Ultrasound Parameters Can Accurately Predict the Risk of Malignancy in Patients with “Indeterminate TIR3b” Cytology Nodules: A Prospective Study
Abstract
1. Introduction
2. Results
2.1. Univariate Analysis
2.2. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Fine Needle Aspiration Procedure
4.2. BRAF Mutation Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vander, J.B.; Gaston, E.A.; Dawber, T.R. The Significance of Nontoxic Thyroid Nodules. Final Report of a 15-Year Study of the Incidence of Thyroid Malignancy. Ann. Intern. Med. 1968, 69, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, W.M.; Evered, D.C.; Hall, R.; Appleton, D.; Brewis, M.; Clark, F.; Evans, J.G.; Young, E.; Bird, T.; Smith, P.A. The Spectrum of Thyroid Disease in a Community: The Whickham Survey. Clin. Endocrinol. Oxf. 1977, 7, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Gharib, H. Thyroid Incidentalomas: Management Approaches to Nonpalpable Nodules Discovered Incidentally on Thyroid Imaging. Ann. Intern. Med. 1997, 126, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Theune, U.; Aberle, J.; Galach, A.; Bamberger, C.M. Very High Prevalence of Thyroid Nodules Detected by High Frequency (13 MHz) Ultrasound Examination. Eur. J. Clin. Investig. 2009, 39, 699–706. [Google Scholar] [CrossRef]
- Hegedüs, L. Clinical Practice. The Thyroid Nodule. N. Engl. J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef]
- Mandel, S.J. A 64-Year-Old Woman with a Thyroid Nodule. JAMA 2004, 292, 2632–2642. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.-K. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. AACE/ACE/AME Task Force on Thyroid Nodules American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules—2016 Update. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22, 622–639. [Google Scholar] [CrossRef]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Renshaw, A.A. Should “Atypical Follicular Cells” in Thyroid Fine-Needle Aspirates Be Subclassified? Cancer Cytopathol. 2010, 118, 186–189. [Google Scholar] [CrossRef]
- Trimboli, P.; Crescenzi, A.; Castellana, M.; Giorgino, F.; Giovanella, L.; Bongiovanni, M. Italian Consensus for the Classification and Reporting of Thyroid Cytology: The Risk of Malignancy between Indeterminate Lesions at Low or High Risk. A Systematic Review and Meta-Analysis. Endocrine 2019, 63, 430–438. [Google Scholar] [CrossRef]
- Trimboli, P.; Ferrarazzo, G.; Cappelli, C.; Piccardo, A.; Castellana, M.; Barizzi, J. Thyroid Nodules with Indeterminate FNAC According to the Italian Classification System: Prevalence, Rate of Operation, and Impact on Risk of Malignancy. An Updated Systematic Review and Meta-Analysis. Endocr. Pathol. 2022, 33, 457–471. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Sparano, C.; Parenti, G.; Cilotti, A.; Bencini, L.; Calistri, M.; Mannucci, E.; Biagini, C.; Vezzosi, V.; Mannelli, M.; Forti, G.; et al. Clinical Impact of the New SIAPEC-IAP Classification on the Indeterminate Category of Thyroid Nodules. J. Endocrinol. Investig. 2019, 42, 1–6. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Rullo, E.; Minelli, G.; Bosco, D.; Nardi, F.; Grani, G.; Durante, C.; Ascoli, V. Indeterminate Thyroid Nodules (TIR3A/TIR3B) According to the New Italian Reporting System for Thyroid Cytology: A Cytomorphological Study. Cytopathol. Off. J. Br. Soc. Clin. Cytol. 2019, 30, 475–484. [Google Scholar] [CrossRef]
- Larcher de Almeida, A.M.; Delfim, R.L.C.; Vidal, A.P.A.; Chaves, M.C.; d’Araujo, C.M.; Santiago, A.C.L.; Gianotti, M.F.; Gonçalves, M.D.d.C.; Vaisman, M.; de Carvalho, D.P.; et al. Combining the American Thyroid Association’s Ultrasound Classification with Cytological Subcategorization Improves the Assessment of Malignancy Risk in Indeterminate Thyroid Nodules. Thyroid Off. J. Am. Thyroid Assoc. 2021, 31, 922–932. [Google Scholar] [CrossRef]
- Boffetta, P.; Memeo, L.; Giuffrida, D.; Ferrante, M.; Sciacca, S. Exposure to Emissions from Mount Etna (Sicily, Italy) and Incidence of Thyroid Cancer: A Geographic Analysis. Sci. Rep. 2020, 10, 21298. [Google Scholar] [CrossRef]
- Malandrino, P.; Russo, M.; Ronchi, A.; Minoia, C.; Cataldo, D.; Regalbuto, C.; Giordano, C.; Attard, M.; Squatrito, S.; Trimarchi, F.; et al. Increased Thyroid Cancer Incidence in a Basaltic Volcanic Area Is Associated with Non-Anthropogenic Pollution and Biocontamination. Endocrine 2016, 53, 471–479. [Google Scholar] [CrossRef]
- Lee, I.A.; Moon, G.; Kang, S.; Lee, K.H.; Lee, S.M.; Kim, J.K.; Lee, C.R.; Kang, S.-W.; Jeong, J.J.; Nam, K.-H.; et al. Predictive Factors Indicative of Hemithyroidectomy and Close Follow-Up versus Bilateral Total Thyroidectomy for Aggressive Variants of Papillary Thyroid Cancer. Cancers 2022, 14, 2757. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Caraci, P.; Sapino, A.; De Rosa, G.; Volante, M.; Papotti, M. Outcome and diagnostic reproducibility of the thyroid cytology “indeterminate categories” SIAPEC/SIE 2014 in a consecutive series of 302 cases. J. Endocrinol. Investig. 2021, 44, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Straccia, P.; Santoro, A.; Rossi, E.D.; Brunelli, C.; Mosseri, C.; Musarra, T.; Pontecorvi, A.; Lombardi, C.P.; Fada, G. Incidence, malignancy rates of diagnoses and cyto-histological correlations in the new Italian Reporting System for Thyroid Cytology: An institutional experience. Cytopathology 2017, 28, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, F.; Arnulfo, G.; Sandrucci, S.; Rossi, C.; Marchese, V.; Saracco, R.; Guzzetti, S.; Taraglio, S.; Mazza, E. A Monocentric Retrospective Study about the Correlation between Histology and Cytology of Thyroid Indeterminate Nodules Classified as TIR 3A and TIR 3B, according to 2014 Italian Consensus for Classification and Reporting of Thyroid Cytology. Adv. Med. 2019, 2019, 3932721. [Google Scholar] [CrossRef]
- Brigante, G.; Craparo, A.; Pignatti, E.; Marino, M.; Monzani, M.L.; De Vincentis, S.; Casarini, L.; Sperduti, S.; Boselli, G.; Margiotta, G.; et al. Real-Life Use of BRAF-V600E Mutation Analysis in Thyroid Nodule Fine Needle Aspiration: Consequences on Clinical Decision-Making. Endocrine 2021, 73, 625–632. [Google Scholar] [CrossRef]
- Ulisse, S.; Bosco, D.; Nardi, F.; Nesca, A.; D’Armiento, E.; Guglielmino, V.; De Vito, C.; Sorrenti, S.; Pironi, D.; Tartaglia, F.; et al. Thyroid Imaging Reporting and Data System Score Combined with the New Italian Classification for Thyroid Cytology Improves the Clinical Management of Indeterminate Nodules. Int. J. Endocrinol. 2017, 2017, 9692304. [Google Scholar] [CrossRef]
- Campanella, P.; Ianni, F.; Rota, C.A.; Corsello, S.M.; Pontecorvi, A. Quantification of Cancer Risk of Each Clinical and Ultrasonographic Suspicious Feature of Thyroid Nodules: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2014, 170, R203–R211. [Google Scholar] [CrossRef]
- Ianni, F.; Campanella, P.; Rota, C.A.; Prete, A.; Castellino, L.; Pontecorvi, A.; Corsello, S.M. A Meta-Analysis-Derived Proposal for a Clinical, Ultrasonographic, and Cytological Scoring System to Evaluate Thyroid Nodules: The “CUT” Score. Endocrine 2016, 52, 313–321. [Google Scholar] [CrossRef]
- Rago, T.; Scutari, M.; Latrofa, F.; Loiacono, V.; Piaggi, P.; Marchetti, I.; Romani, R.; Basolo, F.; Miccoli, P.; Tonacchera, M.; et al. The Large Majority of 1520 Patients with Indeterminate Thyroid Nodule at Cytology Have a Favorable Outcome, and a Clinical Risk Score Has a High Negative Predictive Value for a More Cumbersome Cancer Disease. J. Clin. Endocrinol. Metab. 2014, 99, 3700–3707. [Google Scholar] [CrossRef]
- Alqahtani, S.M.; Alanesi, S.F.; Mahmood, W.S.; Moustafa, Y.M.; Moharram, L.M.; Alharthi, N.F.; Alzahrani, A.M.; Alalawi, Y.S. Clinical and Ultrasonographic Features in Cancer Risk Stratification of Indeterminate Thyroid Nodules. Saudi Med. J. 2022, 43, 473–478. [Google Scholar] [CrossRef]
- Sahin, M.; Gursoy, A.; Tutuncu, N.B.; Guvener, D.N. Prevalence and Prediction of Malignancy in Cytologically Indeterminate Thyroid Nodules. Clin. Endocrinol. Oxf. 2006, 65, 514–518. [Google Scholar] [CrossRef]
- Dutta, S.; Thaha, M.A.; Smith, D.M. Do Sonographic and Cytological Features Predict Malignancy in Cytologically Indeterminate Thyroid Nodules? Ann. R. Coll. Surg. Engl. 2011, 93, 361–364. [Google Scholar] [CrossRef]
- Méndez, W.; Rodgers, S.E.; Lew, J.I.; Montano, R.; Solórzano, C.C. Role of Surgeon-Performed Ultrasound in Predicting Malignancy in Patients with Indeterminate Thyroid Nodules. Ann. Surg. Oncol. 2008, 15, 2487–2492. [Google Scholar] [CrossRef]
- Khoncarly, S.M.; Tamarkin, S.W.; McHenry, C.R. Can Ultrasound Be Used to Predict Malignancy in Patients with a Thyroid Nodule and an Indeterminate Fine-Needle Aspiration Biopsy? Surgery 2014, 156, 967–970. [Google Scholar] [CrossRef]
- Öcal, B.; Korkmaz, M.H.; Yılmazer, D.; Taşkın Türkmenoğlu, T.; Bayır, Ö.; Saylam, G.; Çadallı Tatar, E.; Karahan, S.; Çakal, E. The Malignancy Risk Assessment of Cytologically Indeterminate Thyroid Nodules Improves Markedly by Using a Predictive Model. Eur. Thyroid J. 2019, 8, 83–89. [Google Scholar] [CrossRef]
- Amado, A.; Castro, B.; Torre, A.P.; Graça, S.; Tavares, A.; Póvoa, A.; Soares, C.; Gonçalves, G. Serum TSH as a Predictor of Malignancy in Indeterminate Thyroid Nodules. Ann. R. Coll. Surg. Engl. 2022, 104, 380–384. [Google Scholar] [CrossRef]
- Arena, S.; Benvenga, S. The Risk for Malignancy of the Thyroid Nodule Is Modulated by Gender, Echotexture, and Intranodular Lymphocytic Thyroiditis. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2019, 51, 559–567. [Google Scholar] [CrossRef]
- Cozzolino, A.; Pozza, C.; Pofi, R.; Sbardella, E.; Faggiano, A.; Isidori, A.M.; Giannetta, E.; Pernazza, A.; Rullo, E.; Ascoli, V.; et al. Predictors of Malignancy in High-Risk Indeterminate (TIR3B) Cytopathology Thyroid Nodules. J. Endocrinol. Investig. 2020, 43, 1115–1123. [Google Scholar] [CrossRef]
- Abdelnassef, M.M.; Shalamesh, M.E.; Al-Mahdy, A.M.; Mohamed, E.H. Comparative Study between Total Thyroidectomy and Hemithyroidectomy in Treatment of Solitary Thyroid Nodules Diagnosed as Follicular Lesions by Fine-Needle Aspiration Cytology. Egypt. J. Hosp. Med. 2019, 76, 4119–4123. [Google Scholar] [CrossRef]
- Pizzolanti, G.; Russo, L.; Richiusa, P.; Bronte, V.; Nuara, R.B.; Rodolico, V.; Amato, M.C.; Smeraldi, L.; Sisto, P.S.; Nucera, M.; et al. Fine-Needle Aspiration Molecular Analysis for the Diagnosis of Papillary Thyroid Carcinoma through BRAF V600E Mutation and RET/PTC Rearrangement. Thyroid Off. J. Am. Thyroid Assoc. 2007, 17, 1109–1115. [Google Scholar] [CrossRef]
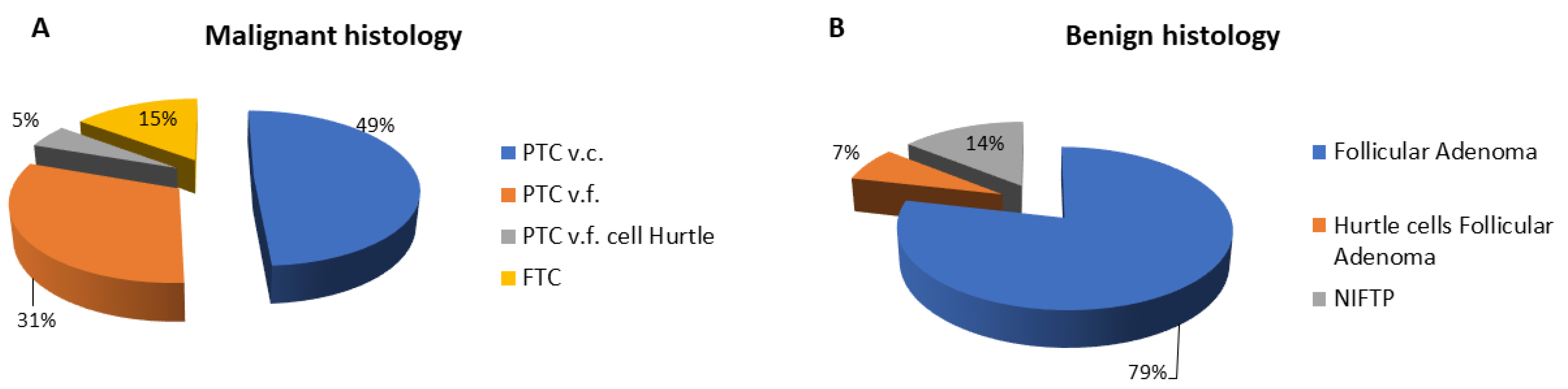
| Benign Histology (N = 42) | Malignant Histology (N = 61) | p | |
|---|---|---|---|
| Subjects (%) | Subjects (%) | ||
| Gender | 0.411 | ||
| Male | 10 (23.8%) | 17 (27.9%) | |
| Female | 32 (76.2%) | 44 (72.1%) | |
| Exposure to ionising radiation | |||
| 2 (4.8%) | 3 (4.69%) | 0.672 | |
| Smoking | 14 (33.3%) | 26 (42.6%) | 0.229 |
| Familial history of thyroid cancer | |||
| 3 (7.1%) | 7 (11.5%) | 0.354 | |
| Familial history of benign nodular pathology | |||
| 24 (57.1%) | 29 (47.5%) | 0.225 | |
| Autoimmune thyroiditis | |||
| 10 (23.8%) | 17 (27.9%) | 0.411 | |
| Ultrasonographic features | |||
| Nodule diameter | 0.002 | ||
| ≤11 mm | 5 (11.9%) | 24 (39.3%) | |
| >11 mm | 37 (88.1%) | 37 (60.7%) | |
| Irregular borders | 7 (16.6%) | 41 (67.2%) | <0.001 |
| Echogenicity of nodule | <0.001 | ||
| Hypoechoic | 13 (31%) | 41 (67.2%) | |
| Isoechoic | 12 (28.6%) | 3 (4.9%) | |
| Iso-hypoechoic | 14 (33.3%) | 14 (23%) | |
| Complex | 3 (7.1%) | 1 (1.6%) | |
| Hyperechoic | 0 | 2 (3.3%) | |
| Vascular Flow | 0.004 | ||
| Absent | 18 (42.9%) | 9 (14.8%) | |
| Perinodular | 17 (40.5%) | 30 (49.2%) | |
| Intra and perinodular | 8 (19%) | 23 (37.7%) | |
| Microcalcifications | 8 (19%) | 32 (52.5%) | 0.001 |
| Taller than wide | 1 (2.4%) | 2 (3.3%) | 0.638 |
| Halo sign | 9 (21.4%) | 3 (4.9%) | 0.012 |
| Mutated BRAF V600E | 0 | 2 (3.2%) | |
| Multinodular goitre | 28 (66.7%) | 38 (62.3%) | 0.405 |
| Pre-intervention therapy | |||
| Levothyroxine | 5 (11.9%) | 11 (18%) | 0.289 |
| Methimazole | 1 (2.4%) | 2 (3.3%) | 0.638 |
| Benign Histology (N = 42) | Malignant Histology (N = 61) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 56.1 ± 14.5 | 52.9 ± 14.1 | 0.267 |
| BMI (kg/m2) | 26.4 ± 4.16 | 27.2 ± 4.86 | 0.389 |
| Waist circumference (cm) | 92.1 ± 12.7 | 94.7 ± 14.2 | 0.341 |
| TSH pre-intervention (µU/mL) | 1.5 ± 0.81 | 1.87 ± 1.01 | 0.041 |
| Calcitonin pre-intervention (pmol/L) | 2.05 ± 1.38 | 2.78 ± 3.24 | 0.126 |
| Variables | Benign Histology (N° = 42) | Malignant Histology (N° = 61) | Crude OR (95% CI) | Adjusted OR (95% CI) | p |
|---|---|---|---|---|---|
| Diameter | |||||
| >11 mm | 5 (11.9%) | 24 (39.3%) | 1 | 1 | |
| ≤11 mm | 37 (88.1%) | 37 (60.7%) | 4.8 (1.65–13.9) | 3.044 (1.10–10.8) | 0.045 |
| TSH | |||||
| ≤2.02 | 34 (81%) | 36 (59%) | 1 | ||
| >2.02 | 8 (19%) | 25 (41%) | 2.95 (1.17–7.43) | ||
| Irregular borders | |||||
| No | 35 (83.3%) | 20 (32.8%) | 1 | 1 | |
| Yes | 7 (16.7%) | 41 (67.2%) | 10.2 (3.87–27.1) | 4.98 (1.71–14.4) | 0.003 |
| Hypoechogenicity of the nodule | |||||
| No | 29 (69%) | 20 (32.8%) | 1 | ||
| Yes | 13 (31%) | 41 (67.2%) | 4.57 (1.95–10.64) | ||
| Intranodular and perivascular flows | |||||
| No | 34 (81%) | 38 (62.3%) | 1 | 1 | |
| Yes | 8 (19%) | 23 (37.7%) | 2.57 (1.13–6.5) | 3.46 (1.17–10.2) | 0.025 |
| Microcalcifications | |||||
| No | 34 (81%) | 29 (47.5%) | 1 | ||
| Yes | 8 (19%) | 32 (52.5%) | 4.69 (1.87–11.76) | ||
| Halo sign | |||||
| Yes | 9 (21.4%) | 3 (4.9%) | 1 | ||
| No | 33 (78.6%) | 58 (95.1%) | −1.66 (−3.31–0.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnotta, V.; La Monica, R.; Ingrao, V.R.; Di Stefano, C.; Salzillo, R.; Pizzolanti, G.; Giannone, A.G.; Almasio, P.L.; Richiusa, P.; Giordano, C. Ultrasound Parameters Can Accurately Predict the Risk of Malignancy in Patients with “Indeterminate TIR3b” Cytology Nodules: A Prospective Study. Int. J. Mol. Sci. 2023, 24, 8296. https://doi.org/10.3390/ijms24098296
Guarnotta V, La Monica R, Ingrao VR, Di Stefano C, Salzillo R, Pizzolanti G, Giannone AG, Almasio PL, Richiusa P, Giordano C. Ultrasound Parameters Can Accurately Predict the Risk of Malignancy in Patients with “Indeterminate TIR3b” Cytology Nodules: A Prospective Study. International Journal of Molecular Sciences. 2023; 24(9):8296. https://doi.org/10.3390/ijms24098296
Chicago/Turabian StyleGuarnotta, Valentina, Roberta La Monica, Vincenza Rita Ingrao, Claudia Di Stefano, Riccardo Salzillo, Giuseppe Pizzolanti, Antonino Giulio Giannone, Piero Luigi Almasio, Pierina Richiusa, and Carla Giordano. 2023. "Ultrasound Parameters Can Accurately Predict the Risk of Malignancy in Patients with “Indeterminate TIR3b” Cytology Nodules: A Prospective Study" International Journal of Molecular Sciences 24, no. 9: 8296. https://doi.org/10.3390/ijms24098296
APA StyleGuarnotta, V., La Monica, R., Ingrao, V. R., Di Stefano, C., Salzillo, R., Pizzolanti, G., Giannone, A. G., Almasio, P. L., Richiusa, P., & Giordano, C. (2023). Ultrasound Parameters Can Accurately Predict the Risk of Malignancy in Patients with “Indeterminate TIR3b” Cytology Nodules: A Prospective Study. International Journal of Molecular Sciences, 24(9), 8296. https://doi.org/10.3390/ijms24098296





