TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy
Abstract
1. Introduction
2. Discovery of the TTT Complex and Its Homologs
3. Tel2 Deletion Reduces the Protein Levels of All PIKKs
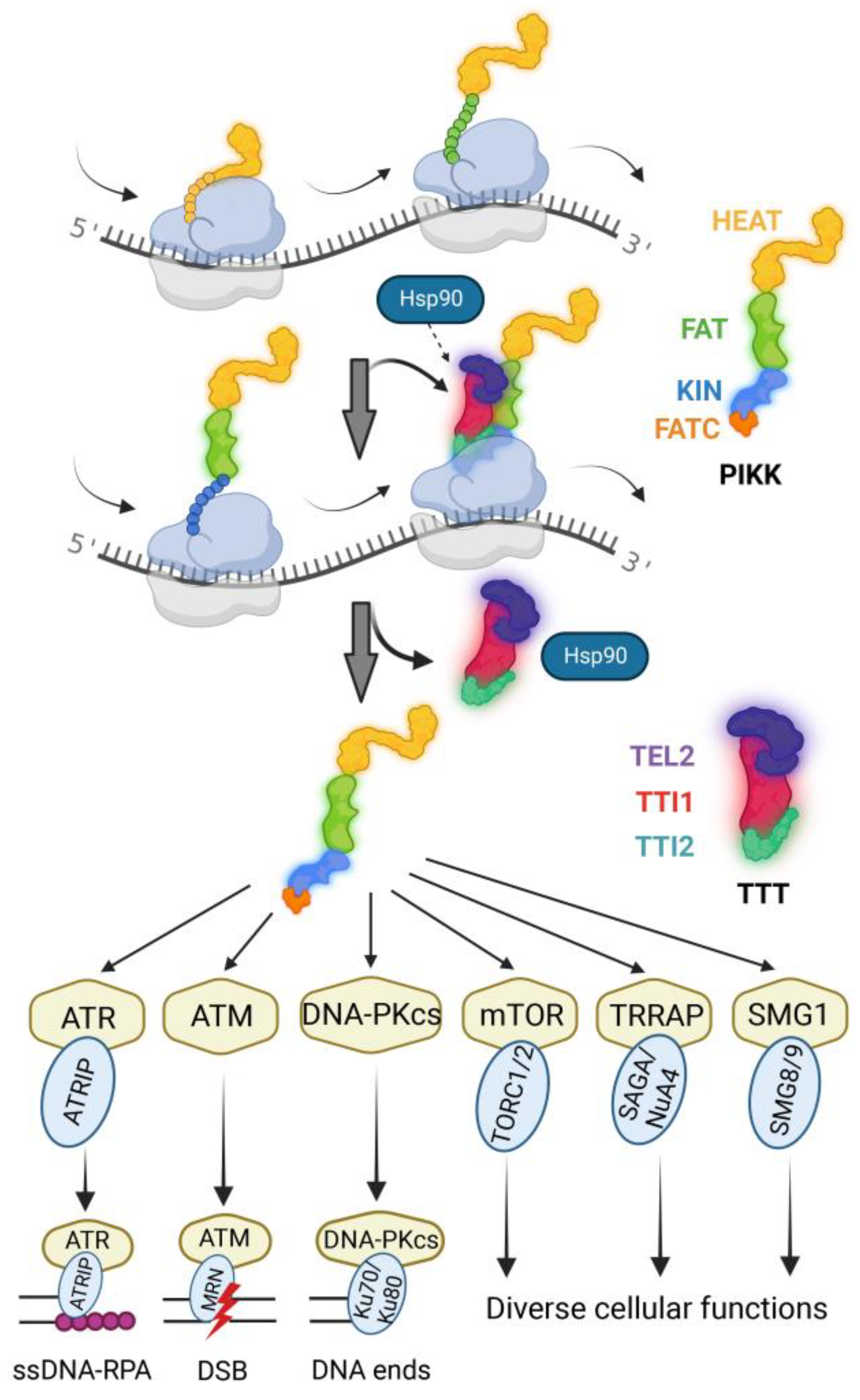
4. TTT Functions as a Co-Chaperone for the Stabilization of PIKKs
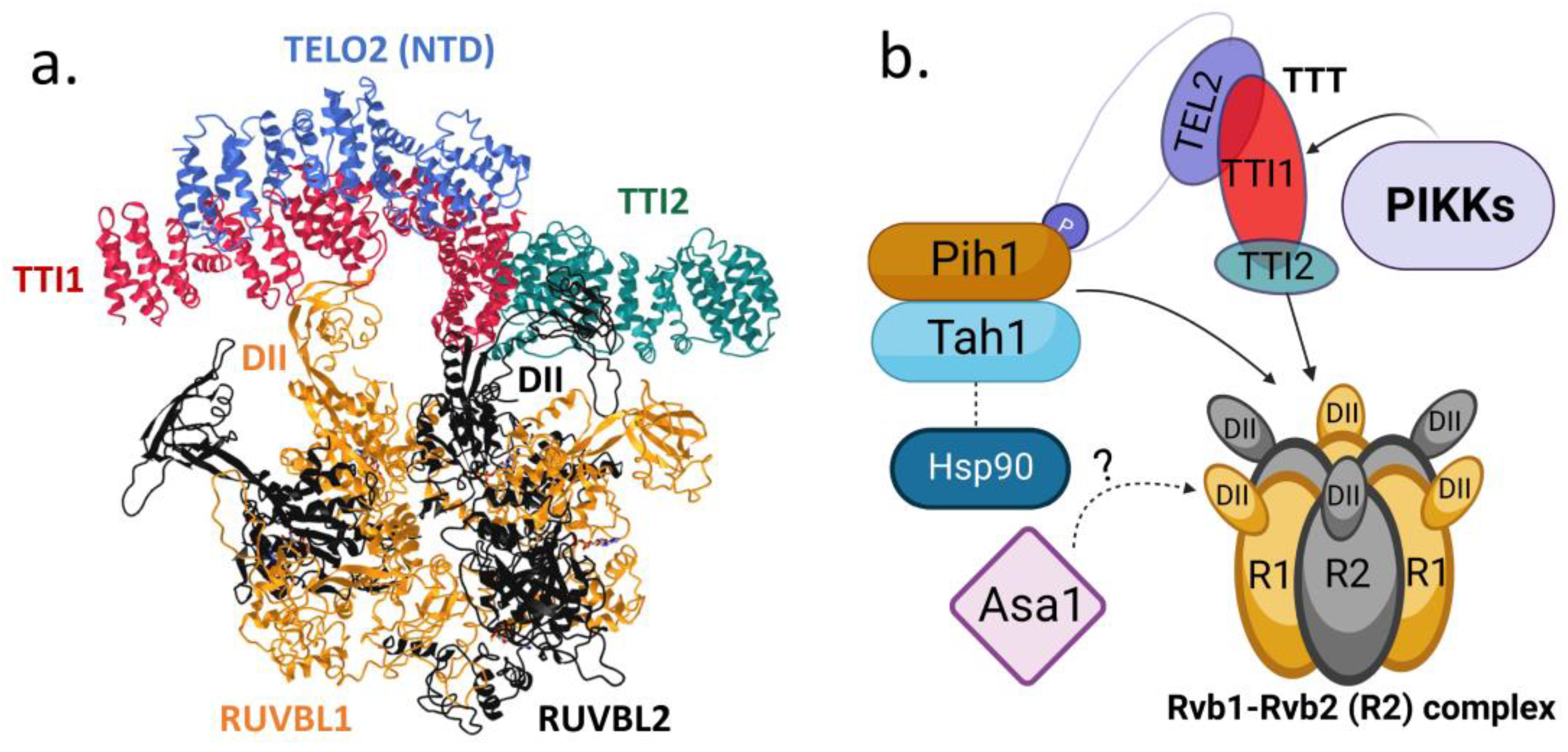
5. TTT Structure and Its Interactions with Hsp90 and R2TP Complex
6. TTT Promotes the Co-Translational Maturation of PIKKs
7. Other Functions of the TTT Complex in Genome Maintenance
7.1. TTT Specifically Regulates the Stability of a Subset of PIKKs via CK2 Phosphorylation
7.2. TTT May Regulate the DNA Replication Checkpoint
8. TTT in Disease and Cancer Chemotherapy
9. Summary and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Bimbó, A.; Jia, Y.; Poh, S.L.; Karuturi, R.K.M.; Elzen, N.D.; Peng, X.; Zheng, L.; O’Connell, M.; Liu, E.T.; Balasubramanian, M.K.; et al. Systematic Deletion Analysis of Fission Yeast Protein Kinases. Eukaryot. Cell 2005, 4, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, A.; Choi, H.; Sharom, J.R.; Boucher, L.; Neduva, V.; Larsen, B.; Lin, Z.-Y.; Breitkreutz, B.-J.; Stark, C.; Liu, G.; et al. A Global Protein Kinase and Phosphatase Interaction Network in Yeast. Science 2010, 328, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Baretić, D.; Williams, R.L. PIKKs—The solenoid nest where partners and kinases meet. Curr. Opin. Struct. Biol. 2014, 29, 134–142. [Google Scholar] [CrossRef]
- Abraham, R.T. PI 3-kinase related kinases: ‘Big’ players in stress-induced signaling pathways. DNA Repair 2004, 3, 883–887. [Google Scholar] [CrossRef]
- Mandal, A.K.; Lee, P.; Chen, J.A.; Nillegoda, N.; Heller, A.; DiStasio, S.; Oen, H.; Victor, J.; Nair, D.M.; Brodsky, J.L.; et al. Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J. Cell Biol. 2007, 176, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative Analysis of Hsp90-Client Interactions Reveals Principles of Substrate Recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef]
- Prince, T.L.; Lang, B.J.; Okusha, Y.; Eguchi, T.; Calderwood, S.K. Cdc37 as a Co-chaperone to Hsp90. In The Networking of Chaperones by Co-Chaperones; Springer International Publishing: Cham, Switzerland, 2022; Volume 101, pp. 141–158. [Google Scholar] [CrossRef]
- Sugimoto, K. Branching the Tel2 pathway for exact fit on phosphatidylinositol 3-kinase-related kinases. Curr. Genet. 2018, 64, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Toullec, D.; Elías-Villalobos, A.; Faux, C.; Noly, A.; Lledo, G.; Séveno, M.; Helmlinger, D. The Hsp90 cochaperone TTT promotes cotranslational maturation of PIKKs prior to complex assembly. Cell Rep. 2021, 37, 109867. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Joo, S.Y.; Kim, B.-G.; Jo, A.; Lee, H.; Cho, Y. Structure of the Human TELO2-TTI1-TTI2 Complex. J. Mol. Biol. 2021, 434, 167370. [Google Scholar] [CrossRef]
- Pal, M.; Muñoz-Hernandez, H.; Bjorklund, D.; Zhou, L.; Degliesposti, G.; Skehel, J.M.; Hesketh, E.L.; Thompson, R.F.; Pearl, L.H.; Llorca, O.; et al. Structure of the TELO2-TTI1-TTI2 complex and its function in TOR recruitment to the R2TP chaperone. Cell Rep. 2021, 36, 109317. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Xie, Y.; de Lange, T.; Pavletich, N.P. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes Dev. 2010, 24, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Sobreira, N.L.; Gable, D.L.; Jurgens, J.; Grange, D.K.; Belnap, N.; Siniard, A.; Szelinger, S.; Schrauwen, I.; Richholt, R.F.; et al. A Syndromic Intellectual Disability Disorder Caused by Variants in TELO2, a Gene Encoding a Component of the TTT Complex. Am. J. Hum. Genet. 2016, 98, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Albokhari, D.; Pritchard, A.B.; Beil, A.; Muss, C.; Bupp, C.; Grange, D.K.; Delplancq, G.; Heeley, J.; Zuteck, M.; Morrow, M.M.; et al. TELO2-related syndrome (You-Hoover-Fong syndrome): Description of 14 new affected individuals and review of the literature. Am. J. Med. Genet. Part A 2023, 191, 1261–1272. [Google Scholar] [CrossRef]
- Ziegler, A.; Bader, P.; McWalter, K.; Douglas, G.; Houdayer, C.; Bris, C.; Rouleau, S.; Coutant, R.; Colin, E.; Bonneau, D. Confirmation that variants in TTI2 are responsible for autosomal recessive intellectual disability. Clin. Genet. 2019, 96, 354–358. [Google Scholar] [CrossRef]
- Serey-Gaut, M.; Cortes, M.; Makrythanasis, P.; Suri, M.; Taylor, A.M.; Sullivan, J.A.; Asleh, A.N.; Mitra, J.; Dar, M.A.; McNamara, A.; et al. Bi-allelic TTI1 variants cause an autosomal-recessive neurodevelopmental disorder with microcephaly. Am. J. Hum. Genet. 2023, 110, 499–515. [Google Scholar] [CrossRef]
- Yonezawa, H.; Ikeda, A.; Takahashi, R.; Endo, H.; Sugawara, Y.; Goto, M.; Kanno, M.; Ogawa, S.; Nakamura, K.; Ujiie, H.; et al. Ivermectin represses Wnt/β-catenin signaling by binding to TELO2, a regulator of phosphatidylinositol 3-kinase-related kinases. iScience 2022, 25, 103912. [Google Scholar] [CrossRef]
- Hartman, P.S.; Herman, R.K. Somatic damage to the X chromosome of the nematode Caenorhabditis elegans induced by gamma radiation. Mol. Genet. Genom. 1982, 187, 116–119. [Google Scholar] [CrossRef]
- Lustig, A.J.; Petes, T.D. Identification of yeast mutants with altered telomere structure. Proc. Natl. Acad. Sci. USA 1986, 83, 1398–1402. [Google Scholar] [CrossRef]
- Lakowski, B.; Hekimi, S. Determination of Life-Span in Caenorhabditis elegans by Four Clock Genes. Science 1996, 272, 1010–1013. [Google Scholar] [CrossRef]
- Hekimi, S.; Boutis, P.; Lakowski, B. Viable maternal-effect mutations that affect the development of the nematode Caenorhabditis elegans. Genetics 1995, 141, 1351–1364. [Google Scholar] [CrossRef]
- Ahmed, S.; Alpi, A.; Hengartner, M.O.; Gartner, A. C. elegans RAD-5/CLK-2 defines a new DNA damage checkpoint protein. Curr. Biol. 2001, 11, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Gartner, A.; Milstein, S.; Ahmed, S.; Hodgkin, J.; Hengartner, M.O. A conserved checkpoint pathway mediates DNA damage–induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 2000, 5, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muse, T.; Boulton, S.J. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 2005, 24, 4345–4355. [Google Scholar] [CrossRef]
- Lim, C.-S.; Mian, I.; Dernburg, A.F.; Campisi, J. C. elegans clk-2, a gene that limits life span, encodes a telomere length regulator similar to yeast telomere binding protein Tel2p. Curr. Biol. 2001, 11, 1706–1710. [Google Scholar] [CrossRef]
- Jiang, N.; Bénard, C.Y.; Kébir, H.; Shoubridge, E.A.; Hekimi, S. Human CLK2 Links Cell Cycle Progression, Apoptosis, and Telomere Length Regulation. J. Biol. Chem. 2003, 278, 21678–21684. [Google Scholar] [CrossRef]
- Runge, K.W.; Zakian, V.A. TEL2, an Essential Gene Required for Telomere Length Regulation and Telomere Position Effect in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996, 16, 3094–3105. [Google Scholar] [CrossRef] [PubMed]
- Kota, R.S.; Runge, K.W. The yeast telomere length regulator TEL2 encodes a protein that binds to telomeric DNA. Nucleic Acids Res. 1998, 26, 1528–1535. [Google Scholar] [CrossRef]
- Anderson, C.M.; Korkin, D.; Smith, D.L.; Makovets, S.; Seidel, J.J.; Sali, A.; Blackburn, E.H. Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break. Genes Dev. 2008, 22, 854–859. [Google Scholar] [CrossRef]
- Anderson, C.M.; Blackburn, E.H. Mec1 function in the DNA damage response does not require its interaction with Tel2. Cell Cycle 2008, 7, 3695–3698. [Google Scholar] [CrossRef]
- Hořejší, Z.; Collis, S.J.; Boulton, S.J. FANCM-FAAP24 and HCLK2: Roles in ATR signalling and the Fanconi Anemia pathway. Cell Cycle 2009, 8, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Collis, S.J.; Barber, L.J.; Clark, A.J.; Martin, J.S.; Ward, J.D.; Boulton, S.J. HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nature 2007, 9, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, J.M.R.; Larsen, D.H.; Schou, K.B.; Freire, R.; Falck, J.; Bartek, J.; Lukas, J. HCLK2 Is Required for Activity of the DNA Damage Response Kinase ATR. J. Biol. Chem. 2009, 284, 4140–4147. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Khan, S.; Didier, A.C.; Wozniak, M.; Liu, Y.; Singh, A.; Nakamura, T.M. A tel2 Mutation That Destabilizes the Tel2-Tti1-Tti2 Complex Eliminates Rad3ATR Kinase Signaling in the DNA Replication Checkpoint and Leads to Telomere Shortening in Fission Yeast. Mol. Cell. Biol. 2019, 39, e00175-19. [Google Scholar] [CrossRef]
- Shikata, M.; Ishikawa, F.; Kanoh, J. Tel2 Is Required for Activation of the Mrc1-mediated Replication Checkpoint. J. Biol. Chem. 2007, 282, 5346–5355. [Google Scholar] [CrossRef]
- Kanoh, J.; Yanagida, M. Tel2: A common partner of PIK-related kinases and a link between DNA checkpoint and nutritional response? Genes Cells 2007, 12, 1301–1304. [Google Scholar] [CrossRef]
- Hayashi, T.; Hatanaka, M.; Nagao, K.; Nakaseko, Y.; Kanoh, J.; Kokubu, A.; Ebe, M.; Yanagida, M. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells 2007, 12, 1357–1370. [Google Scholar] [CrossRef]
- Shevchenko, A.; Roguev, A.; Schaft, D.; Buchanan, L.; Habermann, B.; Sakalar, C.; Thomas, H.; Krogan, N.J.; Shevchenko, A.; Stewart, A.F. Chromatin Central: Towards the comparative proteome by accurate mapping of the yeast proteomic environment. Genome Biol. 2008, 9, R167. [Google Scholar] [CrossRef]
- Hurov, K.E.; Cotta-Ramusino, C.; Elledge, S.J. A genetic screen identifies the Triple T complex required for DNA damage signaling and ATM and ATR stability. Genes Dev. 2010, 24, 1939–1950. [Google Scholar] [CrossRef]
- Takai, H.; Wang, R.C.; Takai, K.K.; Yang, H.; de Lange, T. Tel2 Regulates the Stability of PI3K-Related Protein Kinases. Cell 2007, 131, 1248–1259. [Google Scholar] [CrossRef]
- Goto, G.H.; Ogi, H.; Biswas, H.; Ghosh, A.; Tanaka, S.; Sugimoto, K. Two separate pathways regulate protein stability of ATM/ATR-related protein kinases Mec1 and Tel1 in budding yeast. PLoS Genet. 2017, 13, e1006873. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.S.; Duennwald, M.L.; Karagiannis, J.; Genereaux, J.; McCarton, A.S.; Brandl, C.J. Saccharomyces cerevisiae Tti2 Regulates PIKK Proteins and Stress Response. G3 Genes Genomes Genet. 2016, 6, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Stirling, P.C.; Bloom, M.S.; Solanki-Patil, T.; Smith, S.; Sipahimalani, P.; Li, Z.; Kofoed, M.; Ben-Aroya, S.; Myung, K.; Hieter, P. The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components. PLoS Genet. 2011, 7, e1002057. [Google Scholar] [CrossRef]
- Rozario, D.; Siede, W. Saccharomyces cerevisiae Tel2 plays roles in TORC signaling and telomere maintenance that can be mutationally separated. Biochem. Biophys. Res. Commun. 2012, 417, 1182–1187. [Google Scholar] [CrossRef]
- Grandin, N.; Corset, L.; Charbonneau, M. Genetic and Physical Interactions between Tel2 and the Med15 Mediator Subunit in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e30451. [Google Scholar] [CrossRef]
- Fernández-Sáiz, V.; Targosz, B.-S.; Lemeer, S.; Eichner, R.; Langer, C.; Bullinger, L.; Reiter, C.; Slotta-Huspenina, J.; Schroeder, S.; Knorn, A.-M.; et al. SCFFbxo9 and CK2 direct the cellular response to growth factor withdrawal via Tel2/Tti1 degradation and promote survival in multiple myeloma. Nature 2012, 15, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Izumi, N.; Yamashita, A.; Hirano, H.; Ohno, S. Heat shock protein 90 regulates phosphatidylinositol 3-kinase-related protein kinase family proteins together with the RUVBL1/2 and Tel2-containing co-factor complex. Cancer Sci. 2011, 103, 50–57. [Google Scholar] [CrossRef]
- Kaizuka, T.; Hara, T.; Oshiro, N.; Kikkawa, U.; Yonezawa, K.; Takehana, K.; Iemura, S.-I.; Natsume, T.; Mizushima, N. Tti1 and Tel2 Are Critical Factors in Mammalian Target of Rapamycin Complex Assembly. J. Biol. Chem. 2010, 285, 20109–20116. [Google Scholar] [CrossRef]
- Keith, C.T.; Schreiber, S.L. PIK-Related Kinases: DNA Repair, Recombination, and Cell Cycle Checkpoints. Science 1995, 270, 50–51. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. An expanding role for mTOR in cancer. Trends Mol. Med. 2005, 11, 353–361. [Google Scholar] [CrossRef]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR Signaling in Growth and Metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Cimprich, K.A.; Cortez, D. ATR: An essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 2008, 9, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Imseng, S.; Aylett, C.H.; Maier, T. Architecture and activation of phosphatidylinositol 3-kinase related kinases. Curr. Opin. Struct. Biol. 2018, 49, 177–189. [Google Scholar] [CrossRef]
- Perry, J.; Kleckner, N. The ATRs, ATMs, and TORs Are Giant HEAT Repeat Proteins. Cell 2003, 112, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.; Pavletich, N.P. Structure of the human ATM kinase and mechanism of Nbs1 binding. eLife 2022, 11, e74218. [Google Scholar] [CrossRef]
- Zhu, L.; Li, L.; Qi, Y.; Yu, Z.; Xu, Y. Cryo-EM structure of SMG1–SMG8–SMG9 complex. Cell Res. 2019, 29, 1027–1034. [Google Scholar] [CrossRef]
- Yin, X.; Liu, M.; Tian, Y.; Wang, J.; Xu, Y. Cryo-EM structure of human DNA-PK holoenzyme. Cell Res. 2017, 27, 1341–1350. [Google Scholar] [CrossRef]
- Rao, Q.; Liu, M.; Tian, Y.; Wu, Z.; Hao, Y.; Song, L.; Qin, Z.; Ding, C.; Wang, H.-W.; Wang, J.; et al. Cryo-EM structure of human ATR-ATRIP complex. Cell Res. 2017, 28, 143–156. [Google Scholar] [CrossRef]
- Horejsi, Z.; Takai, H.; Adelman, C.A.; Collis, S.; Flynn, H.; Maslen, S.; Skehel, J.M.; de Lange, T.; Boulton, S.J. CK2 Phospho-Dependent Binding of R2TP Complex to TEL2 Is Essential for mTOR and SMG1 Stability. Mol. Cell 2010, 39, 839–850. [Google Scholar] [CrossRef]
- Back, R.; Dominguez, C.; Rothé, B.; Bobo, C.; Beaufils, C.; Moréra, S.; Meyer, P.; Charpentier, B.; Branlant, C.; Allain, F.H.-T.; et al. High-Resolution Structural Analysis Shows How Tah1 Tethers Hsp90 to the R2TP Complex. Structure 2013, 21, 1834–1847. [Google Scholar] [CrossRef]
- Eckert, K.; Saliou, J.-M.; Monlezun, L.; Vigouroux, A.; Atmane, N.; Caillat, C.; Quevillon-Chéruel, S.; Madiona, K.; Nicaise, M.; Lazereg, S.; et al. The Pih1-Tah1 Cochaperone Complex Inhibits Hsp90 Molecular Chaperone ATPase Activity. J. Biol. Chem. 2010, 285, 31304–31312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kakihara, Y.; Gribun, A.; Huen, J.; Yang, G.; Khanna, M.; Costanzo, M.; Brost, R.L.; Boone, C.; Hughes, T.R.; et al. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J. Cell Biol. 2008, 180, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef]
- Prodromou, C.; Bjorklund, D.M. Advances towards Understanding the Mechanism of Action of the Hsp90 Complex. Biomolecules 2022, 12, 600. [Google Scholar] [CrossRef] [PubMed]
- Borkovich, K.A.; Farrelly, F.W.; Finkelstein, D.B.; Taulien, J.; Lindquist, S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 1989, 9, 3919–3930. [Google Scholar] [CrossRef]
- Cutforth, T. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 1994, 77, 1027–1036. [Google Scholar] [CrossRef]
- Frey, S.; Leskovar, A.; Reinstein, J.; Buchner, J. The ATPase Cycle of the Endoplasmic Chaperone Grp94. J. Biol. Chem. 2007, 282, 35612–35620. [Google Scholar] [CrossRef] [PubMed]
- Leskovar, A.; Wegele, H.; Werbeck, N.D.; Buchner, J.; Reinstein, J. The ATPase Cycle of the Mitochondrial Hsp90 Analog Trap1. J. Biol. Chem. 2008, 283, 11677–11688. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Pearl, L.H. Review: The HSP90 molecular chaperone—An enigmatic ATPase. Biopolymers 2016, 105, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Dollins, D.E.; Warren, J.J.; Immormino, R.M.; Gewirth, D.T. Structures of GRP94-Nucleotide Complexes Reveal Mechanistic Differences between the hsp90 Chaperones. Mol. Cell 2007, 28, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.U.; Roe, S.M.; Vaughan, C.K.; Meyer, P.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 2006, 440, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, C.K.; Gohlke, U.; Sobott, F.; Good, V.M.; Ali, M.M.; Prodromou, C.; Robinson, C.V.; Saibil, H.R.; Pearl, L.H. Structure of an Hsp90-Cdc37-Cdk4 Complex. Mol. Cell 2006, 23, 697–707. [Google Scholar] [CrossRef]
- Oberoi, J.; Guiu, X.A.; Outwin, E.A.; Schellenberger, P.; Roumeliotis, T.I.; Choudhary, J.S.; Pearl, L.H. HSP90-CDC37-PP5 forms a structural platform for kinase dephosphorylation. Nat. Commun. 2022, 13, 7343. [Google Scholar] [CrossRef]
- Keramisanou, D.; Kumar, M.V.; Boose, N.; Abzalimov, R.R.; Gelis, I. Assembly mechanism of early Hsp90-Cdc37-kinase complexes. Sci. Adv. 2022, 8, eabm9294. [Google Scholar] [CrossRef]
- Kimura, Y.; Rutherford, S.L.; Miyata, Y.; Yahara, I.; Freeman, B.C.; Yue, L.; Morimoto, R.I.; Lindquist, S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997, 11, 1775–1785. [Google Scholar] [CrossRef]
- Bandhakavi, S.; McCann, R.O.; Hanna, D.E.; Glover, C.V.C. A Positive Feedback Loop between Protein Kinase CKII and Cdc37 Promotes the Activity of Multiple Protein Kinases. J. Biol. Chem. 2003, 278, 2829–2836. [Google Scholar] [CrossRef]
- Liu, W.; Landgraf, R. Phosphorylated and Unphosphorylated Serine 13 of CDC37 Stabilize Distinct Interactions between Its Client and HSP90 Binding Domains. Biochemistry 2015, 54, 1493–1504. [Google Scholar] [CrossRef]
- Nguyen, L.X.T.; Mitchell, B.S. Akt activation enhances ribosomal RNA synthesis through casein kinase II and TIF-IA. Proc. Natl. Acad. Sci. USA 2013, 110, 20681–20686. [Google Scholar] [CrossRef]
- Ji, H.; Wang, J.; Nika, H.; Hawke, D.; Keezer, S.; Ge, Q.; Fang, B.; Fang, X.; Fang, D.; Litchfield, D.W.; et al. EGF-Induced ERK Activation Promotes CK2-Mediated Disassociation of α-Catenin from β-Catenin and Transactivation of β-Catenin. Mol. Cell 2009, 36, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Donella-Deana, A.; Cesaro, L.; Sarno, S.; Ruzzene, M.; Brunati, A.M.; Marin, O.; Vilk, G.; Doherty-Kirby, A.; Lajoie, G.; Litchfield, D.W.; et al. Tyrosine phosphorylation of protein kinase CK2 by Src-related tyrosine kinases correlates with increased catalytic activity. Biochem. J. 2003, 372, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Park, J.-W.; Bae, Y.-S. Regulation of protein kinase CK2 catalytic activity by protein kinase C and phospholipase D2. Biochimie 2016, 121, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Skjerpen, C.S.; Nilsen, T.; Wesche, J.; Olsnes, S. Binding of FGF-1 variants to protein kinase CK2 correlates with mitogenicity. EMBO J. 2002, 21, 4058–4069. [Google Scholar] [CrossRef]
- Bonnet, H.; Filhol, O.; Truchet, I.; Brethenou, P.; Cochet, C.; Amalric, F.; Bouche, G. Fibroblast Growth Factor-2 Binds to the Regulatory β Subunit of CK2 and Directly Stimulates CK2 Activity toward Nucleolin. J. Biol. Chem. 1996, 271, 24781–24787. [Google Scholar] [CrossRef]
- Rao, F.; Cha, J.; Xu, J.; Xu, R.; Vandiver, M.S.; Tyagi, R.; Tokhunts, R.; Koldobskiy, M.A.; Fu, C.; Barrow, R.; et al. Inositol Pyrophosphates Mediate the DNA-PK/ATM-p53 Cell Death Pathway by Regulating CK2 Phosphorylation of Tti1/Tel2. Mol. Cell 2014, 54, 119–132. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Filhol, O.; Leroy, D.; Bonello, G.; Baudry, M.; Chambaz, E.M.; Cochet, C. The tight association of protein kinase CK2 with plasma membranes is mediated by a specific domain of its regulatory β-subunit. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1998, 1403, 199–210. [Google Scholar] [CrossRef]
- Leroy, D.; Heriché, J.-K.; Filhol, O.; Chambaz, E.M.; Cochet, C. Binding of Polyamines to an Autonomous Domain of the Regulatory Subunit of Protein Kinase CK2 Induces a Conformational Change in the Holoenzyme. J. Biol. Chem. 1997, 272, 20820–20827. [Google Scholar] [CrossRef]
- Leroy, D.; Filhol, O.; Delcros, J.G.; Pares, S.; Chambaz, E.M.; Cochet, C. Chemical Features of the Protein Kinase CK2 Polyamine Binding Site. Biochemistry 1997, 36, 1242–1250. [Google Scholar] [CrossRef]
- Solyakov, L.; Cain, K.; Tracey, B.M.; Jukes, R.; Riley, A.M.; Potter, B.V.L.; Tobin, A.B. Regulation of Casein Kinase-2 (CK2) Activity by Inositol Phosphates. J. Biol. Chem. 2004, 279, 43403–43410. [Google Scholar] [CrossRef]
- Inoue, H.; Sugimoto, S.; Takeshita, Y.; Takeuchi, M.; Hatanaka, M.; Nagao, K.; Hayashi, T.; Kokubu, A.; Yanagida, M.; Kanoh, J. CK2 phospho-independent assembly of the Tel2-associated stress-signaling complexes in Schizosaccharomyces pombe. Genes Cells 2016, 22, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, F.A.; Zanchin, N.I.; Luz, J.S.; Oliveira, C.C. Characterization of Saccharomyces cerevisiae Nop17p, a Novel Nop58p-Interacting Protein that is Involved in Pre-rRNA Processing. J. Mol. Biol. 2005, 346, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Horejsi, Z.L.; Stach, L.; Flower, T.G.; Joshi, D.; Flynn, H.; Skehel, J.M.; O’Reilly, N.J.; Ogrodowicz, R.W.; Smerdon, S.J.; Boulton, S.J. Phosphorylation-Dependent PIH1D1 Interactions Define Substrate Specificity of the R2TP Cochaperone Complex. Cell Rep. 2014, 7, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Morgan, M.; Phelps, S.E.; Roe, S.M.; Parry-Morris, S.; Downs, J.A.; Polier, S.; Pearl, L.H.; Prodromou, C. Structural Basis for Phosphorylation-Dependent Recruitment of Tel2 to Hsp90 by Pih1. Structure 2014, 22, 805–818. [Google Scholar] [CrossRef]
- Zhao, R.; Davey, M.; Hsu, Y.-C.; Kaplanek, P.; Tong, A.; Parsons, A.B.; Krogan, N.; Cagney, G.; Mai, D.; Greenblatt, J.; et al. Navigating the Chaperone Network: An Integrative Map of Physical and Genetic Interactions Mediated by the Hsp90 Chaperone. Cell 2005, 120, 715–727. [Google Scholar] [CrossRef]
- Nano, N.; Houry, W.A. Chaperone-like activity of the AAA+ proteins Rvb1 and Rvb2 in the assembly of various complexes. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110399. [Google Scholar] [CrossRef]
- Yamada, K.; Kunishima, N.; Mayanagi, K.; Ohnishi, T.; Nishino, T.; Iwasaki, H.; Shinagawa, H.; Morikawa, K. Crystal structure of the Holliday junction migration motor protein RuvB from Thermus thermophilus HB8. Proc. Natl. Acad. Sci. USA 2001, 98, 1442–1447. [Google Scholar] [CrossRef]
- Putnam, C.D.; Clancy, S.B.; Tsuruta, H.; Gonzalez, S.; Wetmur, J.G.; Tainer, J.A. Structure and mechanism of the RuvB holliday junction branch migration motor. J. Mol. Biol. 2001, 311, 297–310. [Google Scholar] [CrossRef]
- Gribun, A.; Cheung, K.L.; Huen, J.; Ortega, J.; Houry, W.A. Yeast Rvb1 and Rvb2 are ATP-Dependent DNA Helicases that Form a Heterohexameric Complex. J. Mol. Biol. 2008, 376, 1320–1333. [Google Scholar] [CrossRef]
- Makino, Y.; Kanemaki, M.; Kurokawa, Y.; Koji, T.; Tamura, T.-A. A Rat RuvB-like Protein, TIP49a, Is a Germ Cell-enriched Novel DNA Helicase. J. Biol. Chem. 1999, 274, 15329–15335. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Kanemaki, M.; Making, Y.; Tamura, T.-A. A Notable Example of an Evolutionary Conserved Gene: Studies on a Putative DNA Helicase TIP49. DNA Seq. 1999, 10, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kanemaki, M.; Kurokawa, Y.; Matsu-Ura, T.; Makino, Y.; Masani, M.Y.A.; Okazaki, K.-I.; Morishita, T.; Tamura, T.-A. TIP49b, a New RuvB-like DNA Helicase, Is Included in a Complex Together with Another RuvB-like DNA Helicase, TIP49a. J. Biol. Chem. 1999, 274, 22437–22444. [Google Scholar] [CrossRef] [PubMed]
- Ewens, C.A.; Su, M.; Zhao, L.; Nano, N.; Houry, W.A.; Southworth, D.R. Architecture and Nucleotide-Dependent Conformational Changes of the Rvb1-Rvb2 AAA+ Complex Revealed by Cryoelectron Microscopy. Structure 2016, 24, 657–666. [Google Scholar] [CrossRef]
- Gorynia, S.; Bandeiras, T.M.; Pinho, F.G.; McVey, C.E.; Vonrhein, C.; Round, A.; Svergun, D.I.; Donner, P.; Matias, P.M.; Carrondo, M.A. Structural and functional insights into a dodecameric molecular machine—The RuvBL1/RuvBL2 complex. J. Struct. Biol. 2011, 176, 279–291. [Google Scholar] [CrossRef]
- Lakomek, K.; Stoehr, G.; Tosi, A.; Schmailzl, M.; Hopfner, K.-P. Structural Basis for Dodecameric Assembly States and Conformational Plasticity of the Full-Length AAA+ ATPases Rvb1·Rvb2. Structure 2015, 23, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Torreira, E.; Jha, S.; López-Blanco, J.R.; Arias-Palomo, E.; Chacón, P.; Cañas, C.; Ayora, S.; Dutta, A.; Llorca, O. Architecture of the Pontin/Reptin Complex, Essential in the Assembly of Several Macromolecular Complexes. Structure 2008, 16, 1511–1520. [Google Scholar] [CrossRef]
- Rivera-Calzada, A.; Pal, M.; Muñoz-Hernández, H.; Luque-Ortega, J.R.; Gil-Carton, D.; Degliesposti, G.; Skehel, J.M.; Prodromou, C.; Pearl, L.H.; Llorca, O. The Structure of the R2TP Complex Defines a Platform for Recruiting Diverse Client Proteins to the HSP90 Molecular Chaperone System. Structure 2017, 25, 1145–1152.e4. [Google Scholar] [CrossRef]
- Matias, P.M.; Gorynia, S.; Donner, P.; Carrondo, M.A. Crystal Structure of the Human AAA+ Protein RuvBL1. J. Biol. Chem. 2006, 281, 38918–38929. [Google Scholar] [CrossRef]
- Tian, S.; Yu, G.; He, H.; Zhao, Y.; Liu, P.; Marshall, A.G.; Demeler, B.; Stagg, S.M.; Li, H. Pih1p-Tah1p Puts a Lid on Hexameric AAA+ ATPases Rvb1/2p. Structure 2017, 25, 1519–1529.e4. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.Y.; Huen, J.; Houry, W.A.; Ortega, J. Comparison of the multiple oligomeric structures observed for the Rvb1 and Rvb2 proteins. Biochem. Cell Biol. 2010, 88, 77–88. [Google Scholar] [CrossRef]
- Martino, F.; Pal, M.; Muñoz-Hernández, H.; Rodríguez, C.F.; Núñez-Ramírez, R.; Gil-Carton, D.; Degliesposti, G.; Skehel, J.M.; Roe, S.M.; Prodromou, C.; et al. RPAP3 provides a flexible scaffold for coupling HSP90 to the human R2TP co-chaperone complex. Nat. Commun. 2018, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.A.; Teichmann, S.A. Structure, Dynamics, Assembly, and Evolution of Protein Complexes. Annu. Rev. Biochem. 2015, 84, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.A.; Rees, H.A.; Ahnert, S.E.; Teichmann, S.A. Structural and evolutionary versatility in protein complexes with uneven stoichiometry. Nat. Commun. 2015, 6, 6394. [Google Scholar] [CrossRef] [PubMed]
- Natan, E.; Wells, J.N.; Teichmann, S.A.; Marsh, J.A. Regulation, evolution and consequences of cotranslational protein complex assembly. Curr. Opin. Struct. Biol. 2017, 42, 90–97. [Google Scholar] [CrossRef]
- Panasenko, O.O.; Somasekharan, S.P.; Villanyi, Z.; Zagatti, M.; Bezrukov, F.; Rashpa, R.; Cornut, J.; Iqbal, J.; Longis, M.; Carl, S.H.; et al. Co-translational assembly of proteasome subunits in NOT1-containing assemblysomes. Nat. Struct. Mol. Biol. 2019, 26, 110–120. [Google Scholar] [CrossRef]
- Genereaux, J.; Kvas, S.; Dobransky, D.; Karagiannis, J.; Gloor, G.; Brandl, C.J. Genetic Evidence Links the ASTRA Protein Chaperone Component Tti2 to the SAGA Transcription Factor Tra1. Genetics 2012, 191, 765–780. [Google Scholar] [CrossRef]
- Elías-Villalobos, A.; Toullec, D.; Faux, C.; Séveno, M.; Helmlinger, D. Chaperone-mediated ordered assembly of the SAGA and NuA4 transcription co-activator complexes in yeast. Nat. Commun. 2019, 10, 5237. [Google Scholar] [CrossRef]
- DaSilva, L.F.; Pillon, S.; Genereaux, J.; Davey, M.J.; Gloor, G.B.; Karagiannis, J.; Brandl, C.J. The C-terminal Residues of Saccharomyces cerevisiae Mec1 Are Required for Its Localization, Stability, and Function. G3 Genes Genomes Genet. 2013, 3, 1661–1674. [Google Scholar] [CrossRef]
- Laptenko, O.; Prives, C. p53: Master of life, death, and the epigenome. Genes Dev. 2017, 31, 955–956. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA Damage Sensing by the ATM and ATR Kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Morrison, B.H.; Bauer, J.A.; Kalvakolanu, D.V.; Lindner, D.J. Inositol Hexakisphosphate Kinase 2 Mediates Growth Suppressive and Apoptotic Effects of Interferon-β in Ovarian Carcinoma Cells. J. Biol. Chem. 2001, 276, 24965–24970. [Google Scholar] [CrossRef]
- Nagata, E.; Luo, H.R.; Saiardi, A.; Bae, B.-I.; Suzuki, N.; Snyder, S.H. Inositol Hexakisphosphate Kinase-2, a Physiologic Mediator of Cell Death. J. Biol. Chem. 2005, 280, 1634–1640. [Google Scholar] [CrossRef]
- von Morgen, P.; Burdova, K.; Flower, T.; O’Reilly, N.; Boulton, S.J.; Smerdon, S.; Macurek, L.; Hořejší, Z. MRE11 stability is regulated by CK2-dependent interaction with R2TP complex. Oncogene 2017, 36, 4943–4950. [Google Scholar] [CrossRef]
- Collis, S.; Ciccia, A.; Deans, A.; Horejsi, Z.L.; Martin, J.S.; Maslen, S.L.; Skehel, J.M.; Elledge, S.J.; West, S.; Boulton, S.J. FANCM and FAAP24 Function in ATR-Mediated Checkpoint Signaling Independently of the Fanconi Anemia Core Complex. Mol. Cell 2008, 32, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, R.D.; Staples, C.J.; Patil, A.A.; Myers, K.N.; Maslen, S.; Skehel, J.M.; Boulton, S.J.; Collis, S.J. The leukemia-associated Rho guanine nucleotide exchange factor LARG is required for efficient replication stress signaling. Cell Cycle 2014, 13, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, C.; Duga, V.; Pantaleoni, C.; Esposito, S.; Moroni, I.; Pinelli, M.; Castello, R.; Nigro, V.; Chiapparini, L.; D’Arrigo, S.; et al. Milder presentation of TELO2-related syndrome in two sisters homozygous for the p.Arg609His pathogenic variant. Eur. J. Med. Genet. 2020, 64, 104116. [Google Scholar] [CrossRef]
- Moosa, S.; Altmüller, J.; Lyngbye, T.; Christensen, R.; Li, Y.; Nürnberg, P.; Yigit, G.; Vogel, I.; Wollnik, B. Novel compound heterozygous mutations in TELO2 in a patient with severe expression of You-Hoover-Fong syndrome. Mol. Genet. Genom. Med. 2017, 5, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Del-Prado-Sánchez, C.; Armstrong-Moron, J.; Veiga, C.; Grixolli-Mazzon, S.; García-Cazorla, À.; Juliá-Palacios, N.; Morales-Ballús, M. Cataract in You-Hoover-Fong syndrome: TELO2 deficiency. Ophthalmic Genet. 2020, 41, 656–658. [Google Scholar] [CrossRef]
- Langouët, M.; Saadi, A.; Rieunier, G.; Moutton, S.; Siquier-Pernet, K.; Fernet, M.; Nitschke, P.; Munnich, A.; Stern, M.-H.; Chaouch, M.; et al. Mutation in TTI2 Reveals a Role for Triple T Complex in Human Brain Development. Hum. Mutat. 2013, 34, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Najmabadi, H.; Hu, H.; Garshasbi, M.; Zemojtel, T.; Abedini, S.S.; Chen, W.; Hosseini, M.; Behjati, F.; Haas, S.; Jamali, P.; et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 2011, 478, 57–63. [Google Scholar] [CrossRef]
- Karaca, E.; Harel, T.; Pehlivan, D.; Jhangiani, S.N.; Gambin, T.; Akdemir, Z.C.; Gonzaga-Jauregui, C.; Erdin, S.; Bayram, Y.; Campbell, I.M.; et al. Genes that Affect Brain Structure and Function Identified by Rare Variant Analyses of Mendelian Neurologic Disease. Neuron 2015, 88, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Besterman, A.D.; Althoff, T.; Elfferich, P.; Gutierrez-Mejia, I.; Sadik, J.; Bernstein, J.A.; van Ierland, Y.; Kattentidt-Mouravieva, A.A.; Nellist, M.; Abramson, J.; et al. Functional and structural analyses of novel Smith-Kingsmore Syndrome-Associated MTOR variants reveal potential new mechanisms and predictors of pathogenicity. PLoS Genet. 2021, 17, e1009651. [Google Scholar] [CrossRef] [PubMed]
- Baynam, G.; Overkov, A.; Davis, M.; Mina, K.; Schofield, L.; Allcock, R.; Laing, N.; Cook, M.; Dawkins, H.; Goldblatt, J. A germline MTOR mutation in Aboriginal Australian siblings with intellectual disability, dysmorphism, macrocephaly, and small thoraces. Am. J. Med. Genet. Part A 2015, 167, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Gala, M.K.; Mizukami, Y.; Le, L.P.; Moriichi, K.; Austin, T.; Yamamoto, M.; Lauwers, G.Y.; Bardeesy, N.; Chung, D.C. Germline Mutations in Oncogene-Induced Senescence Pathways Are Associated With Multiple Sessile Serrated Adenomas. Gastroenterology 2014, 146, 520–529.e6. [Google Scholar] [CrossRef]
- Morais-Rodrigues, F.; Silύerio-Machado, R.; Kato, R.B.; Rodrigues, D.L.N.; Valdez-Baez, J.; Fonseca, V.; San, E.J.; Gomes, L.G.R.; dos Santos, R.G.; Viana, M.V.C.; et al. Analysis of the microarray gene expression for breast cancer progression after the application modified logistic regression. Gene 2019, 726, 144168. [Google Scholar] [CrossRef]
- Xu, P.; Du, G.; Guan, H.; Xiao, W.; Sun, L.; Yang, H. A role of TTI1 in the colorectal cancer by promoting proliferation. Transl. Cancer Res. 2021, 10, 1378–1388. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Wu, Z.; Liao, Z.; Wang, D.; Chen, S.; Lu, F.; Wu, Y.; Zhu, S. TTI1 promotes non-small-cell lung cancer progression by regulating the mTOR signaling pathway. Cancer Sci. 2022, 114, 855–869. [Google Scholar] [CrossRef]
- Li, Z.-N.; Luo, Y. HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review). Oncol. Rep. 2022, 49, 6. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Wang, D.; Chen, Y.; Zhang, N. Recent Advances in the Discovery of CK2 Allosteric Inhibitors: From Traditional Screening to Structure-Based Design. Molecules 2020, 25, 870. [Google Scholar] [CrossRef]
- Salvi, M.; Borgo, C.; Pinna, L.A.; Ruzzene, M. Targeting CK2 in cancer: A valuable strategy or a waste of time? Cell Death Discov. 2021, 7, 325. [Google Scholar] [CrossRef]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 742–755. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, F.; Li, S.; Cheng, K.-W.; Zhu, Y.; Huo, R.; Abdukirim, E.; Kang, G.; Chou, T.-F. Discovery of small-molecule inhibitors of RUVBL1/2 ATPase. Bioorg. Med. Chem. 2022, 62, 116726. [Google Scholar] [CrossRef]
- Elkaim, J.; Castroviejo, M.; Bennani, D.; Taouji, S.; Allain, N.; Laguerre, M.; Rosenbaum, J.; Dessolin, J.; Lestienne, P. First identification of small-molecule inhibitors of Pontin by combining virtual screening and enzymatic assay. Biochem. J. 2012, 443, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin—Old Drug, New Tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2020, 163, 105207. [Google Scholar] [CrossRef]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Wagstaff, K.; Sivakumaran, H.; Heaton, S.; Harrich, D.; Jans, D. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Juarez, M.; Schcolnik-Cabrera, A.; Dominguez-Gomez, G.; Chavez-Blanco, A.; Diaz-Chavez, J.; Duenas-Gonzalez, A. Antitumor effects of ivermectin at clinically feasible concentrations support its clinical development as a repositioned cancer drug. Cancer Chemother. Pharmacol. 2020, 85, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Juarez, M.; Schcolnik-Cabrera, A.; Duenas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331. [Google Scholar]
- Didier, A.; Loor, F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anti-Cancer Drugs 1996, 7, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-B.; Lin, Y.-L.; Thome, K.C.; Pian, P.; Schlegel, B.P.; Weremowicz, S.; Parvin, J.D.; Dutta, A. An Eukaryotic RuvB-like Protein (RUVBL1) Essential for Growth. J. Biol. Chem. 1998, 273, 27786–27793. [Google Scholar] [CrossRef] [PubMed]
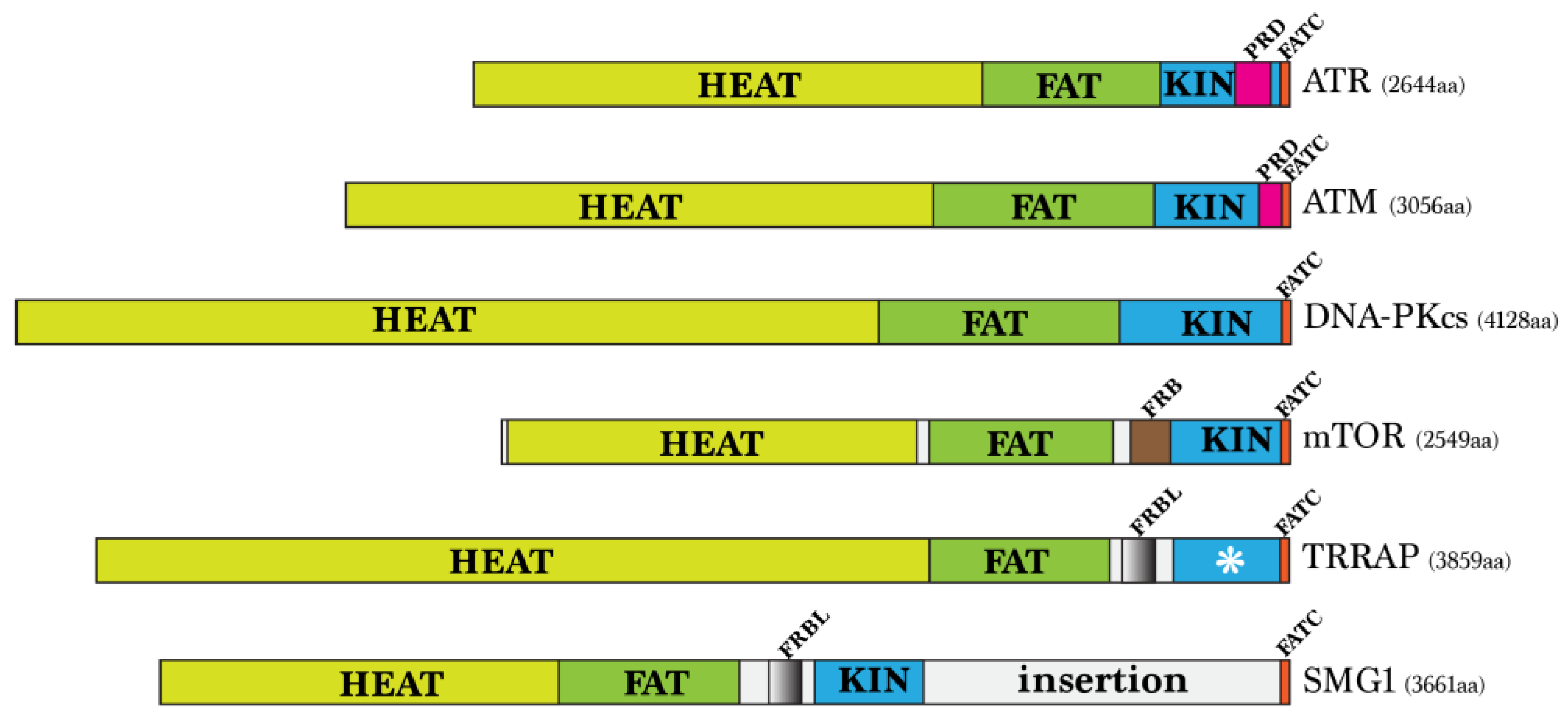
| Complex | Human | Budding Yeast | Fission Yeast | C. elegans |
|---|---|---|---|---|
| TELO2 | Tel2 | Tel2 | CLK-2/RAD-5 | |
| TTT | TTI1 | Tti1 | Tti1 | R10H10.7 |
| TTI2 | Tti2 | Tti2 | C28H8.3? | |
| Hsp90 | HSP90α/HSP90β | Hsp82/Hsc82 | Hsp90 | HSP-90 |
| R2 | RUVBL1 | Rvb1 | Rvb1 | RUVB-1 |
| RUVBL2 | Rvb2 | Rvb2 | RUVB-2 | |
| TP | RPAP3 | Tah1 | ? | RPAP-3 |
| PIH1D1 | Pih1 | ? | ? | |
| Asa1 | GNB1L | Asa1 | Asa1 | ? |
| ATR | Mec1 | Rad3 | ATL-1 | |
| ATM | Tel1 | Tel1 | ATM-1 | |
| PIKKs | DNA-PKcs | ? | ? | ? |
| mTOR | Tor1, Tor2 | Tor1, Tor2 | LET-363 | |
| SMG1 | ? | ? | SMG-1 | |
| TRRAP | Tra1, Tra2 | Tra1, Tra2 | TRR-1 | |
| ATRIP | ATRIP | Ddc2 | Rad26 | ? |
| MRE11 | Mre11 | Mre11 | MRE-11 | |
| MRN | RAD50 | Rad50 | Rad50 | RAD-50 |
| NBS1 | Xrs2 | Nbs1 | NBS-1 | |
| KU70/80 | KU70/80 | Ku70/Ku80 | Pku70/Pku80 | CKU-70/CKU-80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhadra, S.; Xu, Y.-j. TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy. Int. J. Mol. Sci. 2023, 24, 8268. https://doi.org/10.3390/ijms24098268
Bhadra S, Xu Y-j. TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy. International Journal of Molecular Sciences. 2023; 24(9):8268. https://doi.org/10.3390/ijms24098268
Chicago/Turabian StyleBhadra, Sankhadip, and Yong-jie Xu. 2023. "TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy" International Journal of Molecular Sciences 24, no. 9: 8268. https://doi.org/10.3390/ijms24098268
APA StyleBhadra, S., & Xu, Y.-j. (2023). TTT (Tel2-Tti1-Tti2) Complex, the Co-Chaperone of PIKKs and a Potential Target for Cancer Chemotherapy. International Journal of Molecular Sciences, 24(9), 8268. https://doi.org/10.3390/ijms24098268




