical shape. Phys. Rev. B 1988, 38, 9797. [Google Scholar] [CrossRef]
- D’Acunto, M.; Cioni, P.; Gabellieri, E.; Ciuttini, G. Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology 2021, 32, 192001. [Google Scholar] [CrossRef]
- Matusiak, J.; Grządka, E. Stability of colloidal systems—A review of the stability measurements methods. Ann. Univ. Mariae Curie-Sklodowska 2017, 72, 33. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Rodrigues, J.; Pereira, S.O.; Zanoni, J.; Falcão, B.P.; Santos, N.F.; Moura, J.P.; Soares, M.R.; Rino, L.; Costa, F.M.; Monteiro, T. The impact of physiological buffer solutions on zinc oxide nanostructures: Zinc phosphate conversion. Mater. Today Chem. 2022, 23, 100629. [Google Scholar] [CrossRef]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and theoretical studies of light-to-heat conversion and collective heating effects in metal nanoparticle solutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef]


| Colloid | Turbiscan Stability Index (TSI) | ||
|---|---|---|---|
| Raw Colloid | pH = 7.2 | pH = 7.4 | |
| Sphere-shaped | 30.61 | 62.44 | 65.36 |
| Sphere-Fl-PEG | 2.94 | 4.38 | 3.26 |
| Rod-shaped | 14.71 | 41.99 | 30.52 |
| Star-shaped | 36.85 | 54.79 | 47.79 |
| NPs | Tss (°C) | T0 (°C) | Aλ (532 nm) | η (%) | ||
|---|---|---|---|---|---|---|
| Spheres | 36 | 22 | 0.2037 | 0.0023447 | 27.70 | |
| Spheres- FI-PEG | 37 | 22 | 0.1189 | 0.0020737 | 34.78 | |
| Rods | 37 | 22 | 0.3928 | 0.0048498 | 36.93 | |
| Stars | 28 | 22 | 0.0537 | 0.0025936 | 37.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amézaga González, M.F.; Acosta Bezada, J.; Gómez Flores, V.; Chapa González, C.; Farias Mancilla, J.R.; Castillo, S.J.; Avila Orta, C.; García-Casillas, P.E. Effect of Physiological Fluid on the Photothermal Properties of Gold Nanostructured. Int. J. Mol. Sci. 2023, 24, 8339. https://doi.org/10.3390/ijms24098339
Amézaga González MF, Acosta Bezada J, Gómez Flores V, Chapa González C, Farias Mancilla JR, Castillo SJ, Avila Orta C, García-Casillas PE. Effect of Physiological Fluid on the Photothermal Properties of Gold Nanostructured. International Journal of Molecular Sciences. 2023; 24(9):8339. https://doi.org/10.3390/ijms24098339
Chicago/Turabian StyleAmézaga González, María Fernanda, Jazzely Acosta Bezada, Víctor Gómez Flores, Christian Chapa González, Jose Rurik Farias Mancilla, S. J. Castillo, Carlos Avila Orta, and Perla E. García-Casillas. 2023. "Effect of Physiological Fluid on the Photothermal Properties of Gold Nanostructured" International Journal of Molecular Sciences 24, no. 9: 8339. https://doi.org/10.3390/ijms24098339
APA StyleAmézaga González, M. F., Acosta Bezada, J., Gómez Flores, V., Chapa González, C., Farias Mancilla, J. R., Castillo, S. J., Avila Orta, C., & García-Casillas, P. E. (2023). Effect of Physiological Fluid on the Photothermal Properties of Gold Nanostructured. International Journal of Molecular Sciences, 24(9), 8339. https://doi.org/10.3390/ijms24098339