Role of the ABCA4 Gene Expression in the Clearance of Toxic Vitamin A Derivatives in Human Hair Follicle Stem Cells and Keratinocytes
Abstract
1. Introduction
2. Results
2.1. ABCA4 Protein Is Expressed in the Basal Layer of the Epidermis
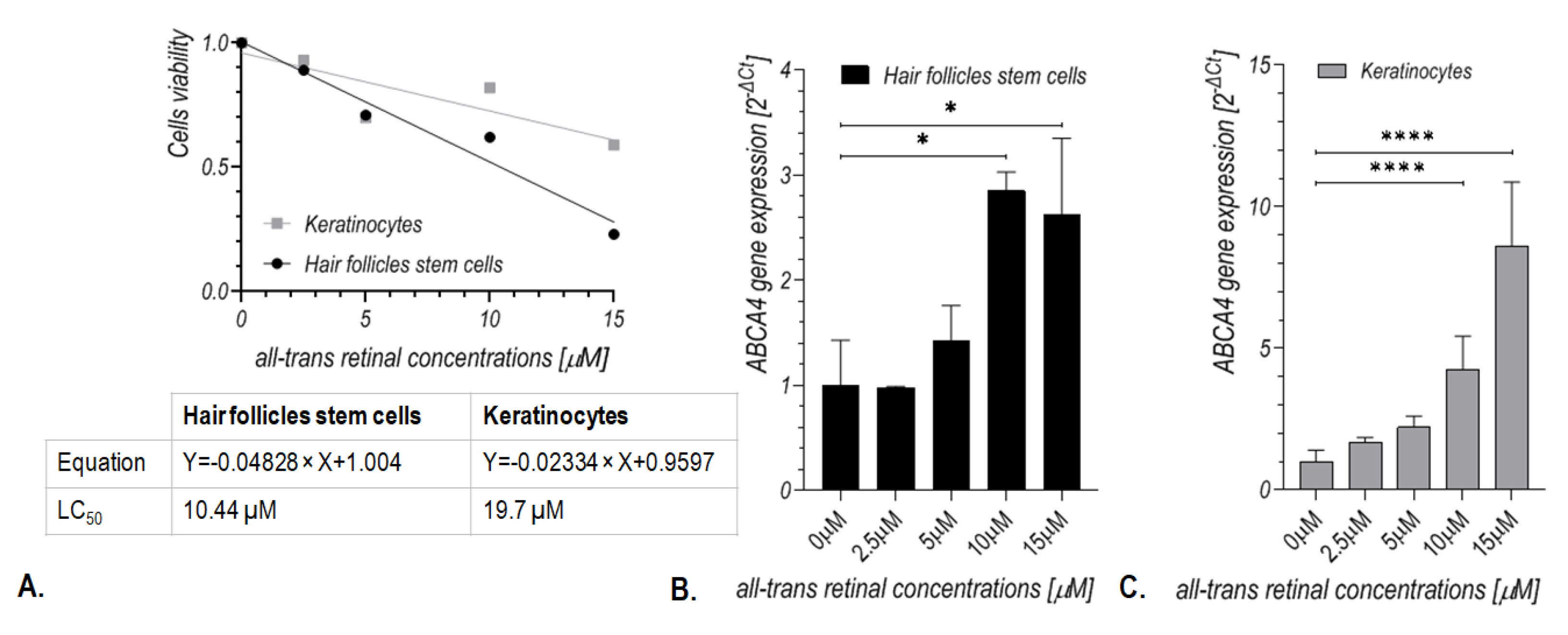
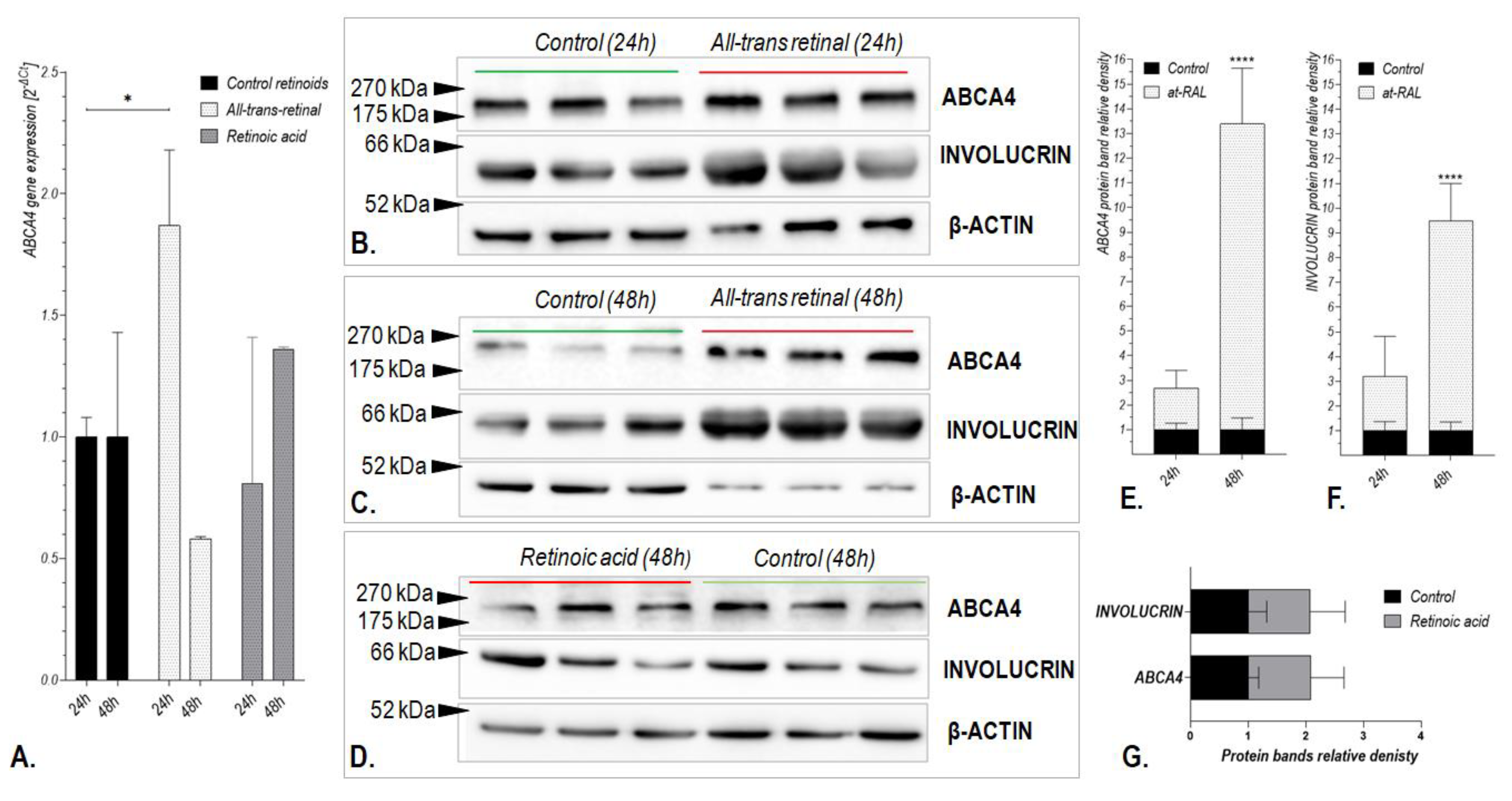
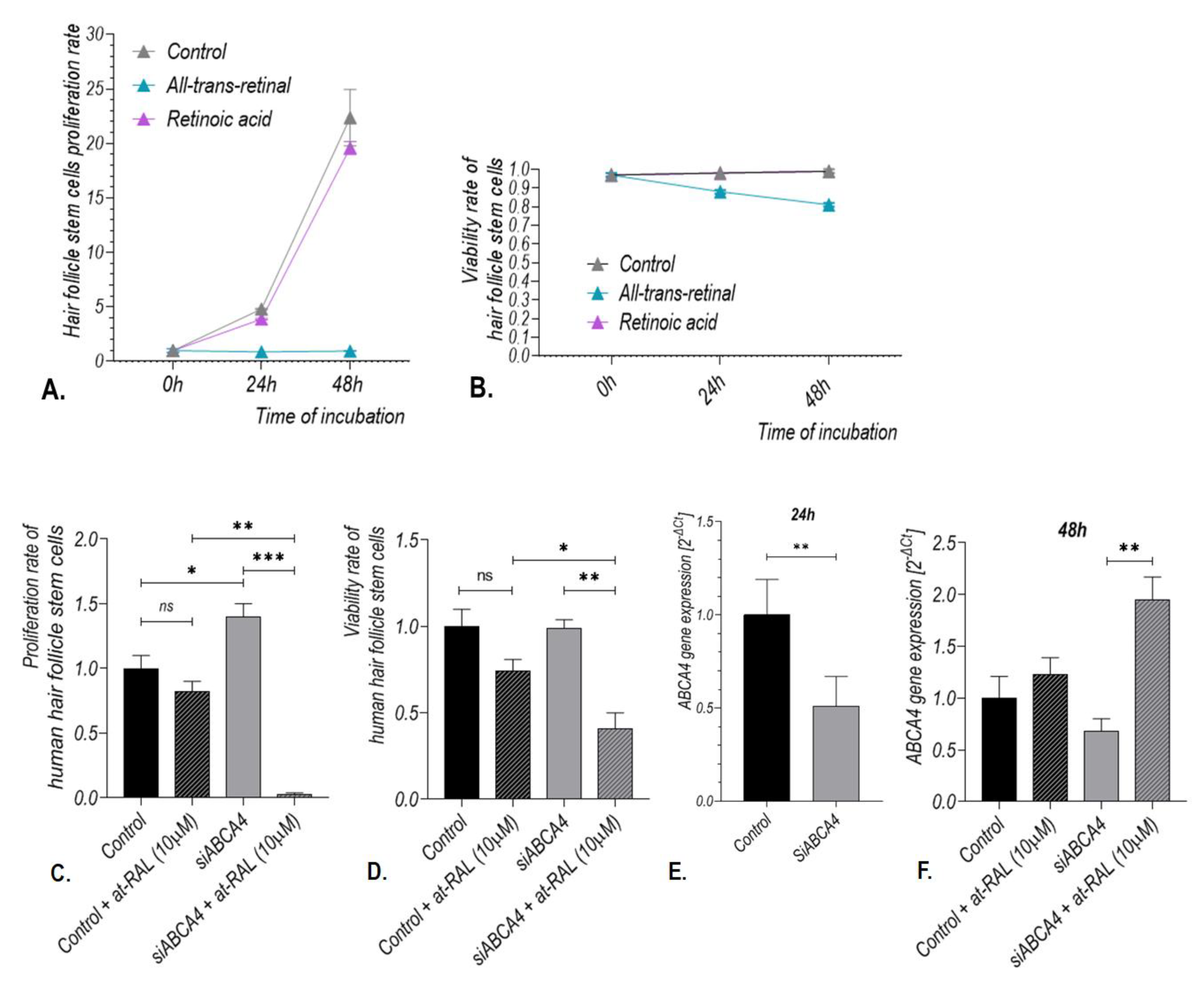
2.2. Treatment with All-Trans-Retinal induces Expression of the ABCA4 Gene in Human Hair Follicle Stem Cells
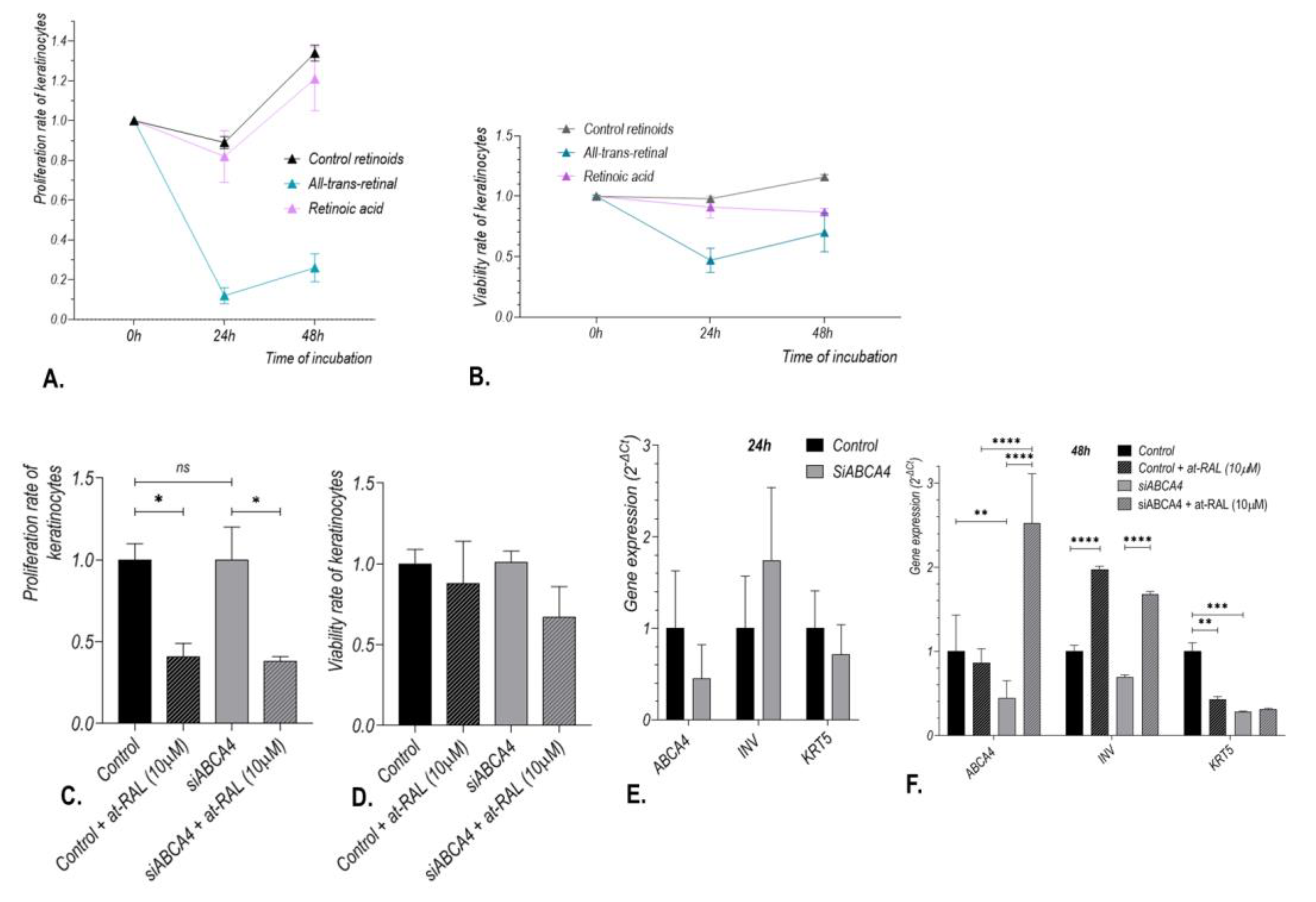
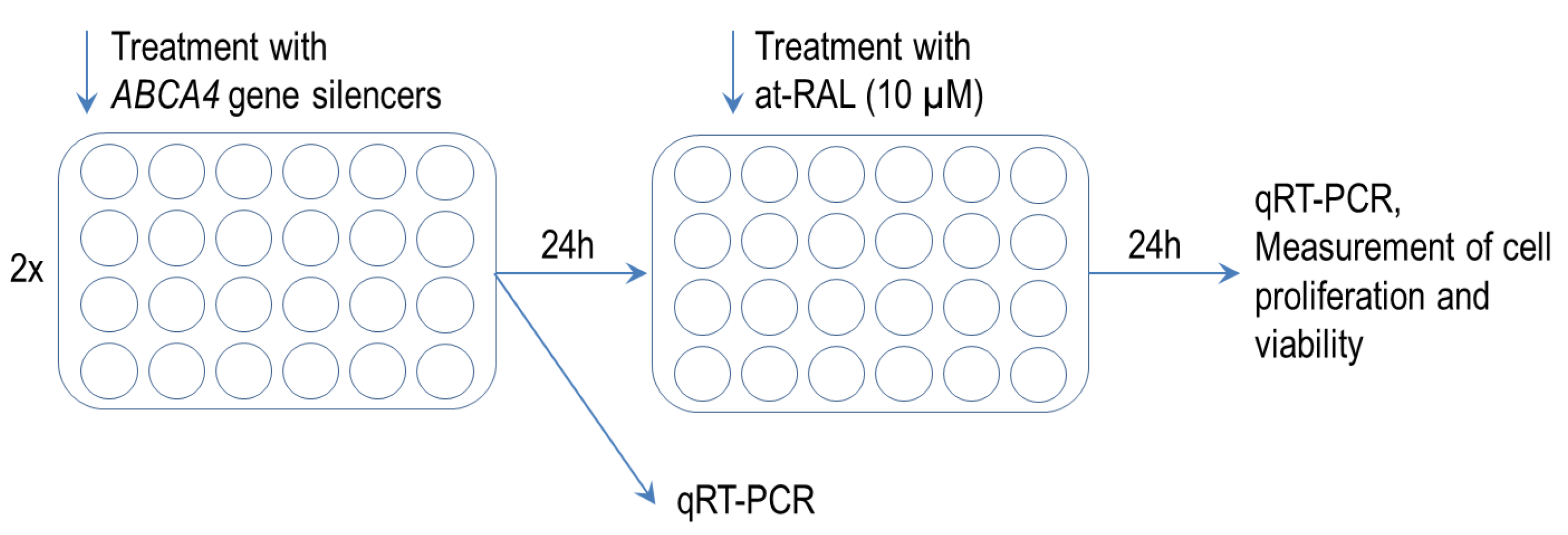
2.3. ABCA4 Gene Silencing Enhances Toxic Effect of All-Trans-Retinal on Human Hair Follicle Stem Cells
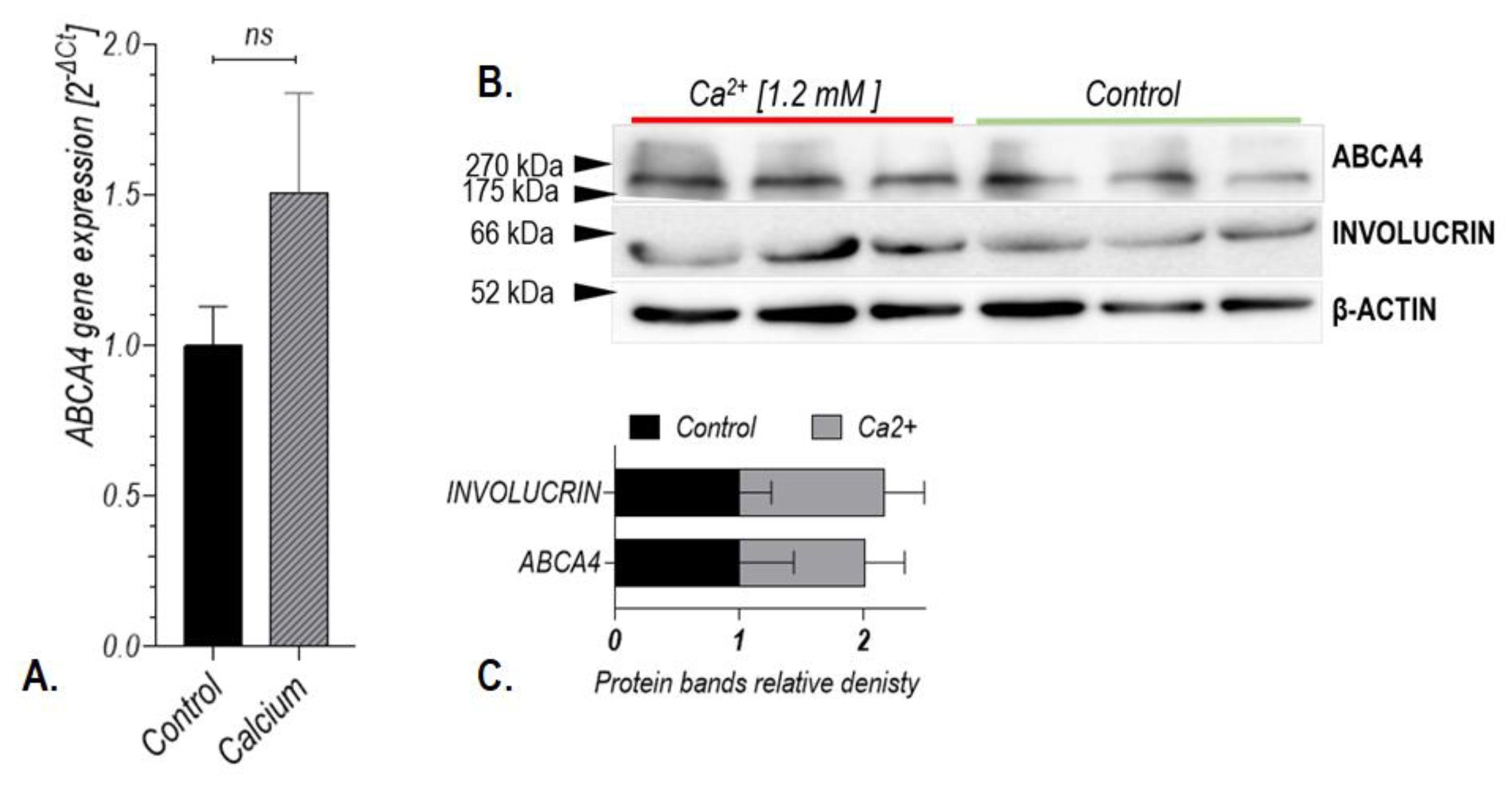
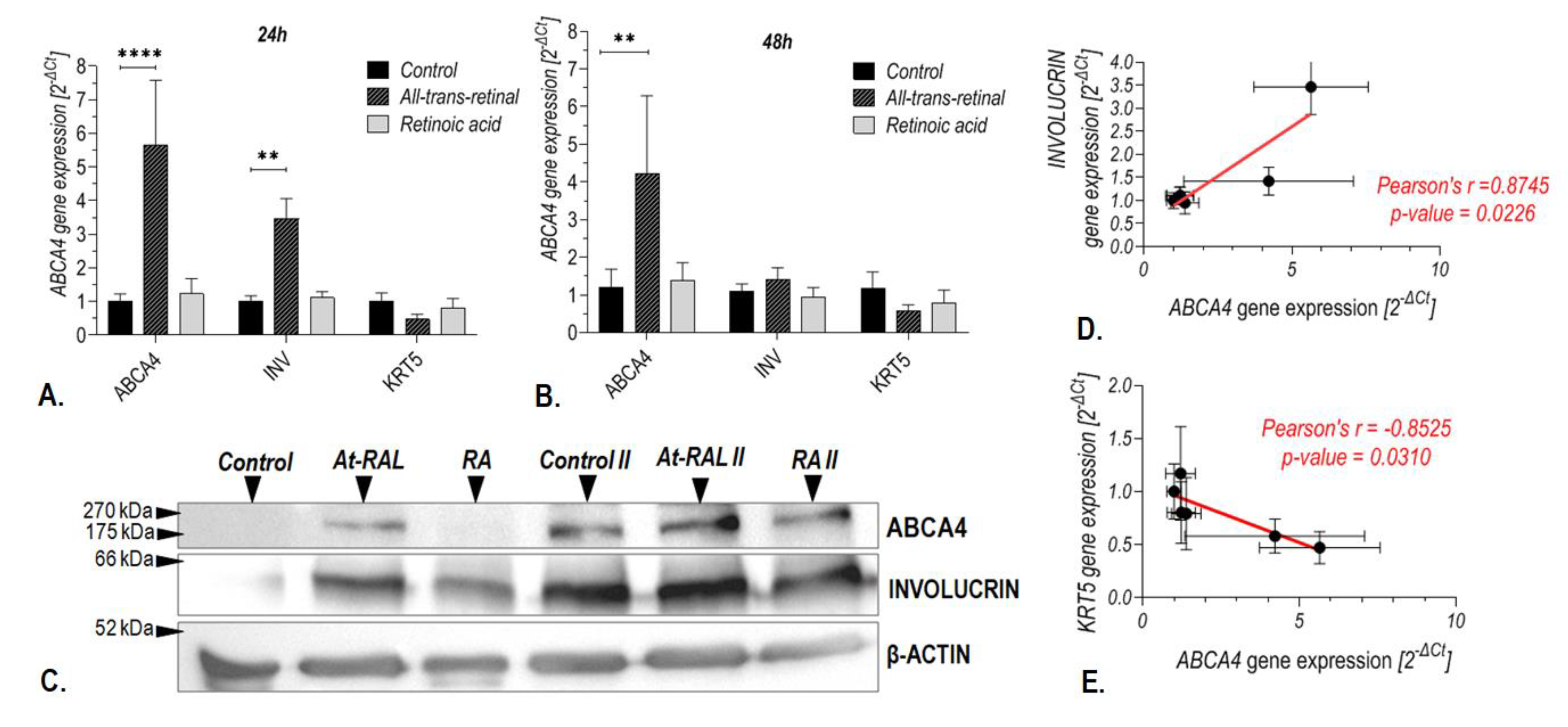
2.4. Treatment with All-Trans-Retinal Induced Expression of the ABCA4 Gene in Human Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Tissue Collection and Cell Cultures
4.2. ABCA4 Gene Silencing
4.3. Proliferation and Viability Assays
4.4. Isolation of Total RNA and cDNA Synthesis
4.5. Quantitative Reverse Transcription PCR (qRT-PCR)
4.6. Western-Blot Analysis
4.7. Immunofluorescence Staining
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molday, R.S.; Zhang, K. Defective lipid transport and biosynthesis in recessive and dominant Stargardt macular degeneration. Prog. Lipid Res. 2010, 49, 476–492. [Google Scholar] [CrossRef]
- Maugeri, A.; Klevering, B.J.; Rohrschneider, K.; Blankenagel, A.; Brunner, H.G.; Deutman, A.F.; Hoyng, C.B.; Cremers, F.P. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am. J. Hum. Genet. 2000, 67, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Lenis, T.L.; Hu, J.; Ng, S.Y.; Jiang, Z.; Sarfare, S.; Lloyd, M.B.; Esposito, N.J.; Samuel, W.; Jaworski, C.; Bok, D.; et al. Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E11120–E11127. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, Q.; Wei, X.; Ma, W.; Luo, W.; Gu, H.; Liu, D.; He, Y.; Huang, T.; Liu, Y.; et al. miR-351-3p promotes rat amniotic fluid-derived mesenchymal stromal cell proliferation via targeting the coding sequence of Abca4. Stem Cells 2021, 39, 1192–1206. [Google Scholar] [CrossRef] [PubMed]
- Sciezynska, A.; Soszynska, M.; Komorowski, M.; Podgorska, A.; Krzesniak, N.; Nogowska, A.; Smolinska, M.; Szulborski, K.; Szaflik, J.P.; Noszczyk, B.; et al. Molecular Analysis of the ABCA4 Gene Mutations in Patients with Stargardt Disease Using Human Hair Follicles. Int. J. Mol. Sci. 2020, 21, 3430. [Google Scholar] [CrossRef]
- Buscone, S.; Mardaryev, A.N.; Raafs, B.; Bikker, J.W.; Sticht, C.; Gretz, N.; Farjo, N.; Uzunbajakava, N.E.; Botchkareva, N.V. A new path in defining light parameters for hair growth: Discovery and modulation of photoreceptors in human hair follicle. Lasers Surg. Med. 2017, 49, 705–718. [Google Scholar] [CrossRef]
- Olinski, L.E.; Lin, E.M.; Oancea, E. Illuminating insights into opsin 3 function in the skin. Adv. Biol. Regul. 2020, 75, 100668. [Google Scholar] [CrossRef]
- Kelley, J.L.; Davies, W.I.L. The Biological Mechanisms and Behavioral Functions of Opsin-Based Light Detection by the Skin. Front. Ecol. Evol. 2016, 4, 106. [Google Scholar] [CrossRef]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg. Med. 2019, 51, 370–382. [Google Scholar] [CrossRef]
- Haltaufderhyde, K.; Ozdeslik, R.N.; Wicks, N.L.; Najera, J.A.; Oancea, E. Opsin expression in human epidermal skin. Photochem. Photobiol. 2015, 91, 117–123. [Google Scholar] [CrossRef]
- Feldman, T.; Ostrovskiy, D.; Yakovleva, M.; Dontsov, A.; Borzenok, S.; Ostrovsky, M. Lipofuscin-Mediated Photic Stress Induces a Dark Toxic Effect on ARPE-19 Cells. Int. J. Mol. Sci. 2022, 23, 12234. [Google Scholar] [CrossRef] [PubMed]
- Antille, C.; Tran, C.; Sorg, O.; Saurat, J.H. Topical beta-carotene is converted to retinyl esters in human skin ex vivo and mouse skin in vivo. Exp. Dermatol. 2004, 13, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.; Vahlquist, A.; Rosdahl, I. Beta-carotene uptake and bioconversion to retinol differ between human melanocytes and keratinocytes. Nutr. Cancer 2001, 39, 300–306. [Google Scholar] [CrossRef]
- Torma, H.; Berne, B.; Vahlquist, A. UV irradiation and topical vitamin A modulate retinol esterification in hairless mouse epidermis. Acta Derm. Venereol. 1988, 68, 291–299. [Google Scholar] [PubMed]
- Torma, H.; Vahlquist, A. Vitamin A esterification in human epidermis: A relation to keratinocyte differentiation. J. Invest. Dermatol. 1990, 94, 132–138. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care 2014, 3, 272–280. [Google Scholar] [CrossRef]
- Haslam, I.S.; El-Chami, C.; Faruqi, H.; Shahmalak, A.; O’Neill, C.A.; Paus, R. Differential expression and functionality of ATP-binding cassette transporters in the human hair follicle. Br. J. Dermatol. 2015, 172, 1562–1572. [Google Scholar] [CrossRef]
- Puntoni, M.; Bigazzi, F.; Sabatino, L.; Sbrana, F.; Musio, A.; Dal Pino, B.; Ragusa, A.; Corsano, E.; Sampietro, T. Early senescence in heterozygous ABCA1 mutation skin fibroblasts: A gene dosage effect beyond HDL deficiency? Biochem. Biophys. Res. Commun. 2014, 447, 231–236. [Google Scholar] [CrossRef]
- Akiyama, M. The roles of ABCA12 in epidermal lipid barrier formation and keratinocyte differentiation. Biochim. Biophys. Acta 2014, 1841, 435–440. [Google Scholar] [CrossRef]
- Akiyama, M.; Sugiyama-Nakagiri, Y.; Sakai, K.; McMillan, J.R.; Goto, M.; Arita, K.; Tsuji-Abe, Y.; Tabata, N.; Matsuoka, K.; Sasaki, R.; et al. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J. Clin. Investig. 2005, 115, 1777–1784. [Google Scholar] [CrossRef]
- Kobayashi, T.; Vischer, U.M.; Rosnoblet, C.; Lebrand, C.; Lindsay, M.; Parton, R.G.; Kruithof, E.K.; Gruenberg, J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell. 2000, 11, 1829–1843. [Google Scholar] [CrossRef]
- Kedishvili, N.Y. Retinoic Acid Synthesis and Degradation. Subcell. Biochem. 2016, 81, 127–161. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Krajewska-Wlodarczyk, M.; Kruszewska, A.; Banasiak, L.; Placek, W.; Maksymowicz, W.; Wojtkiewicz, J. Therapeutic Potential of Stem Cells in Follicle Regeneration. Stem Cells Int. 2018, 2018, 1049641. [Google Scholar] [CrossRef]
- Jia, H.Y.; Shi, Y.; Luo, L.F.; Jiang, G.; Zhou, Q.; Xu, S.Z.; Lei, T.C. Asymmetric stem-cell division ensures sustained keratinocyte hyperproliferation in psoriatic skin lesions. Int. J. Mol. Med. 2016, 37, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Radner, F.P.; Grond, S.; Haemmerle, G.; Lass, A.; Zechner, R. Fat in the skin: Triacylglycerol metabolism in keratinocytes and its role in the development of neutral lipid storage disease. Dermato-Endocrinology 2011, 3, 77–83. [Google Scholar] [CrossRef]
- Haslam, I.S.; Pitre, A.; Schuetz, J.D.; Paus, R. Protection against chemotherapy-induced alopecia: Targeting ATP-binding cassette transporters in the hair follicle? Trends Pharmacol. Sci. 2013, 34, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Wu, Y.C.; Kuo, Y.F.; Chang, S.F.; Chang, C.H.; Wu, R.; Lu, Y.C. Growth and characterization of normal human keratinocytes in F12 serum-free medium. J. Formos. Med. Assoc. 1990, 89, 559–564. [Google Scholar] [PubMed]
- Harrell, C.R.; Gazdic, M.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Therapeutic Potential of Amniotic Fluid Derived Mesenchymal Stem Cells Based on their Differentiation Capacity and Immunomodulatory Properties. Curr. Stem Cell Res. Ther. 2019, 14, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Q.; Dong, H. Proteomic evidence that ABCA4 is vital for traumatic proliferative vitreoretinopathy formation and development. Exp. Eye Res. 2019, 181, 232–239. [Google Scholar] [CrossRef]
- Liu, L.P.; Zheng, D.X.; Xu, Z.F.; Zhou, H.C.; Wang, Y.C.; Zhou, H.; Ge, J.Y.; Sako, D.; Li, M.; Akimoto, K.; et al. Transcriptomic and Functional Evidence Show Similarities between Human Amniotic Epithelial Stem Cells and Keratinocytes. Cells 2021, 11, 70. [Google Scholar] [CrossRef]
- Siegenthaler, G.; Saurat, J.H.; Ponec, M. Retinol and retinal metabolism. Relationship to the state of differentiation of cultured human keratinocytes. Biochem. J. 1990, 268, 371–378. [Google Scholar] [CrossRef] [PubMed]
- VanBuren, C.A.; Everts, H.B. Vitamin A in Skin and Hair: An Update. Nutrients 2022, 14, 2952. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.X.; Porte, S.; Pares, X.; Farres, J. Biological role of aldo-keto reductases in retinoic Acid biosynthesis and signaling. Front. Pharmacol. 2012, 3, 58. [Google Scholar] [CrossRef]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-Associated Endoplasmic Reticulum Membranes in Cardiovascular Diseases. Front. Cell. Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxid Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Xia, Q.; Chen, Y.; Li, J. All-trans-retinal induces autophagic cell death via oxidative stress and the endoplasmic reticulum stress pathway in human retinal pigment epithelial cells. Toxicol. Lett. 2020, 322, 77–86. [Google Scholar] [CrossRef]
- Park, K.; Lee, S.E.; Shin, K.O.; Uchida, Y. Insights into the role of endoplasmic reticulum stress in skin function and associated diseases. FEBS J. 2019, 286, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.; Sivaprakasam, C.; Prinz, W.A.; Nachiappan, V. Endoplasmic reticulum stress affects the transport of phosphatidylethanolamine from mitochondria to the endoplasmic reticulum in S. cerevisiae. Biochim. Biophys. Acta 2016, 1861, 1959–1967. [Google Scholar] [CrossRef]
- Wiley, L.; Kaalberg, E.; Penticoff, J.; Mullins, R.; Stone, E.; Tucker, B. Expression of the retina-specific flippase, ABCA4, in epidermal keratinocytes [version 1; peer review: 2 approved with reservations]. F1000Research 2016, 5, 193. [Google Scholar] [CrossRef]
- Zhong, M.; Molday, L.L.; Molday, R.S. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J. Biol. Chem. 2009, 284, 3640–3649. [Google Scholar] [CrossRef]
- Molday, L.L.; Wahl, D.; Sarunic, M.V.; Molday, R.S. Localization and functional characterization of the p.Asn965Ser (N965S) ABCA4 variant in mice reveal pathogenic mechanisms underlying Stargardt macular degeneration. Hum. Mol. Genet. 2018, 27, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Jahoda, C.A. An improved method of human keratinocyte culture from skin explants: Cell expansion is linked to markers of activated progenitor cells. Exp. Dermatol. 2009, 18, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścieżyńska, A.; Łuszczyński, K.; Radziszewski, M.; Komorowski, M.; Soszyńska, M.; Krześniak, N.; Shevchenko, K.; Lutyńska, A.; Malejczyk, J. Role of the ABCA4 Gene Expression in the Clearance of Toxic Vitamin A Derivatives in Human Hair Follicle Stem Cells and Keratinocytes. Int. J. Mol. Sci. 2023, 24, 8275. https://doi.org/10.3390/ijms24098275
Ścieżyńska A, Łuszczyński K, Radziszewski M, Komorowski M, Soszyńska M, Krześniak N, Shevchenko K, Lutyńska A, Malejczyk J. Role of the ABCA4 Gene Expression in the Clearance of Toxic Vitamin A Derivatives in Human Hair Follicle Stem Cells and Keratinocytes. International Journal of Molecular Sciences. 2023; 24(9):8275. https://doi.org/10.3390/ijms24098275
Chicago/Turabian StyleŚcieżyńska, Aneta, Krzysztof Łuszczyński, Marcin Radziszewski, Michał Komorowski, Marta Soszyńska, Natalia Krześniak, Kateryna Shevchenko, Anna Lutyńska, and Jacek Malejczyk. 2023. "Role of the ABCA4 Gene Expression in the Clearance of Toxic Vitamin A Derivatives in Human Hair Follicle Stem Cells and Keratinocytes" International Journal of Molecular Sciences 24, no. 9: 8275. https://doi.org/10.3390/ijms24098275
APA StyleŚcieżyńska, A., Łuszczyński, K., Radziszewski, M., Komorowski, M., Soszyńska, M., Krześniak, N., Shevchenko, K., Lutyńska, A., & Malejczyk, J. (2023). Role of the ABCA4 Gene Expression in the Clearance of Toxic Vitamin A Derivatives in Human Hair Follicle Stem Cells and Keratinocytes. International Journal of Molecular Sciences, 24(9), 8275. https://doi.org/10.3390/ijms24098275




