Severity- and Time-Dependent Activation of Microglia in Spinal Cord Injury
Abstract
1. Introduction
2. Results
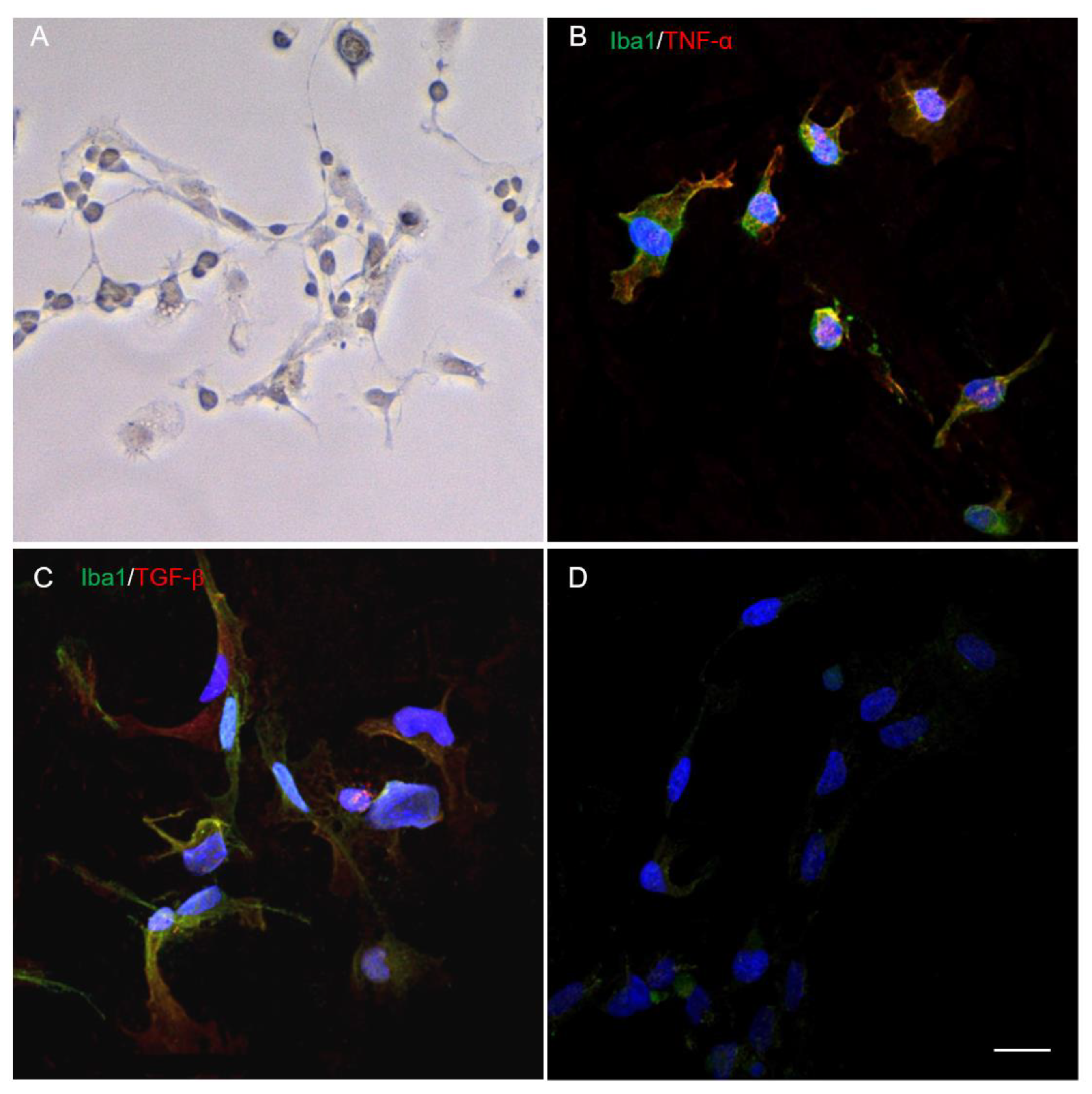
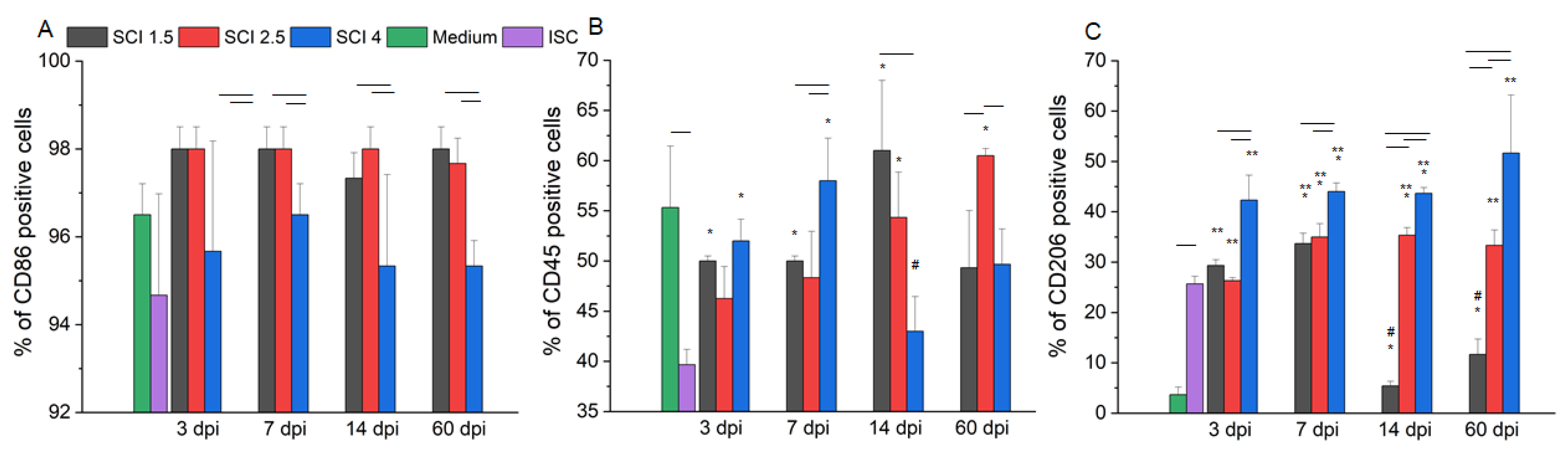
2.1. Morphology and Phenotype of Cultivated Microglia
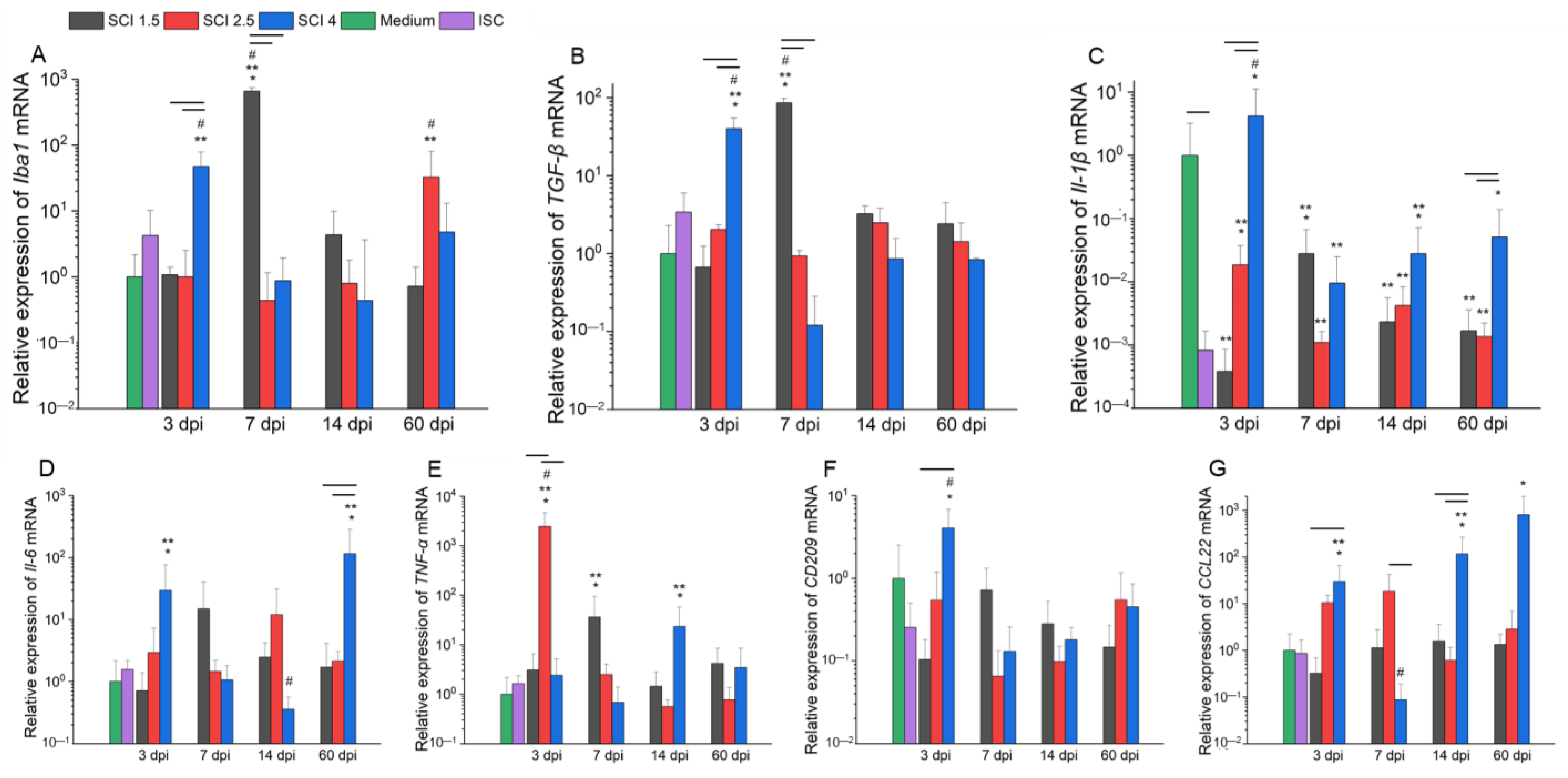
2.2. Effect of SCE on Microglia Gene Expression
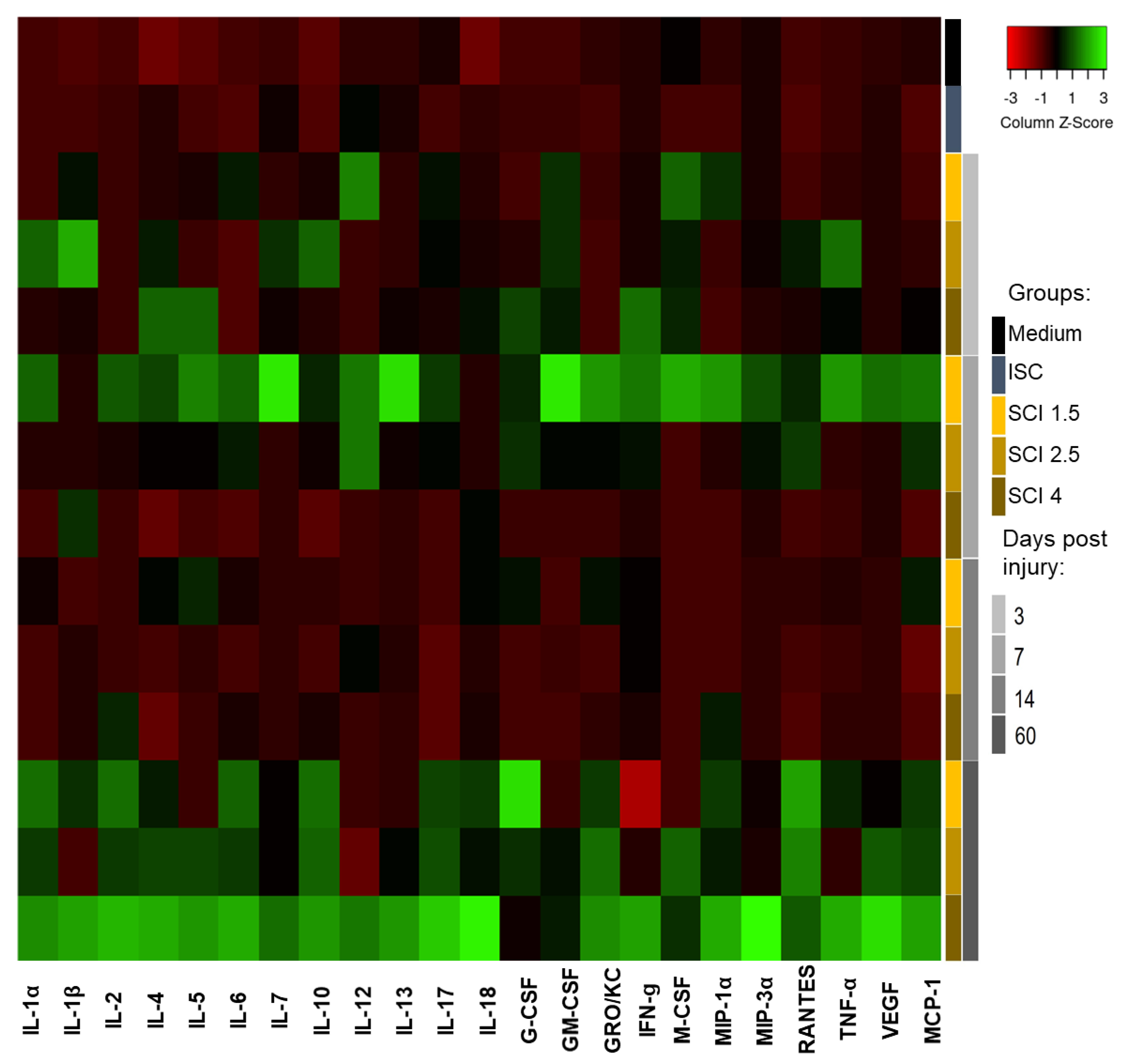
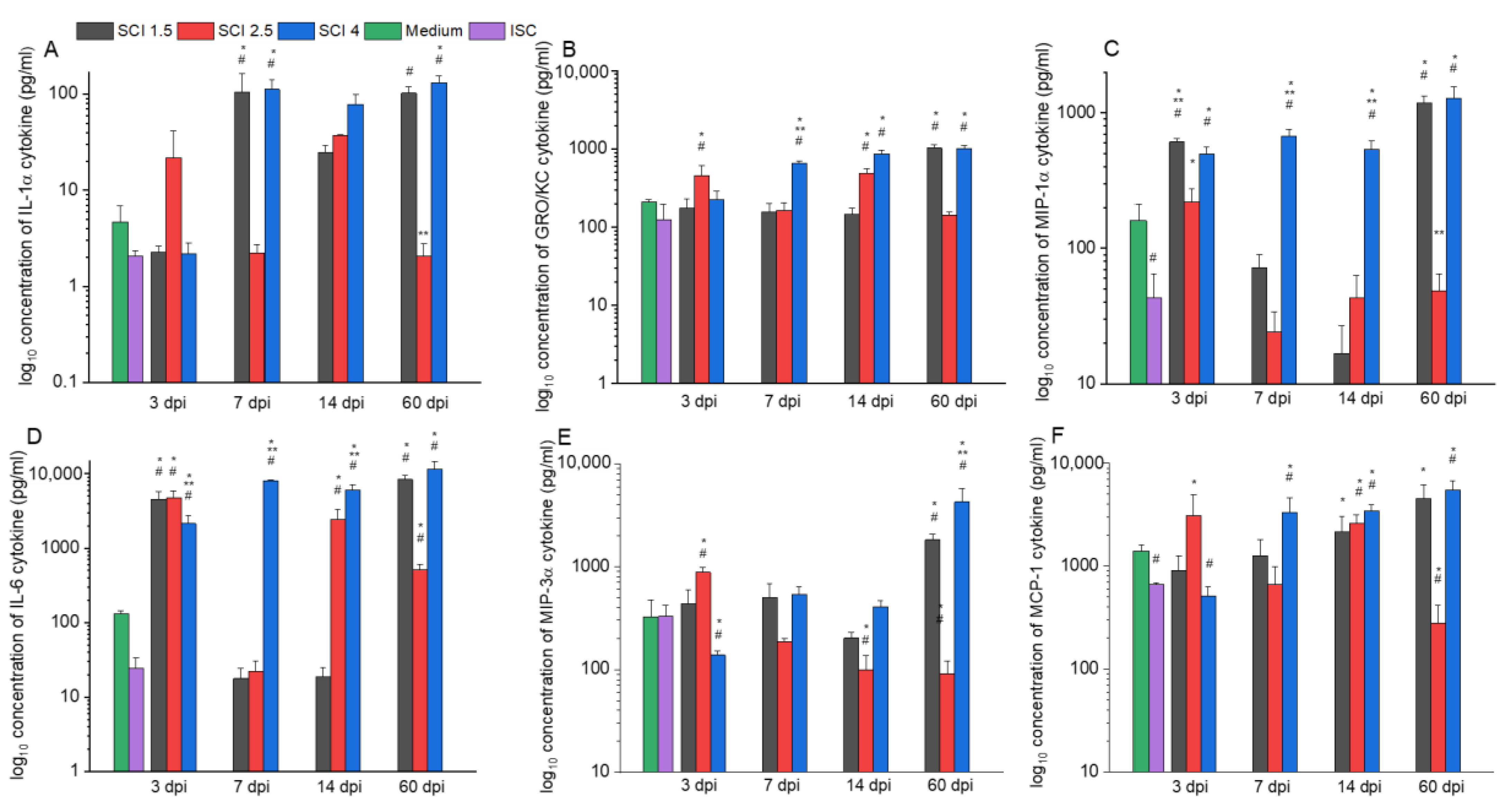
2.3. Effect of SCE on Cytokine Profile of Microglia
2.4. Effect of SCE on Microglia Proliferation
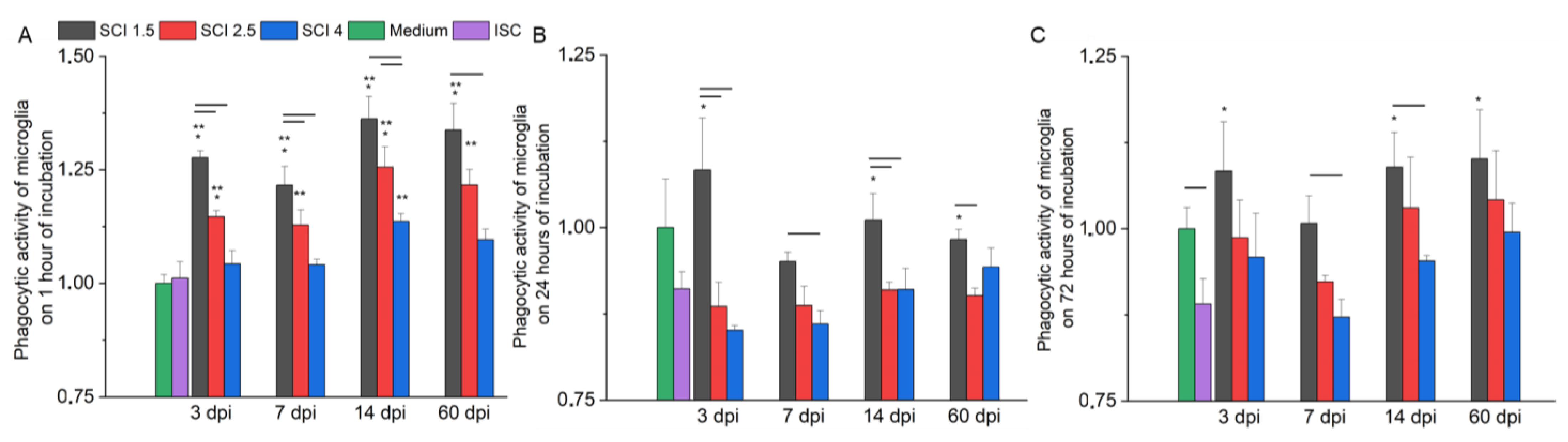
2.5. Effect of SCE on Microglia Phagocytic Activity
3. Discussion
4. Materials and Methods
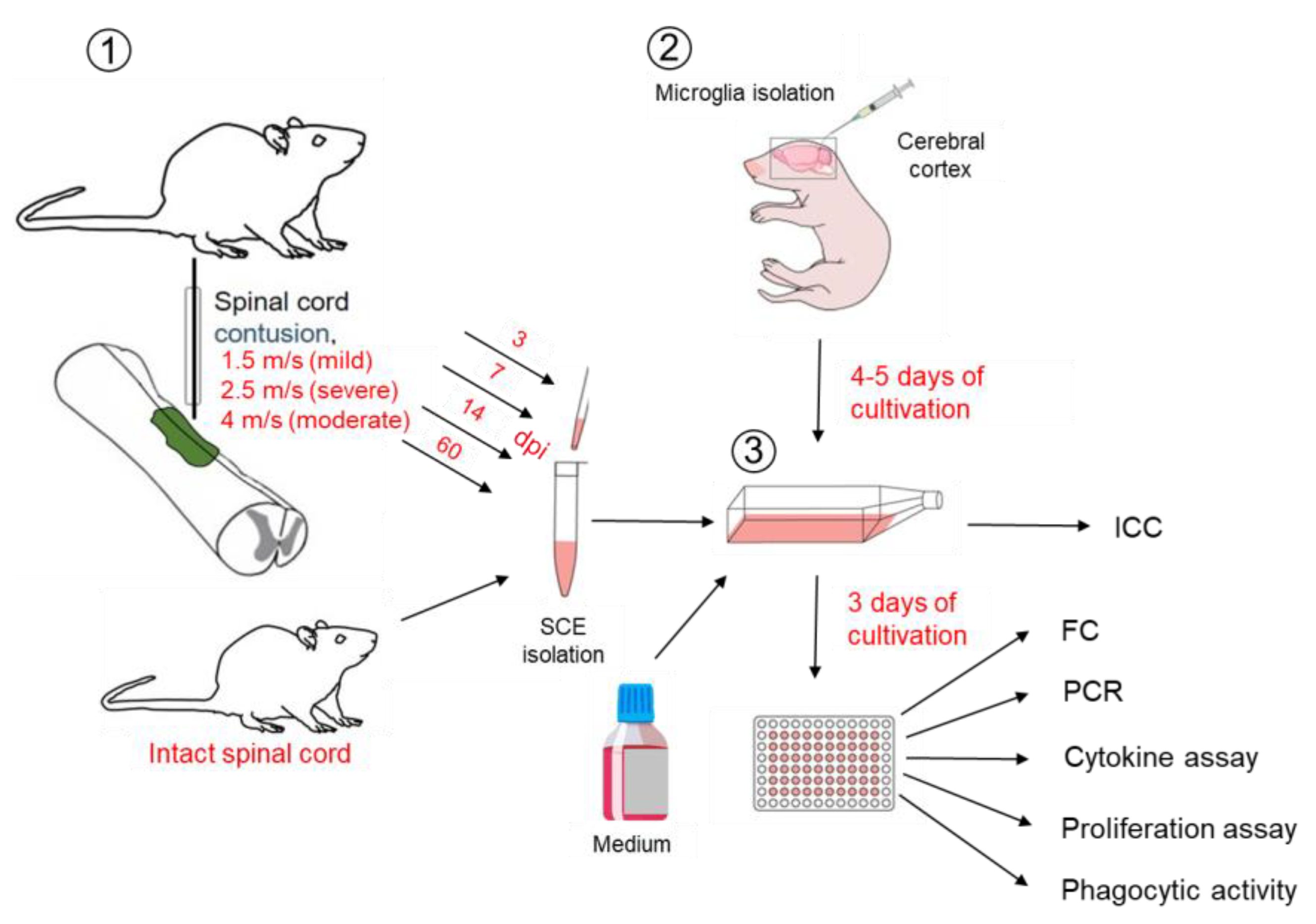
4.1. Microglia Isolation and Cultivation
4.2. Immunocytochemistry
4.3. Spinal Cord Injury
4.4. Spinal Cord Extracts (SCE) Isolation
4.5. Modeling of Spinal Cord Injury In Vitro
4.6. Flow Cytometry
4.7. RNA Isolation and Real-Time PCR Analysis
4.8. Cytokine Assay
4.9. Microglia Proliferation Assay
4.10. Phagocytic Activity
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakajima, H.; Honjoh, K.; Watanabe, S.; Kubota, A.; Matsumine, A. Distribution and polarization of microglia and macrophages at injured sites and the lumbar enlargement after spinal cord injury. Neurosci. Lett. 2020, 737, 135152. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.S.; Zhang, C.J.; Xia, N.; Tian, H.; Li, D.Y.; Lin, J.Q.; Mei, X.F.; Wu, C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021, 126, 211–223. [Google Scholar] [CrossRef]
- McCarthy, K.D.; De Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Biber, K.; Vinet, J.W.G.M.; Wgm Boddeke, H. Microglia phenotype diversity. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2011, 10, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Grassivaro, F.; Menon, R.; Acquaviva, M.; Ottoboni, L.; Ruffini, F.; Bergamaschi, A.; Muzio, L.; Farina, C.; Martino, G. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J. Neurosci. 2020, 40, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Li, H.; Jiang, T.; Li, M.Q.; Zheng, X.L.; Zhao, G.J. Transcriptional regulation of macrophages polarization by MicroRNAs. Front. Immunol. 2018, 9, 1175. [Google Scholar] [CrossRef]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Detloff, M.R.; Fisher, L.C.; McGaughy, V.; Longbrake, E.E.; Popovich, P.G.; Basso, D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008, 212, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef]
- Hains, B.C.; Waxman, S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.R.; Mukhamedshina, Y.O.; Zhuravleva, M.N.; Galieva, L.R.; Kostennikov, A.A.; Garanina, E.E.; Rizvanov, A.A. Transplantation of microglia in the area of spinal cord injury in an acute period increases tissue sparing, but not functional recovery. Front. Cell. Neurosci. 2018, 12, 507. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, Y.; Hu, D.; Wang, S.; Yu, T.; Zhang, L. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yu, H.; Zhang, B.; Wang, D.; Li, B.; Zhu, J.; Zhu, W. Promising neuroprotective function for M2 microglia in Kainic Acid-induced neurotoxicity via the down-regulation of NF-κB and caspase 3 signaling pathways. Neuroscience 2019, 406, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kisucká, A.; Bimbová, K.; Bačová, M.; Gálik, J.; Lukáčová, N. Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury. Cells 2021, 10, 1943. [Google Scholar] [CrossRef] [PubMed]
- Nissl, F. Über einige Beziehungen zwischen Nervenzellerkrankungen und gliösen Erscheinungen bei verschiedenen Psychosen. Arch. Für Psychiatr. Nervenkrankh. 1899, 32, 656–676. [Google Scholar]
- Loayza, M.; Lin, S.; Carter, K.; Ojeda, N.; Fan, L.W.; Ramarao, S.; Bhatt, A.; Pang, Y. Maternal immune activation alters fetal and neonatal microglia phenotype and disrupts neurogenesis in mice. Pediatric Res. 2022, 93, 1216–1225. [Google Scholar] [CrossRef]
- Kroner, A.; Almanza, J.R. Role of microglia in spinal cord injury. Neurosci. Lett. 2019, 709, 134370. [Google Scholar] [CrossRef]
- Olson, J.K. Immune response by microglia in the spinal cord. Ann. N. Y. Acad. Sci. 2010, 1198, 271–278. [Google Scholar] [CrossRef]
- Zrzavy, T.; Schwaiger, C.; Wimmer, I.; Berger, T.; Bauer, J.; Butovsky, O.; Schwab, J.M.; Lassmann, H.; Höftberger, R. Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain 2021, 144, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jones, N.R.; Blumbergs, P.C.; Van Den Heuvel, C.; Moore, E.J.; Manavis, J.; Sarvestani, G.T.; Ghabriel, M.N. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J. Clin. Neurosci. 2005, 12, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Garay, J.; Di Giovanni, S.; De Biase, A.; Knoblach, S.M.; Hoffman, E.P.; Movsesyan, V.; Faden, A.I. Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia 2006, 53, 420–433. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef]
- Pease, J.E. Tails of the unexpected—An atypical receptor for the chemokine RANTES/CCL5 expressed in brain. Br. J. Pharmacol. 2006, 149, 460–462. [Google Scholar] [CrossRef]
- Mukaino, M.; Nakamura, M.; Yamada, O.; Okada, S.; Morikawa, S.; Renault-Mihara, F.; Iwanami, A.; Ikegami, T.; Ohsugi, Y.; Tsuji, O.; et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp. Neurol. 2010, 224, 403–414. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K. Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Sato, K. Activated microglia disrupt the blood-brain barrier and induce chemokines and cytokines in a rat in vitro model. Front. Cell. Neurosci. 2018, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.Y.; Weng, J.Y.; Lai, H.L.; Liao, F.; Sun, S.H.; Tu, P.H.; Dennis, D.W.; Chern, Y. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J. Neurosci. 2008, 28, 3277–3290. [Google Scholar] [CrossRef]
- Lin, M.S.; Hung, K.S.; Chiu, W.T.; Sun, Y.Y.; Tsai, S.H.; Lin, J.W.; Lee, Y.H. Curcumin enhances neuronal survival in N-methyl-d-aspartic acid toxicity by inducing RANTES expression in astrocytes via PI-3K and MAPK signaling pathways. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 931–938. [Google Scholar] [CrossRef]
- Neher, J.J.; Neniskyte, U.; Zhao, J.W.; Bal-Price, A.; Tolkovsky, A.M.; Brown, G.C. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J. Immunol. 2011, 186, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, A.D.; David, S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 2014, 34, 6316–6322. [Google Scholar] [CrossRef]
- Fleming, J.C.; Norenberg, M.D.; Ramsay, D.A.; Dekaban, G.A.; Marcillo, A.E.; Saenz, A.D.; Pasquale-Styles, M.; Dietrich, W.D.; Weaver, L.C. The cellular inflammatory response in human spinal cords after injury. Brain 2006, 129, 3249–3269. [Google Scholar] [CrossRef]
- Mukhamedshina, Y.O.; Akhmetzyanova, E.R.; Martynova, E.V.; Khaiboullina, S.F.; Galieva, L.R.; Rizvanov, A.A. Systemic and local cytokine profile following spinal cord injury in rats: A multiplex analysis. Front. Neurol. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.; Zhuravleva, M.; Sergeev, M.; Zakirova, E.; Gracheva, O.; Mukhutdinova, D.; Rizvanov, A. Improving Culture Conditions, Proliferation, and Migration of Porcine Mesenchymal Stem Cells on Spinal Cord Contusion Injury Model in vitro. Cells Tissues Organs 2020, 209, 236–247. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetzyanova, E.R.; Zhuravleva, M.N.; Timofeeva, A.V.; Tazetdinova, L.G.; Garanina, E.E.; Rizvanov, A.A.; Mukhamedshina, Y.O. Severity- and Time-Dependent Activation of Microglia in Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 8294. https://doi.org/10.3390/ijms24098294
Akhmetzyanova ER, Zhuravleva MN, Timofeeva AV, Tazetdinova LG, Garanina EE, Rizvanov AA, Mukhamedshina YO. Severity- and Time-Dependent Activation of Microglia in Spinal Cord Injury. International Journal of Molecular Sciences. 2023; 24(9):8294. https://doi.org/10.3390/ijms24098294
Chicago/Turabian StyleAkhmetzyanova, Elvira Ruslanovna, Margarita Nikolaevna Zhuravleva, Anna Viktorovna Timofeeva, Leisan Gazinurovna Tazetdinova, Ekaterina Evgenevna Garanina, Albert Anatolevich Rizvanov, and Yana Olegovna Mukhamedshina. 2023. "Severity- and Time-Dependent Activation of Microglia in Spinal Cord Injury" International Journal of Molecular Sciences 24, no. 9: 8294. https://doi.org/10.3390/ijms24098294
APA StyleAkhmetzyanova, E. R., Zhuravleva, M. N., Timofeeva, A. V., Tazetdinova, L. G., Garanina, E. E., Rizvanov, A. A., & Mukhamedshina, Y. O. (2023). Severity- and Time-Dependent Activation of Microglia in Spinal Cord Injury. International Journal of Molecular Sciences, 24(9), 8294. https://doi.org/10.3390/ijms24098294