Intracellular Zinc Trafficking during Crotalus atrox Venom Wound Development
Abstract
1. Introduction
2. Results
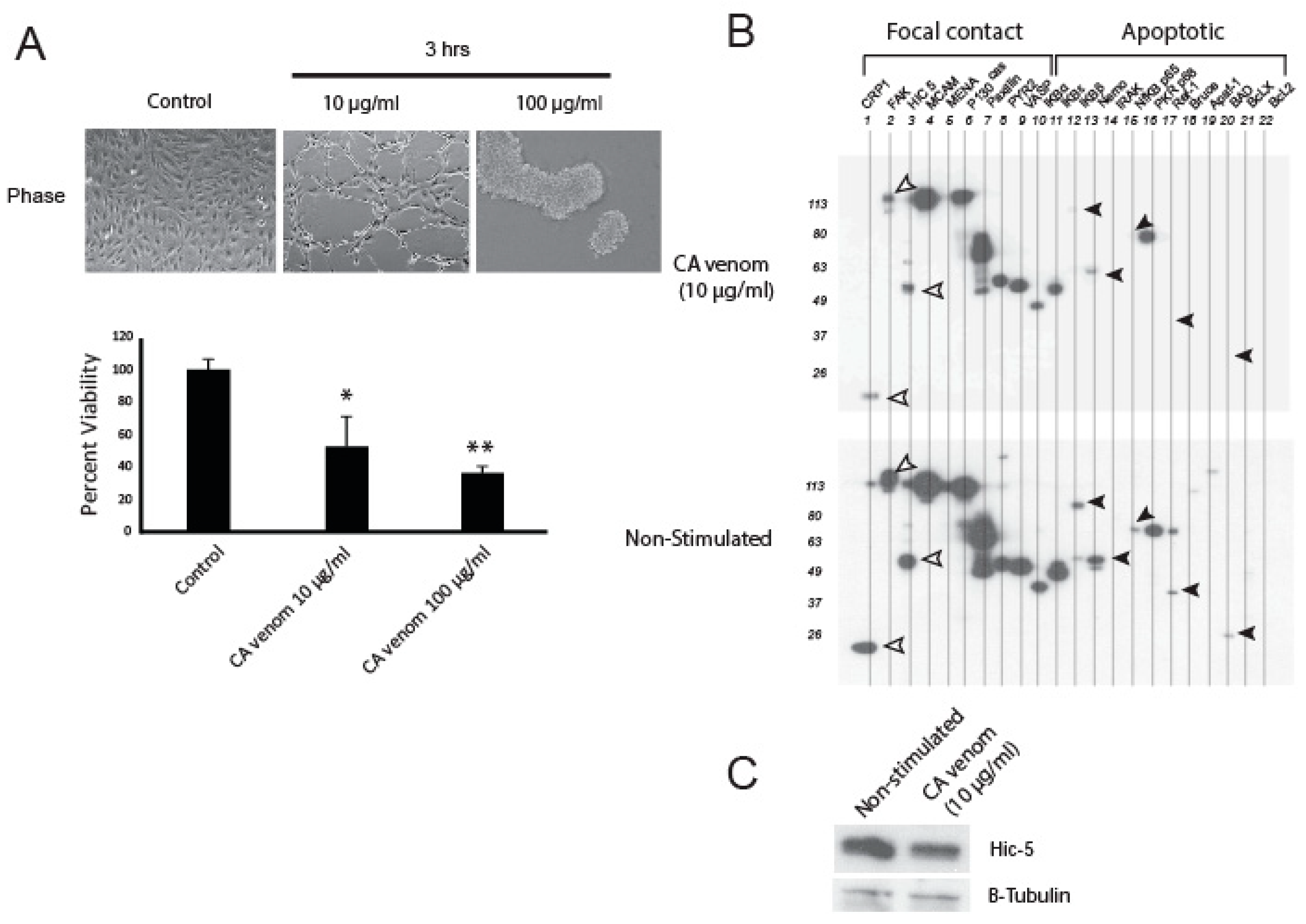
2.1. CA Venom Decreases Cell Viability and Apoptotic Related Proteins
2.2. CA Venom Cellular Injury Induces Increases in Intracellular Labile Zinc
3. Discussion
4. Material and Methods
4.1. Endothelial Cell Culture and Venom Preparation
4.2. MTS Assay
4.3. Quantitative PCR
4.4. Western Blotting
4.5. Fluorescent Intensity Assay and Microscopy Imaging
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes: Cellular Mechanisms of Wound Repair. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, B.; Tedesco, A.; Lebig, E.G.D.; Ruegger, P.M.; Xu, K.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress and Skin Microbiome Are Critical for Initiation and Development of Chronic Wounds in Diabetic Mice. Sci. Rep. 2019, 9, 19318. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the Venom Proteome of the Western Diamondback Rattlesnake, Crotalus Atrox, via Snake Venomics and Combinatorial Peptide Ligand Library Approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef]
- Olaoba, O.T.; Karina dos Santos, P.; Selistre-de-Araujo, H.S.; Ferreira de Souza, D.H. Snake Venom Metalloproteinases (SVMPs): A Structure-Function Update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef]
- Jia, L.-G.; Wang, X.-M.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Function of Disintegrin-like/Cysteine-Rich Domains of Atrolysin A. J. Biol. Chem. 1997, 272, 13094–13102. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Ohta, T.; Kaji, K.; Fox, J.W.; Hayashi, H.; Araki, S. CDNA Cloning and Characterization of Vascular Apoptosis-Inducing Protein 1. Biochem. Biophys. Res. Commun. 2000, 278, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hifumi, T.; Sakai, A.; Kondo, Y.; Yamamoto, A.; Morine, N.; Ato, M.; Shibayama, K.; Umezawa, K.; Kiriu, N.; Kato, H.; et al. Venomous snake bites: Clinical diagnosis and treatment. J. Intensiv. Care 2015, 3, 16. [Google Scholar] [CrossRef]
- Igarashi, T.; Araki, S.; Mori, H.; Takeda, S. Crystal Structures of Catrocollastatin/VAP2B Reveal a Dynamic, Modular Architecture of ADAM/Adamalysin/Reprolysin Family Proteins. FEBS Lett. 2007, 581, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef]
- Selistre-de-Araujo, H.S.; Pontes, C.L.S.; Montenegro, C.F.; Martin, A.C.B.M. Snake Venom Disintegrins and Cell Migration. Toxins 2010, 2, 2606–2621. [Google Scholar] [CrossRef]
- Galán, J.A.; Sánchez, E.E.; Rodríguez-Acosta, A.; Soto, J.G.; Bashir, S.; McLane, M.A.; Paquette-Straub, C.; Pérez, J.C. Inhibition of Lung Tumor Colonization and Cell Migration with the Disintegrin Crotatroxin 2 Isolated from the Venom of Crotalus Atrox. Toxicon 2008, 51, 1186–1196. [Google Scholar] [CrossRef]
- Scarborough, R.M.; Rose, J.W.; Naughton, M.A.; Phillips, D.R.; Nannizzi, L.; Arfsten, A.; Campbell, A.M.; Charo, I.F. Characterization of the Integrin Specificities of Disintegrins Isolated from American Pit Viper Venoms. J. Biol. Chem. 1993, 268, 1058–1065. [Google Scholar] [CrossRef]
- Arruda Macedo, J.; Fox, J.; Souza Castro, M. Disintegrins from Snake Venoms and Their Applications in Cancer Research and Therapy. Curr. Protein Pept. Sci. 2015, 16, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef]
- Eble, J.A.; Beermann, B.; Hinz, H.J.; Schmidt-Hederich, A. A2β1 Integrin Is Not Recognized by Rhodocytin but Is the Specific, High Affinity Target of Rhodocetin, an RGD-Independent Disintegrin and Potent Inhibitor of Cell Adhesion to Collagen. J. Biol. Chem. 2001, 276, 12274–12284. [Google Scholar] [CrossRef]
- Torii, S.; Naito, M.; Tsuruo, T. Apoxin I, a Novel Apoptosis-Inducing Factor with L-Amino Acid Oxidase Activity Purified from Western Diamondback Rattlesnake Venom. J. Biol. Chem. 1997, 272, 9539–9542. [Google Scholar] [CrossRef]
- Nunes, E.S.; Souza, M.A.A.; Vaz, A.F.M.; Silva, T.G.; Aguiar, J.S.; Batista, A.M.; Guerra, M.M.P.; Guarnieri, M.C.; Coelho, L.C.B.B.; Correia, M.T.S. Cytotoxic Effect and Apoptosis Induction by Bothrops Leucurus Venom Lectin on Tumor Cell Lines. Toxicon 2012, 59, 667–671. [Google Scholar] [CrossRef]
- Ginsberg, M.H. Integrin activation. BMB Rep. 2014, 47, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Dadsena, S.; King, L.E.; García-Sáez, A.J. Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183716. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.L.; Cui, W. Proliferation, Survival and Metabolism: The Role of PI3K/AKT/MTOR Signalling in Pluripotency and Cell Fate Determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Eron, S.J.; Macpherson, D.J.; Dagbay, K.B.; Hardy, J.A. Multiple Mechanisms of Zinc-Mediated Inhibition for the Apoptotic Caspases-3, -6, -7, and -8. ACS Chem. Biol. 2018, 13, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Wang, Y.X.; Li, L.X.; Liu, Y.Q. Neuronal death/apoptosis induced by intracellular zinc deficiency associated with changes in amino-acid neurotransmitters and glutamate receptor subtypes. J. Inorg. Biochem. 2018, 179, 54–59. [Google Scholar] [CrossRef]
- Untergasser, G.; Rumpold, H.; Plas, E.; Witkowski, M.; Pfister, G.; Berger, P. High Levels of Zinc Ions Induce Loss of Mitochondrial Potential and Degradation of Antiapoptotic Bcl-2 Protein in in Vitro Cultivated Human Prostate Epithelial Cells. Biochem. Biophys. Res. Commun. 2000, 279, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Subramanian Vignesh, K.; Deepe, G.S., Jr. Metallothioneins: Emerging Modulators in Immunity and Infection. Int. J. Mol. Sci. 2017, 18, 2197. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Albrecht, E.A.; Dhanasekaran, S.M.; Tomlins, S. Immediate Early Inflammatory Gene Responses of Human Umbilical Vein Endothelial Cells to Hemorrhagic Venom. Inflamm. Res. 2011, 60, 213–217. [Google Scholar] [CrossRef]
- Carraway, R.E.; Dobner, P.R. Zinc Pyrithione Induces ERK- and PKC-Dependent Necrosis Distinct from TPEN-Induced Apoptosis in Prostate Cancer Cells. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 544–557. [Google Scholar] [CrossRef]
- Inoue, K.; Branigan, D.; Xiong, Z.G. Zinc-Induced Neurotoxicity Mediated by Transient Receptor Potential Melastatin 7 Channels. J. Biol. Chem. 2010, 285, 7430–7439. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, T. Intracellular Zinc Homeostasis and Zinc Signaling. Cancer Sci. 2008, 99, 1515–1522. [Google Scholar] [CrossRef]
- Sensi, S.L.; Ton-That, D.; Sullivan, P.G.; Jonas, E.A.; Gee, K.R.; Kaczmarek, L.K.; Weiss, J.H. Modulation of Mitochondrial Function by Endogenous Zn2+ Pools. Proc. Natl. Acad. Sci. USA 2003, 100, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, T.; Tawaramoto, M.; Opare Kennedy, D.; Kojima, A.; Matsui-Yuasa, I. Apoptosis Induced by Chelation of Intracellular Zinc Is Associated with Depletion of Cellular Reduced Glutathione Level in Rat Hepatocytes. Chem. Biol. Interact. 2000, 125, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Lees, G.J.; Cuajungco, M.P.; Leong, W. Effect of Metal Chelating Agents on the Direct and Seizure-Related Neuronal Death Induced by Zinc and Kainic Acid. Brain Res. 1998, 799, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.A.L.; Vignesh, K.S.; Deepe, G.S.; Caruso, J. Selectivity and Specificity of Small Molecule Fluorescent Dyes/Probes Used for the Detection of Zn2+ and Ca2+ in Cells. Metallomics 2014, 6, 301–315. [Google Scholar] [CrossRef]
- Nowakowski, A.; Petering, D. Sensor Specific Imaging of Proteomic Zn2+ with Zinquin and TSQ after Cellular Exposure to N-Ethylmaleimide. Metallomics 2012, 4, 448–456. [Google Scholar] [CrossRef]
- Tang, Z.-L.; Wasserloos, K.; Croix, C.M.S.; Pitt, B.R. Role of Zinc in Pulmonary Endothelial Cell Response to Oxidative Stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L243–L249. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental Pathology of Local Tissue Damage Induced by Bothrops Asper Snake Venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef]
- Díaz, C.; Valverde, L.; Brenes, O.; Rucavado, A.; Gutiérrez, J.M. Characterization of Events Associated with Apoptosis/Anoikis Induced by Snake Venom Metalloproteinase BaP1 on Human Endothelial Cells. J. Cell Biochem. 2005, 94, 520–528. [Google Scholar] [CrossRef]
- Barkett, M.; Gilmore, T.D. Control of Apoptosis by Rel/NF-ΚB Transcription Factors. Oncogene 1999, 18, 6910–6924. [Google Scholar] [CrossRef]
- Gilmore, T.D. The Rel/NF-ΚB/IκB Signal Transduction Pathway and Cancer. In Signal Transduction in Cancer; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-ΚB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Coccia, M.; Rossi, A.; Riccio, A.; Trotta, E.; Santoro, M.G. Human NF-ΚB Repressing Factor Acts as a Stress-Regulated Switch for Ribosomal RNA Processing and Nucleolar Homeostasis Surveillance. Proc. Natl. Acad. Sci. USA 2017, 114, 1045–1050. [Google Scholar] [CrossRef]
- Koh, J.Y. Zinc and Disease of the Brain. Mol. Neurobiol. 2001, 24, 99–106. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, C.; Wu, W.; Shi, L.; Jiang, C.; Wang, L.; Ding, Z.; Liu, Y. Zinc Transporter ZIP12 Maintains Zinc Homeostasis and Protects Spermatogonia from Oxidative Stress during Spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Yong, K. Exposure to zinc induces lysosomal-mitochondrial axis-mediated apoptosis in PK-15 cells. Ecotoxicol. Environ. Saf. 2022, 241, 113716. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, N.; Jia, R.; Zhang, S.; Miao, J. Integrin Β4 Is a Target of Rattlesnake Venom during Inducing Apoptosis of Vascular Endothelial Cells. Vascul. Pharmacol. 2004, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rainwater, R.; Parks, D.; Anderson, M.E.; Tegtmeyer, P.; Mann, K. Role of Cysteine Residues in Regulation of P53 Function. Mol. Cell Biol. 1995, 15, 3892–3903. [Google Scholar] [CrossRef]
- Zhao, Q.; Araki, S.; Zhang, S.L.; Miao, J. Rattlesnake Venom Induces Apoptosis by Stimulating PC-PLC and Upregulating the Expression of Integrin Β4, P53 in Vascular Endothelial Cells. Toxicon 2004, 44, 161–168. [Google Scholar] [CrossRef]
- Francis, B.; Seebart, C.; Kaiser, I.I. Citrate Is an Endogenous Inhibitor of Snake Venom Enzymes by Metal-Ion Chelation. Toxicon 1992, 30, 1239–1246. [Google Scholar] [CrossRef]
- Nanjaraj Urs, A.N.; Ramakrishnan, C.; Joshi, V.; Suvilesh, K.N.; Gowda, T.V.; Velmurugan, D.; Vishwanath, B.S. Progressive Hemorrhage and Myotoxicity Induced by Echis Carinatus Venom in Murine Model: Neutralization by Inhibitor Cocktail of N,N,N′,N′-Tetrakis (2-Pyridylmethyl) Ethane-1,2-Diamine and Silymarin. PLoS ONE 2015, 10, e0135843. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, E.A.; Carter, J.D.; Garbar, V.; Choudhary, A.; Tomlins, S.A. Intracellular Zinc Trafficking during Crotalus atrox Venom Wound Development. Int. J. Mol. Sci. 2023, 24, 6763. https://doi.org/10.3390/ijms24076763
Albrecht EA, Carter JD, Garbar V, Choudhary A, Tomlins SA. Intracellular Zinc Trafficking during Crotalus atrox Venom Wound Development. International Journal of Molecular Sciences. 2023; 24(7):6763. https://doi.org/10.3390/ijms24076763
Chicago/Turabian StyleAlbrecht, Eric A., Jasmine D. Carter, Veronica Garbar, Abeeha Choudhary, and Scott A. Tomlins. 2023. "Intracellular Zinc Trafficking during Crotalus atrox Venom Wound Development" International Journal of Molecular Sciences 24, no. 7: 6763. https://doi.org/10.3390/ijms24076763
APA StyleAlbrecht, E. A., Carter, J. D., Garbar, V., Choudhary, A., & Tomlins, S. A. (2023). Intracellular Zinc Trafficking during Crotalus atrox Venom Wound Development. International Journal of Molecular Sciences, 24(7), 6763. https://doi.org/10.3390/ijms24076763





