MicroRNA Dysregulation in Early Breast Cancer Diagnosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
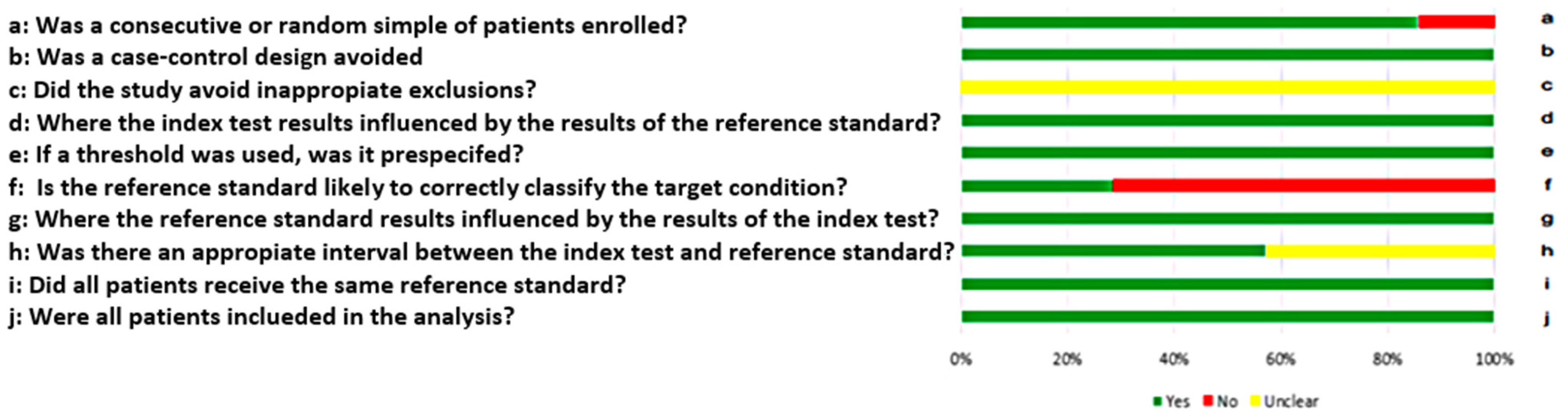
2.3. Quality Assessment
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Included Studies
3.2. RNA Quantification
3.3. Data Normalization
3.4. Quality of Included Studies
3.5. Differentially Expressed miRNAs
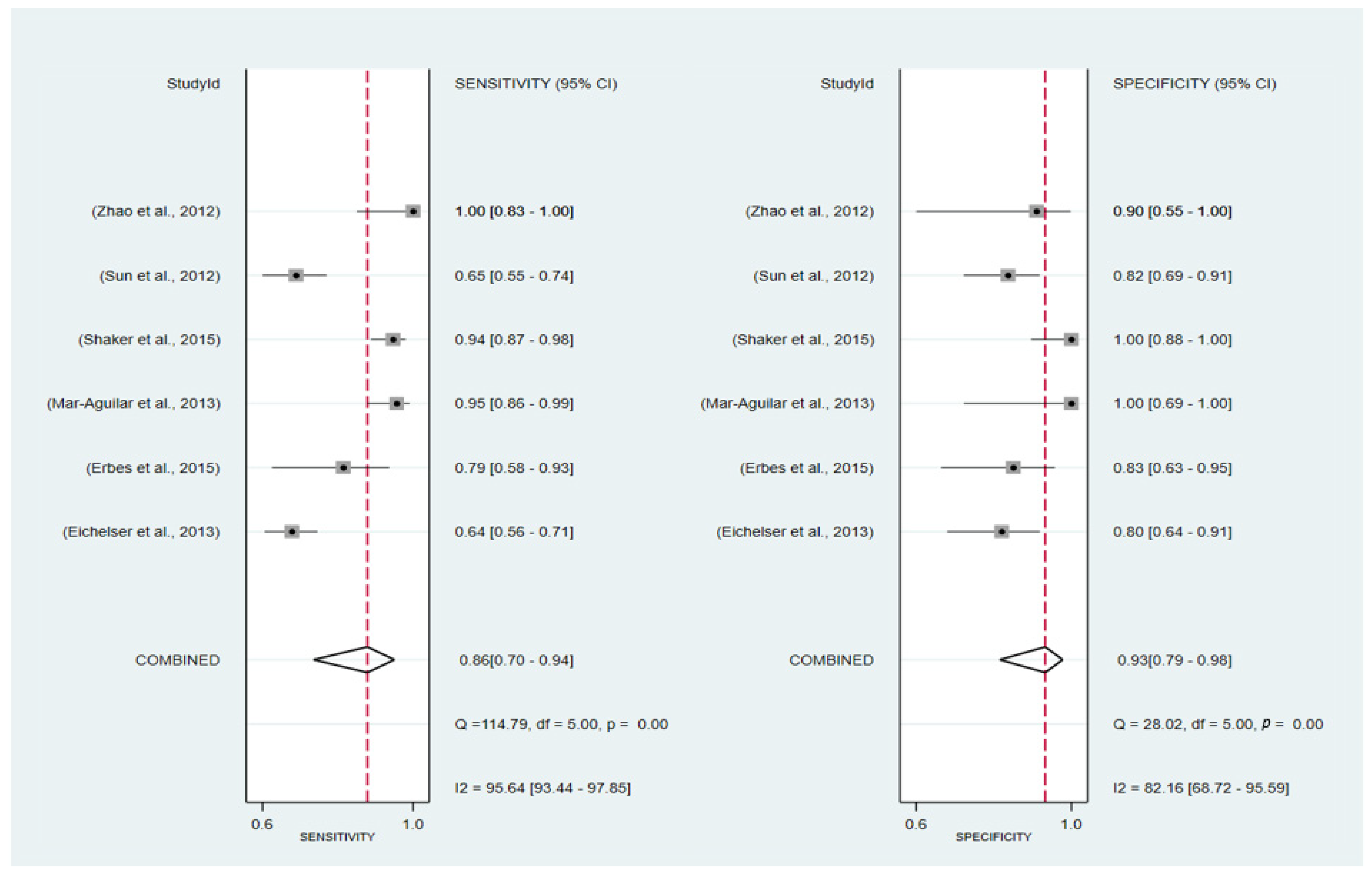
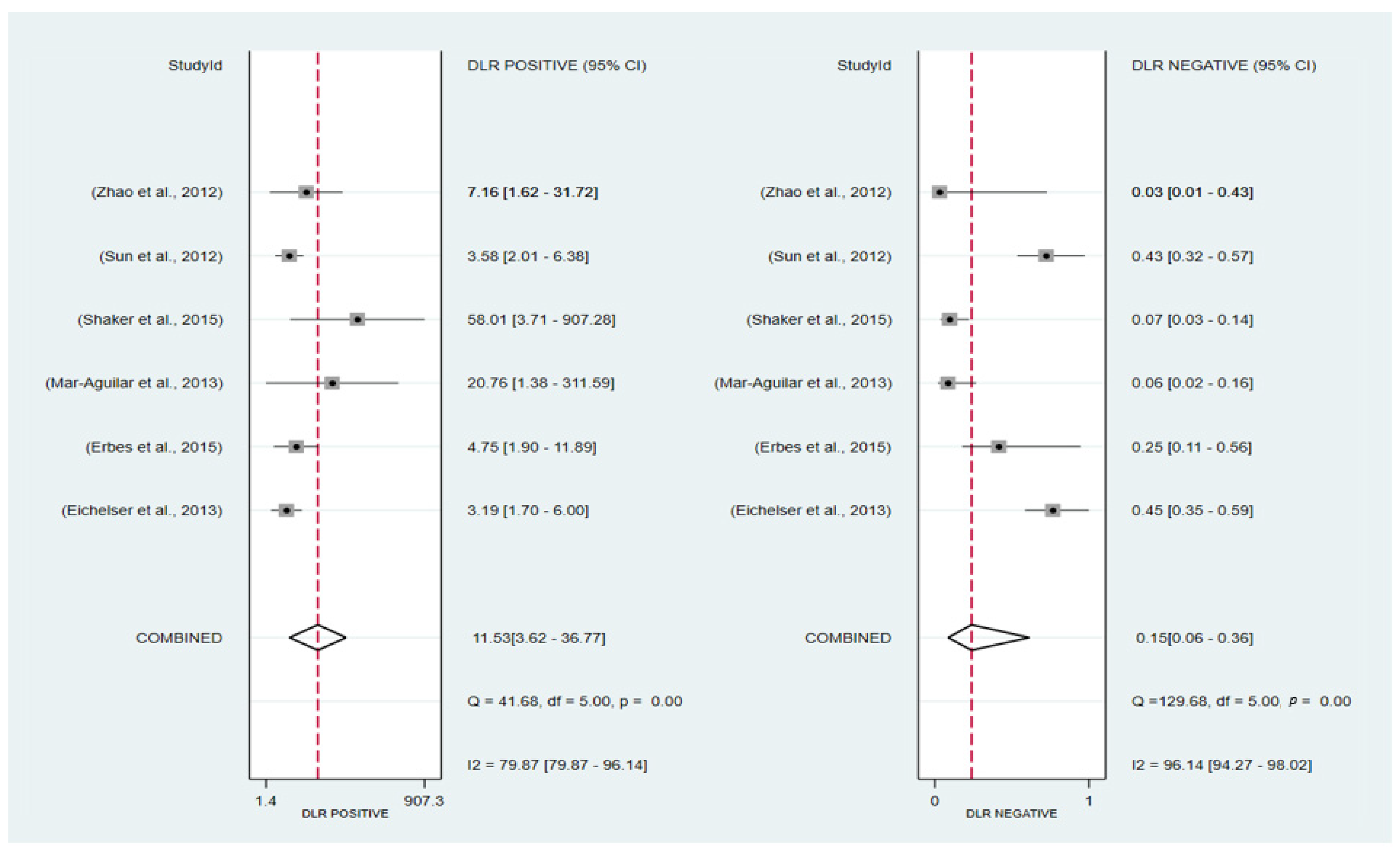
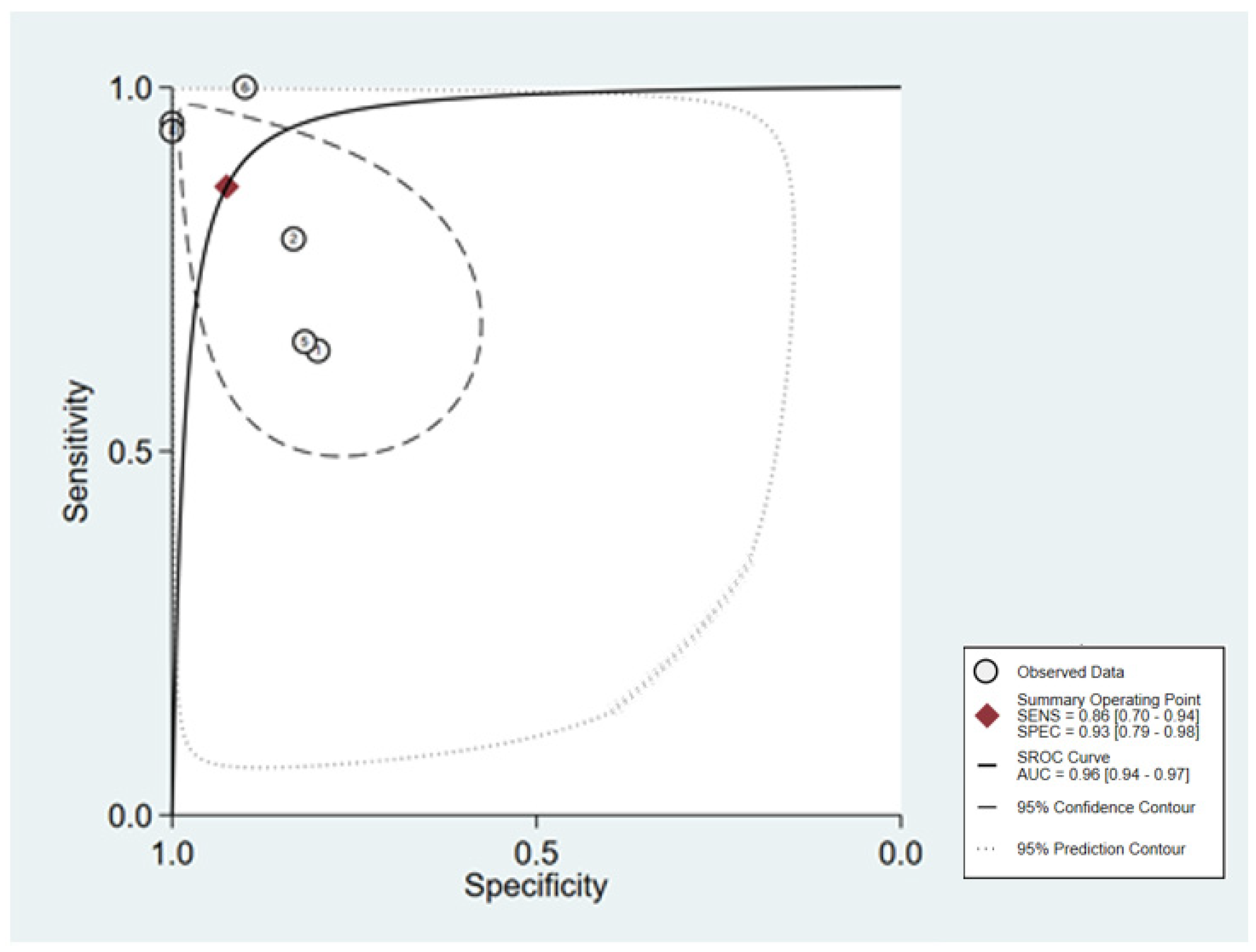
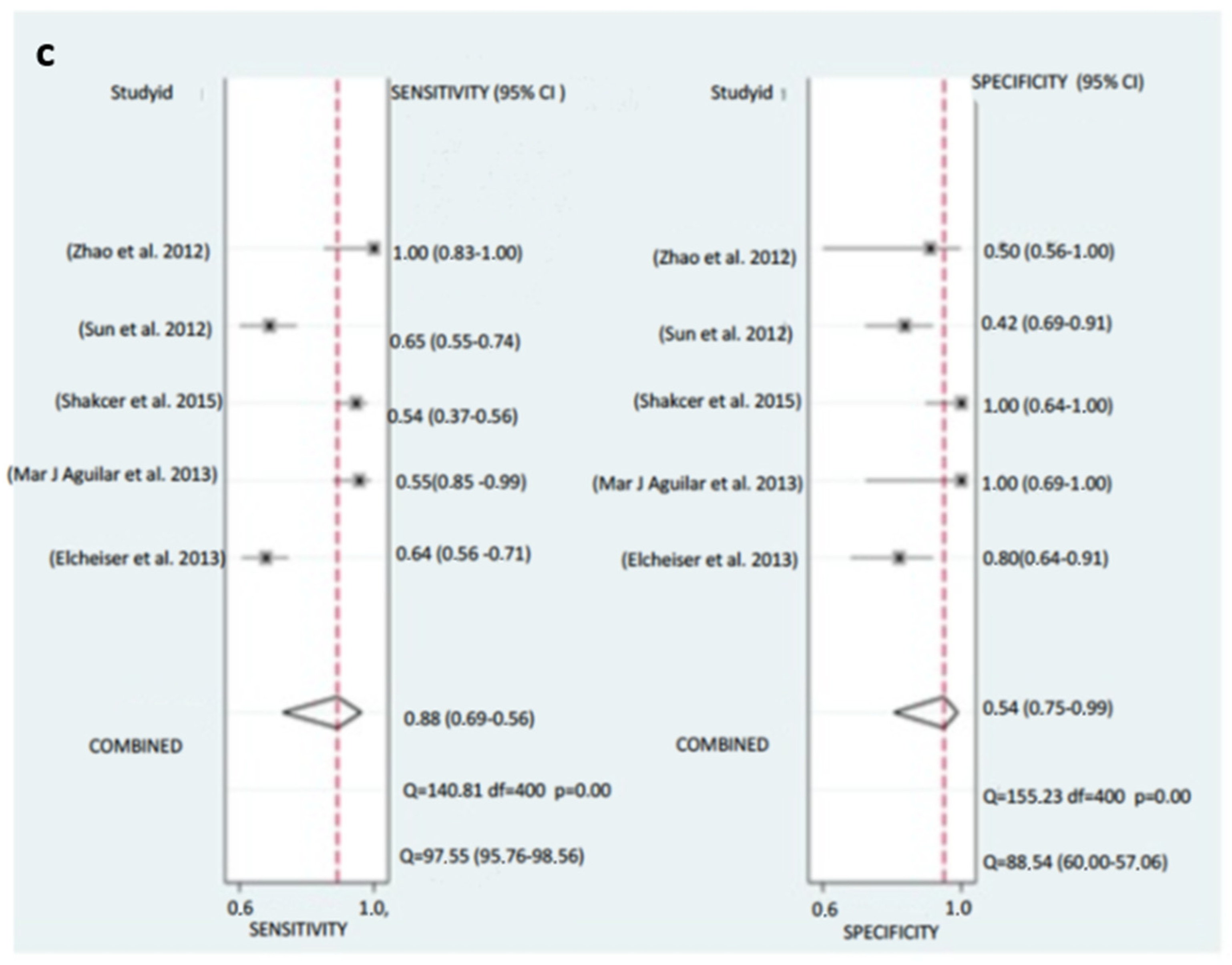
3.6. Diagnosis Test Accuracy Meta-Analysis
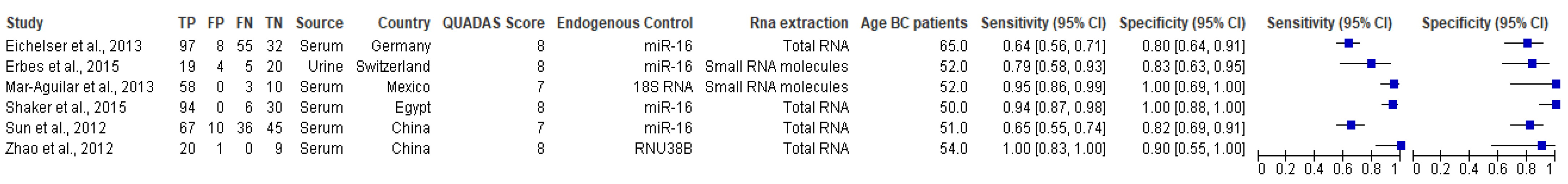
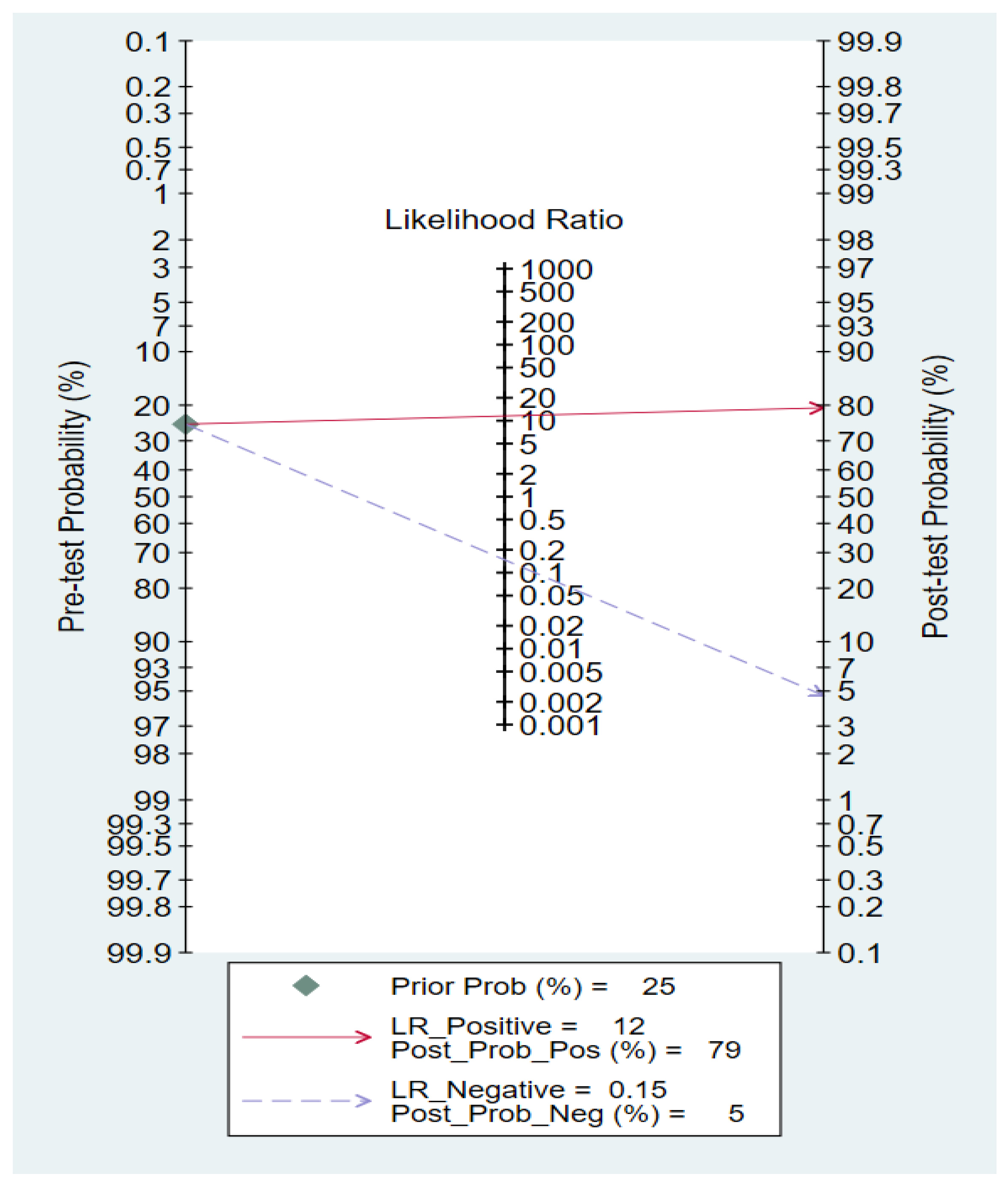
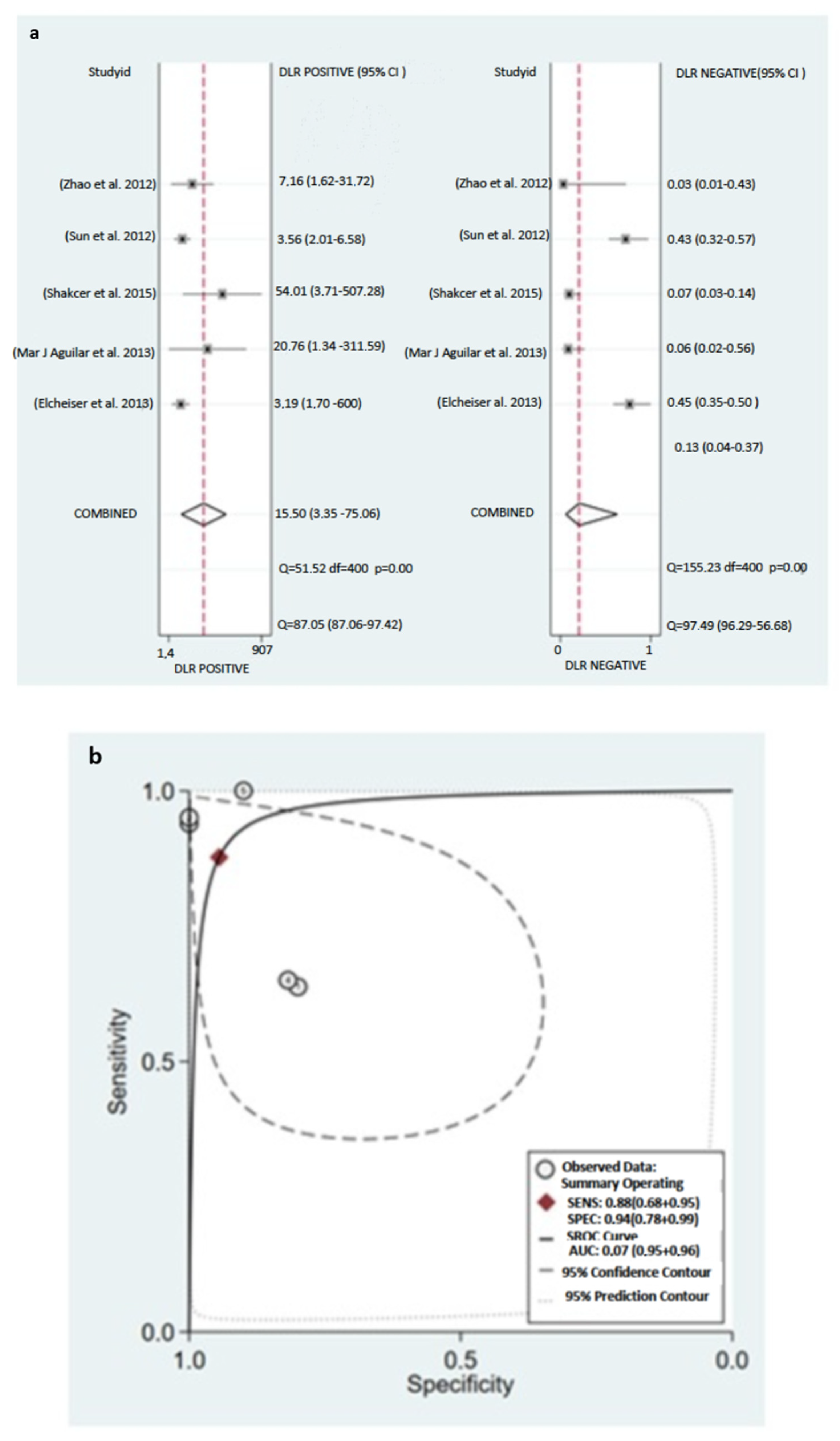
3.6.1. Pooled Diagnostic Value of miR-155 in Breast Cancer
3.6.2. Subgroup Analysis
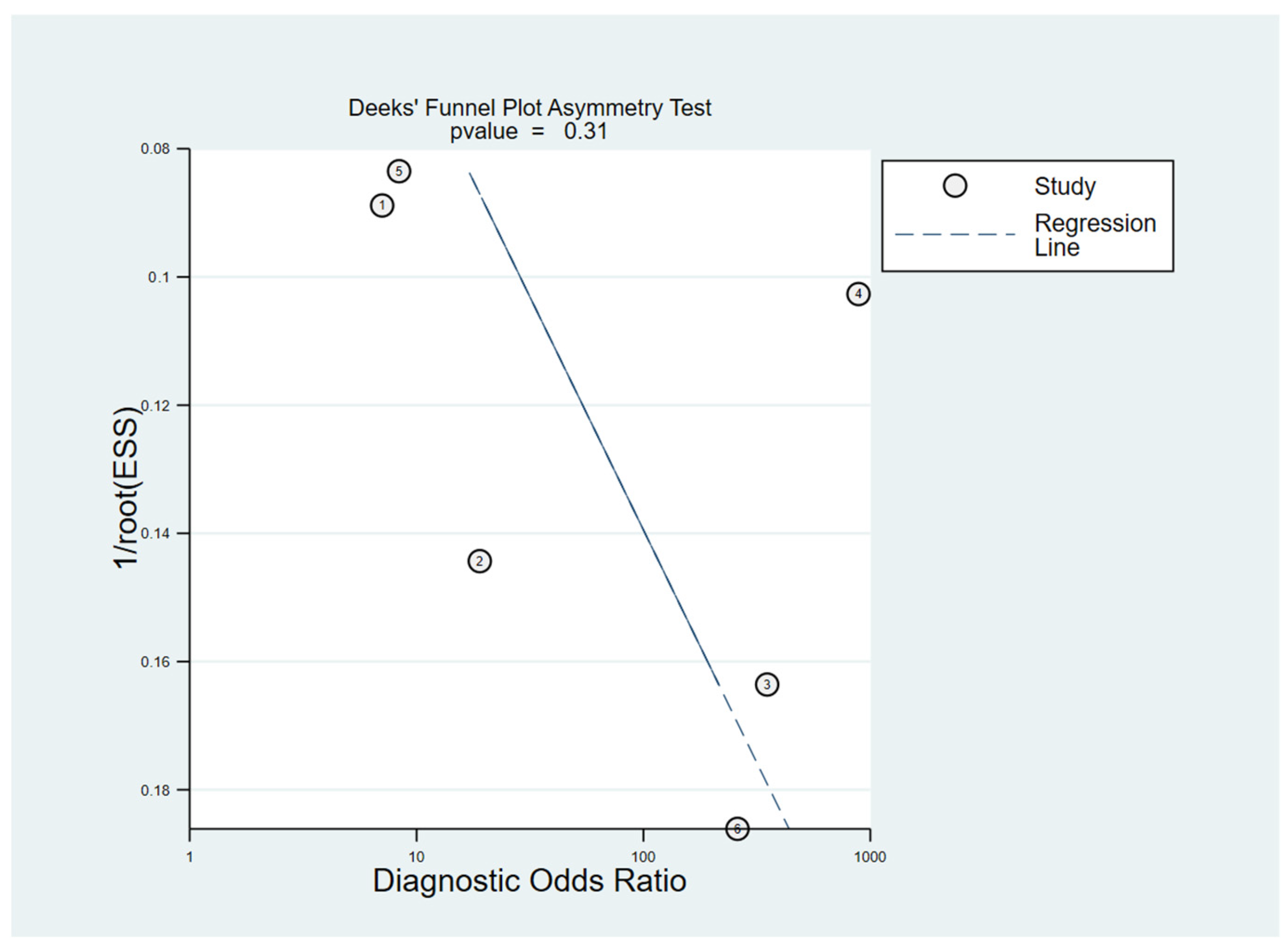
3.6.3. Publication Bias
4. Discussion
4.1. Size and Angiogenesis
4.2. Angiogenesis and Tumor Spreading
4.3. Heterogeneity: A Major Challenge to Overcome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Franco, M.M.; Leon-Rodriguez, E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer Basic Clin. Res. 2018, 12, 1178223417752677. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, N.; Dapp, R.; Zapf, M.; Gemmeke, H.; Ruiter, N.V.; van Dongen, K.W.A. Comparing different ultrasound imaging methods for breast cancer detection. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; El-Shenawee, M. Review of Electromagnetic Techniques for Breast Cancer Detection. IEEE Rev. Biomed. Eng. 2011, 4, 103–118. [Google Scholar] [CrossRef]
- Roganovic, D.; Djilas, D.; Vujnovic, S.; Pavic, D.; Stojanov, D. Breast MRI, digital mammography and breast tomosynthesis: Comparison of three methods for early detection of breast cancer. Bosn. J. Basic Med. Sci. 2015, 15, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mohd Norsuddin, N.; Maslan, F.A. Association between breast density, lesions characteristic and diagnosis error in mammography. In Proceedings of the 15th International Workshop on Breast Imaging (IWBI2020), Leuven, Belgium, 25–27 May 2020; Volume 82. [Google Scholar] [CrossRef]
- Johansson, A.L.V.; Andersson, T.M.L.; Hsieh, C.C.; Jirström, K.; Dickman, P.; Cnattingius, S.; Lambe, M. Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC). Breast Cancer Res. Treat. 2013, 139, 183–192. [Google Scholar] [CrossRef]
- Rojas, K.E.; Bilbro, N.; Manasseh, D.M.; Borgen, P.I. A Review of Pregnancy-Associated Breast Cancer: Diagnosis, Local and Systemic Treatment, and Prognosis. J. Women’s Health 2019, 28, 778–784. [Google Scholar] [CrossRef]
- Shao, C.; Yu, Z.; Xiao, J.; Liu, L.; Hong, F.; Zhang, Y.; Jia, H. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer 2020, 20, 746. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Mulrane, L.; McGee, S.F.; Gallagher, W.M.; O’Connor, D.P. miRNA dysregulation in breast cancer. Cancer Res. 2013, 73, 6554–6562. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Asaga, S.; Kuo, C.; Nguyen, T.; Terpenning, M.; Giuliano, A.E.; Hoon, D.S.B. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin. Chem. 2011, 57, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J. Cancer Res. Clin. Oncol. 2012, 138, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cano, I.; Constâncio, V.; Adam-Artigues, A.; Lameirinhas, A.; Simón, S.; Ortega, B.; Martínez, M.T.; Hernando, C.; Bermejo, B.; Lluch, A.; et al. Circulating mir-99a-5p expression in plasma: A potential biomarker for early diagnosis of breast cancer. Int. J. Mol. Sci. 2020, 21, 7427. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Lin, G.; Sun, S.; Li, X.; Qi, J.; Li, J. Serum MicroRNA-155 as a Potential Biomarker to Track Disease in Breast Cancer. PLoS ONE 2012, 7, e47003. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Wu, Q.; Gao, F.; Zhang, C.B.; Yang, X.W. Serum microRNA-155 as a potential biomarker for breast cancer screening. Chin. Sci. Bull. 2012, 57, 3466–3468. [Google Scholar] [CrossRef]
- Anwar, S.L.; Tanjung, D.S.; Fitria, M.S.; Kartika, A.I.; Sari, D.N.I.; Rakhmina, D.; Wardana, T.; Astuti, I.; Haryana, S.M.; Aryandono, T. Dynamic Changes of Circulating Mir-155 Expression and the Potential Application as a Non-Invasive Biomarker in Breast Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 491–497. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Hwang, D.; Kim, S.I.; Lee, H. Diagnostic value of circulating miR-202 in early-stage breast cancer in South Korea. Medicina 2020, 56, 340. [Google Scholar] [CrossRef]
- Han, S.; Li, P.; Wang, D.; Yan, H. Dysregulation of serum miR-1204 and its potential as a biomarker for the diagnosis and prognosis of breast cancer. Rev. Assoc. Med. Bras. 2020, 66, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Rack, B.; Müller, V.; Janni, W.; Pantel, K.; Schwarzenbach, H. Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 2010, 12, R90. [Google Scholar] [CrossRef]
- Mar-Aguilar, F.; Mendoza-Ramírez, J.A.; Malagón-Santiago, I.; Espino-Silva, P.K.; Santuario-Facio, S.K.; Ruiz-Flores, P.; Rodríguez-Padilla, C.; Reséndez-Pérez, D. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis. Markers 2013, 34, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, Z.; Guo, J.; Ding, X. Correlation and quantitation of microRNA aberrant expression in tissues and sera from patients with breast tumor. Gynecol. Oncol. 2010, 119, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.K.O.; Li, R.; Shin, V.Y.; Jin, H.C.; Leung, C.P.H.; Ma, E.S.K.; Pang, R.; Chua, D.; Chu, K.M.; Law, W.L.; et al. Circulating microRNAs as Specific Biomarkers for Breast Cancer Detection. PLoS ONE 2013, 8, e53141. [Google Scholar] [CrossRef]
- Kumar, S.; Keerthana, R.; Pazhanimuthu, A.; Perumal, P. Overexpression of circulating miRNA-21 and miRNA-146a in plasma samples of breast cancer patients. Indian J. Biochem. Biophys. 2013, 50, 210–214. [Google Scholar]
- Si, H.; Sun, X.; Chen, Y.; Cao, Y.; Chen, S.; Wang, H.; Hu, C. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 223–229. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Trigoso, Y.D.; Calloway, C.L.; Zhao, Y.D.; Lum, D.H.; Welm, A.L.; Zhao, Z.J.; Blick, K.E.; Dooley, W.C.; Ding, W.Q. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016, 18, 90. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Kelly, R.; Newell, J.; Kerin, M.J. Systemic miRNA-195 Differentiates Breast Cancer from Other Malignancies and Is a Potential Biomarker for Detecting Noninvasive and Early Stage Disease. Oncologist 2010, 15, 673–682. [Google Scholar] [CrossRef]
- Sun, E.H.; Zhou, Q.; Liu, K.S.; Wei, W.; Wang, C.M.; Liu, X.F.; Lu, C.; Ma, D.Y. Screening miRNAs related to different subtypes of breast cancer with miRNAs microarray. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2783–2788. [Google Scholar]
- Shimomura, A.; Shiino, S.; Kawauchi, J.; Takizawa, S.; Sakamoto, H.; Matsuzaki, J.; Ono, M.; Takeshita, F.; Niida, S.; Shimizu, C.; et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016, 107, 326–334. [Google Scholar] [CrossRef]
- Elshimy, R.A.A.; El-Mahdy, H.A.; Mansour, O.A.; Badr, M.M.A.; Ali, A.M. MiR-133a and MiR-155 as Potential Minimally Invasive Biomarkers in Breast Cancer. Cancer Biol. 2017, 7, 96–105. [Google Scholar] [CrossRef]
- Ando, W.; Kikuchi, K.; Uematsu, T.; Yokomori, H.; Takaki, T.; Sogabe, M.; Kohgo, Y.; Otori, K.; Ishikawa, S.; Okazaki, I. Novel breast cancer screening: Combined expression of miR-21 and MMP-1 in urinary exosomes detects 95% of breast cancer without metastasis. Sci. Rep. 2019, 9, 13595. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zou, X.; Xia, T.; Wang, T.; Liu, P.; Zhou, X.; Wang, S.; Zhu, W. A five-miRNA panel in plasma was identified for breast cancer diagnosis. Cancer Med. 2019, 8, 7006–7017. [Google Scholar] [CrossRef]
- Aksan, H.; Kundaktepe, B.P.; Sayili, U.; Velidedeoglu, M.; Simsek, G.; Koksal, S.; Gelisgen, R.; Yaylim, I.; Uzun, H. Circulating miR-155, let-7c, miR-21, and PTEN levels in differential diagnosis and prognosis of idiopathic granulomatous mastitis and breast cancer. BioFactors 2020, 46, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.; Maher, M.; Nassar, Y.; Morcos, G.; Gad, Z. Role of microRNAs -29b-2, −155, −197 and −205 as diagnostic biomarkers in serum of breast cancer females. Gene 2015, 560, 77–82. [Google Scholar] [CrossRef]
- Ashirbekov, Y.; Abaildayev, A.; Omarbayeva, N.; Botbayev, D.; Belkozhayev, A.; Askandirova, A.; Neupokoyeva, A.; Utegenova, G.; Sharipov, K.; Aitkhozhina, N. Combination of circulating miR-145-5p/miR-191-5p as biomarker for breast cancer detection. PeerJ 2020, 8, e10494. [Google Scholar] [CrossRef]
- Zaleski, M.; Kobilay, M.; Schroeder, L.; Debald, M.; Semaan, A.; Hettwer, K.; Uhlig, S.; Kuhn, W.; Hartmann, G.; Holdenrieder, S. Improved sensitivity for detection of breast cancer by combination of miR-34a and tumor markers CA 15-3 or CEA. Oncotarget 2018, 9, 22523–22536. [Google Scholar] [CrossRef]
- Li, X.; Zou, W.; Wang, Y.; Liao, Z.; Li, L.; Zhai, Y.; Zhang, L.; Gu, S.; Zhao, X. Plasma-based microRNA signatures in early diagnosis of breast cancer. Mol. Genet. Genomic Med. 2020, 8, e1092. [Google Scholar] [CrossRef]
- Hu, Z.; Dong, J.; Wang, L.E.; Ma, H.; Liu, J.; Zhao, Y.; Tang, J.; Chen, X.; Dai, J.; Wei, Q.; et al. Serum microRNA profiling and breast cancer risk: The use of miR-484/191 as endogenous controls. Carcinogenesis 2012, 33, 828–834. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, Y.S.; Kang, K.N.; Kim, K.H.; Park, Y.J.; Kim, C.W. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol. Clin. Oncol. 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kodahl, A.R.; Lyng, M.B.; Binder, H.; Cold, S.; Gravgaard, K.; Knoop, A.S.; Ditzel, H.J. Novel circulating microRNA signature as a potential non-invasive multi-marker test in ER-positive early-stage breast cancer: A case control study. Mol. Oncol. 2014, 8, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Metawae, E.; Abd-Alhamid, N.; Abd-Allatif, S.; Abd-Elghaffar, N.; El-Ghoroury, E.; Abd-Elrahman, A.; Ashmawy, I.; Aboafya, Z. Evaluation of microRNA-155 as a diagnostic serum-based biomarker in patients with breast cancer. Al-Azhar Assiut Med. J. 2018, 16, 81. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating micrornas as novel minimally invasive biomarkers for breast cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef]
- Hagrass, H.A.; Sharaf, S.; Pasha, H.F.; Tantawy, E.A.; Mohamed, R.H.; Kassem, R. Circulating microRNAs-a new horizon in molecular diagnosis of breast cancer. Genes Cancer 2015, 6, 281–287. [Google Scholar] [CrossRef]
- Eichelser, C.; Flesch-Janys, D.; Chang-Claude, J.; Pantel, K.; Schwarzenbach, H. Deregulated serum concentrations of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin. Chem. 2013, 59, 1489–1496. [Google Scholar] [CrossRef]
- Sochor, M.; Basova, P.; Pesta, M.; Dusilkova, N.; Bartos, J.; Burda, P.; Pospisil, V.; Stopka, T. Oncogenic MicroRNAs: MiR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer 2014, 14, 448. [Google Scholar] [CrossRef]
- Erbes, T.; Hirschfeld, M.; Rücker, G.; Jaeger, M.; Boas, J.; Iborra, S.; Mayer, S.; Gitsch, G.; Stickeler, E. Feasibility of urinary microRNA detection in breast cancer patients and its potential as an innovative non-invasive biomarker. BMC Cancer 2015, 15, 193. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Q.; Xu, J.; Guo, L.; Li, X. Clinical significance of serum miR-21 in breast cancer compared with CA153 and CEA. Chin. J. Cancer Res. 2013, 25, 743–748. [Google Scholar] [CrossRef]
- Wu, Q.; Lu, Z.; Li, H.; Lu, J.; Guo, L.; Ge, Q. Next-generation sequencing of microRNAs for breast cancer detection. J. Biomed. Biotechnol. 2011, 2011, 597145. [Google Scholar] [CrossRef]
- National Cancer Institute Female Breast Cancer—Cancer Stat Facts. SEER 17 2012–2018. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 30 January 2023).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mulrane, L.; Klinger, R.; McGee, S.F.; Gallagher, W.M.; O’Connor, D.P. microRNAs: A new class of breast cancer biomarkers. Expert Rev. Mol. Diagn. 2014, 14, 347–363. [Google Scholar] [CrossRef]
- Li, J.; Guan, X.; Fan, Z.; Ching, L.M.; Li, Y.; Wang, X.; Cao, W.M.; Liu, D.X. Non-invasive biomarkers for early detection of breast cancer. Cancers 2020, 12, 2767. [Google Scholar] [CrossRef] [PubMed]
- Higgs, G.; Slack, F. The multiple roles of microRNA-155 in oncogenesis. J. Clin. Bioinforma. 2013, 3, 17. [Google Scholar] [CrossRef]
- Kazerounian, S.; Lawler, J. Integration of pro- and anti-angiogenic signals by endothelial cells. J. Cell Commun. Signal. 2018, 12, 171–179. [Google Scholar] [CrossRef]
- Hussen, B.M.; Abdullah, S.T.; Rasul, M.F.; Salihi, A.; Ghafouri-Fard, S.; Hidayat, H.J.; Taheri, M. MicroRNAs: Important Players in Breast Cancer Angiogenesis and Therapeutic Targets. Front. Mol. Biosci. 2021, 8, 764025. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.P. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin. J. Cancer 2011, 30, 603–611. [Google Scholar] [CrossRef]
- Alhasan, L. MiR-126 Modulates Angiogenesis in Breast Cancer by Targeting VEGF-A -mRNA. Asian Pacific J. Cancer Prev. 2019, 20, 193–197. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Xie, H.; Zhou, Z.; Chen, H.; Hu, T.; Bai, Y.; Shen, Y.; Yuan, W.; Jing, Q.; et al. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol. Cell. Biochem. 2011, 351, 157–164. [Google Scholar] [CrossRef]
- Pan, S.; Zhao, X.; Shao, C.; Fu, B.; Huang, Y.; Zhang, N.; Dou, X.; Zhang, Z.; Qiu, Y.; Wang, R.; et al. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis. 2021, 12, 38. [Google Scholar] [CrossRef]
- Isanejad, A.; Alizadeh, A.M.; Amani Shalamzari, S.; Khodayari, H.; Khodayari, S.; Khori, V.; Khojastehnjad, N. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 2016, 151, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-H.; Zhou, H.-C.; Zeng, C.; Yang, J.; Liu, Y.; Huang, X.; Zhang, J.-P.; Guan, X.-Y.; Zhuang, S.-M. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology 2011, 54, 1729–1740. [Google Scholar] [CrossRef]
- Kong, X.; Gao, R.; Wang, Z.; Wang, X.; Fang, Y.; Gao, J.; Reiter, R.J.; Wang, J. Melatonin: A Potential Therapeutic Option for Breast Cancer. Trends Endocrinol. Metab. 2020, 31, 859–871. [Google Scholar] [CrossRef]
- Bakr, N.M.; Mahmoud, M.S.; Nabil, R.; Boushnak, H.; Swellam, M. Impact of circulating miRNA-373 on breast cancer diagnosis through targeting VEGF and cyclin D1 genes. J. Genet. Eng. Biotechnol. 2021, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutat. Res. Mutat. Res. 2011, 728, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Agarwal, P.; Bhowmick, N.A. MicroRNA applications for prostate, ovarian and breast cancer in the era of precision medicine. Endocr. Relat. Cancer 2017, 24, R157–R172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, S.; Mao, L.; Zhang, H.; Sun, D.; Zhang, J.; Li, J.; Tang, J. Crosstalk between TGF-β signaling and miRNAs in breast cancer metastasis. Tumor Biol. 2016, 37, 10011–10019. [Google Scholar] [CrossRef]
- Theys, J.; Jutten, B.; Habets, R.; Paesmans, K.; Groot, A.J.; Lambin, P.; Wouters, B.G.; Lammering, G.; Vooijs, M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother. Oncol. 2011, 99, 392–397. [Google Scholar] [CrossRef]
- Han, M.; Wang, F.; Gu, Y.; Pei, X.; Guo, G.; Yu, C.; Li, L.; Zhu, M.; Xiong, Y.; Wang, Y. MicroRNA-21 induces breast cancer cell invasion and migration by suppressing smad7 via EGF and TGF-β pathways. Oncol. Rep. 2016, 35, 73–80. [Google Scholar] [CrossRef]
- Han, X.; Yan, S.; Weijie, Z.; Feng, W.; Liuxing, W.; Mengquan, L.; Qingxia, F. Critical role of miR-10b in transforming growth factor-β1-induced epithelial–mesenchymal transition in breast cancer. Cancer Gene Ther. 2014, 21, 60–67. [Google Scholar] [CrossRef]
- Kong, W.; Yang, H.; He, L.; Zhao, J.; Coppola, D.; Dalton, W.S.; Cheng, J.Q. MicroRNA-155 Is Regulated by the Transforming Growth Factor β/Smad Pathway and Contributes to Epithelial Cell Plasticity by Targeting RhoA. Mol. Cell. Biol. 2008, 28, 6773–6784. [Google Scholar] [CrossRef]
- Nagpal, N.; Ahmad, H.M.; Chameettachal, S.; Sundar, D.; Ghosh, S.; Kulshreshtha, R. HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFβ-signaling in hypoxic microenvironment. Sci. Rep. 2015, 5, 9650. [Google Scholar] [CrossRef]
- Burk, U.; Schubert, J.; Wellner, U.; Schmalhofer, O.; Vincan, E.; Spaderna, S.; Brabletz, T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008, 9, 582–589. [Google Scholar] [CrossRef]
- Tiago, D.M.; Conceição, N.; Caiado, H.; Laizé, V.; Cancela, M.L. Matrix Gla protein repression by miR-155 promotes oncogenic signals in breast cancer MCF-7 cells. FEBS Lett. 2016, 590, 1234–1241. [Google Scholar] [CrossRef]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110. [Google Scholar] [CrossRef]
- Liu, F.; Kong, X.; Lv, L.; Gao, J. TGF-β1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial–mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015, 359, 288–298. [Google Scholar] [CrossRef]
- Kong, W.; He, L.; Coppola, M.; Guo, J.; Esposito, N.N.; Coppola, D.; Cheng, J.Q. MicroRNA-155 Regulates Cell Survival, Growth, and Chemosensitivity by Targeting FOXO3a in Breast Cancer. J. Biol. Chem. 2010, 285, 17869–17879. [Google Scholar] [CrossRef]
- Shen, R.; Wang, Y.; Wang, C.X.; Yin, M.; Liu, H.L.; Chen, J.P.; Han, J.Q.; Wang, W.B. MiRNA-155 mediates TAM resistance by modulating SOCS6-STAT3 signalling pathway in breast cancer. Am. J. Transl. Res. 2015, 7, 2115–2126. [Google Scholar]
- Li, C.I.; Malone, K.E.; Daling, J.R. Differences in Breast Cancer Stage, Treatment, and Survival by Race and Ethnicity. Arch. Intern. Med. 2003, 163, 49. [Google Scholar] [CrossRef]
- Maskarinec, G.; Sen, C.; Koga, K.; Conroy, S.M. Ethnic Differences in Breast Cancer Survival: Status and Determinants. Women’s Health 2011, 7, 677–687. [Google Scholar] [CrossRef]
- Ooi, S.L.; Martinez, M.E.; Li, C.I. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res. Treat. 2011, 127, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, S.L.; Koru-Sengul, T.; Miao, F.; Byrne, M.M. Disparities in survival after female breast cancer diagnosis: A population-based study. Cancer Causes Control 2013, 24, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Hirko, K.A.; Rocque, G.; Reasor, E.; Taye, A.; Daly, A.; Cutress, R.I.; Copson, E.R.; Lee, D.-W.; Lee, K.-H.; Im, S.-A.; et al. The impact of race and ethnicity in breast cancer—Disparities and implications for precision oncology. BMC Med. 2022, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Chen, Z.; Anderson, G.L.; Rohan, T.; Aragaki, A.; Lane, D.; Dolan, N.C.; Paskett, E.D.; McTiernan, A.; Hubbell, F.A.; et al. Ethnicity and Breast Cancer: Factors Influencing Differences in Incidence and Outcome. JNCI J. Natl. Cancer Inst. 2005, 97, 439–448. [Google Scholar] [CrossRef]
- Hill, D.A.; Prossnitz, E.R.; Royce, M.; Nibbe, A. Temporal trends in breast cancer survival by race and ethnicity: A population-based cohort study. PLoS ONE 2019, 14, e0224064. [Google Scholar] [CrossRef]
- Gathani, T.; Reeves, G.; Broggio, J.; Barnes, I. Ethnicity and the tumour characteristics of invasive breast cancer in over 116,500 women in England. Br. J. Cancer 2021, 125, 611–617. [Google Scholar] [CrossRef]
- Cho, B.; Han, Y.; Lian, M.; Colditz, G.A.; Weber, J.D.; Ma, C.; Liu, Y. Evaluation of Racial/Ethnic Differences in Treatment and Mortality Among Women With Triple-Negative Breast Cancer. JAMA Oncol. 2021, 7, 1016. [Google Scholar] [CrossRef]
- Rinnerthaler, G.; Hackl, H.; Hamacher, F.; Gampenrieder, S.P.; Hufnagl, C.; Romeder, F.; Monzo Fuentes, C.; Hauser-Kronberger, C.; Mlineritsch, B.; Greil, R. MiR-16 is the most stable-expressed housekeeping microRNA in breast cancer tissues from primary and metastatic sites. Ann. Oncol. 2015, 26, iii10. [Google Scholar] [CrossRef]
- McDermott, A.M.; Kerin, M.J.; Miller, N. Identification and Validation of miRNAs as Endogenous Controls for RQ-PCR in Blood Specimens for Breast Cancer Studies. PLoS ONE 2013, 8, e83718. [Google Scholar] [CrossRef]
- Davoren, P.A.; McNeill, R.E.; Lowery, A.J.; Kerin, M.J.; Miller, N. Identification of suitable endogenous control genes for microRNA gene expression analysis in human breast cancer. BMC Mol. Biol. 2008, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Eichelser, C.; Stückrath, I.; Müller, V.; Milde-Langosch, K.; Wikman, H.; Pantel, K.; Schwarzenbach, H. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget 2014, 5, 9650–9663. [Google Scholar] [CrossRef] [PubMed]

| miRNA/s | References | Normalization | Sample | n | Cases | Controls | Conclusions |
|---|---|---|---|---|---|---|---|
| miR-21 | [15] | miR-16 | Serum | 122 | 102 | 20 | miRNA-21 concentrations differed between cases and controls and enabled the differentiation of localized disease from metastases. |
| miR-21 | [16] | miR-16 | Serum | 89 | 50 | 39 | The mean serum levels of miR-21 were higher in different types of cancer compared to control (including breast cancer). The expression of miR-21 did not correlate to other aspects of breast cancer such as estrogen receptor, progesterone receptor, menopause status, and KI-67. Similarly, miR-21 expression was not associated with metastasis status. There were nine males in the control group. |
| miR-99a-5p | [17] | RNU38B | Serum and tissue | 203 | 105 | 98 | The data reflected a significant underexpression of miR-99a-5p in breast cancer tissue versus healthy tissue. However, serum levels reflected overexpression in cases versus controls. Only the testing cohort was considered. |
| miR-155 | [18] | miR-39 | Serum | 158 | 103 | 55 | MiR-155 levels were significantly elevated in the case group compared to control. The ROC curve = 0.801 with a specificity of 81.8%, suggesting that the expression of miR-155 allows one to discriminate the cases from the controls, and its expression decreases with a good response to treatment. |
| miR-155 | [19] | RNU38B | Serum | 30 | 20 | 10 | miR-155 levels were significantly elevated in the cases group. |
| miR-155 | [20] | miR-16 | Serum | 117 | 102 | 15 | The expression of miR-155 was significantly higher in the cases group. After receiving treatment (surgery and chemotherapy), the expression decreased significantly. miR-155 overexpression was also related to tumor size and stage. |
| miR-202 | [21] | miR-16 | Serum | 60 | 30 | 30 | miR-202 was found to be significantly overexpressed in the cases. Sensitivity and specificity were 90 and 93%, respectively. The positivity was 100% for Stage I, 90% for Stage II, and 80% for Stage III, demonstrating great utility for the diagnosis of BC in early stages, in addition to showing predictive value (risk of 9.6 times higher, p < 0.001) |
| miR-1204 | [22] | GAPDH | Serum and tissue | 182 | 144 | 38 | The expression of miR-1204 was significantly higher both in the tissue and in the serum of the cases compared to the controls, allowing the cases to be discriminated from the controls with a ROC curve of 0.854. |
| miR-34a miR-10b | [23] | miR-16 | Serum | 118 | 89 | 29 | The microRNAs analyzed were significantly elevated in the cases. miR-10b and miR-34a were significantly higher in patients with metastases compared to patients with localized breast cancer. Elevation of miR-34a correlated with advanced stages. |
| miR-10b miR-21 miR-145 miR-155 miR-191 miR-382 | [24] | 18S RNA | Serum | 71 | 61 | 10 | The concentrations of all miRNAs were significantly higher in cases. The ROC curve showed that three of them (miR-145, miR-155, and miR-382) are potential biomarkers. Possible differences in the expression of the cases were also analyzed, although they were not significant (different stages) |
| miR-21 miR-106a miR-126 miR-155 miR-199a miR-335 | [25] | miR-16 | Serum | 108 | 68 | 40 | The concentrations of the microRNAs were significantly different between the cases and the controls. miR-21, miR-106a, and miR-155 were overexpressed and miR-126, miR-199a, and miR-335 were underexpressed. |
| miR-16 miR-21 miR-145 miR-451 | [26] | RNU6B | Serum | 270 | 170 | 100 | miR-21 and miR-451 were found to be significantly overexpressed in the cases, while miR-145 was underexpressed with respect to the controls. The combination of miR-145 and miR-451 presented a ROC curve of 96%, with an optimal sensitivity of 90% and specificity of 96%. These markers are not elevated for other types of cancer and are useful in different stages of breast cancer, with higher diagnostic values in some types of breast neoplasms, such as DCI (ductal carcinoma in situ), which obtained a ROC curve of 98% |
| miR-21 miR-146a | [27] | miR-16 | Serum | 22 | 14 | 8 | The expression of miR-21 and miR-146a were found to be significantly higher in the cases compared to the controls. |
| miR-21 miR-92a | [28] | RNU6 | Serum | 120 | 100 | 20 | Significant overexpression of miR-21 and significant underexpression of miR-92a. Subsequent analysis showed that low miR-92a and high miR-21 levels were associated with tumor size and lymph node staging. |
| miR-21 miR-1246 | [29] | miR-54 | Serum and tissue | 32 | 16 | 16 | Several microRNAs were encapsulated or highly enriched and selectively secreted by tumor exosomes. Both miR-21 and miR-1246 showed significantly high expression in the cases. miR-122 and let-7a are also considered to be potential biomarkers as they are selectively secreted. |
| let-7a miR-10b miR-16 miR-21 miR-145 miR-155 miR-195 | [30] | miR-16 | Serum | 146 | 83 | 63 | Only miR-195 was found to be significantly overexpressed in patients with breast cancer specifically, compared to cases of other neoplasms and healthy controls. Furthermore, a positive correlation between miR-195 levels and tumour size was demonstrated. On the other hand, let-7a was significantly elevated in the cases (with the exception of malignant melanoma). In patients with breast neoplasms, the simultaneous use of three miRNAs (let-7a, miR-195, and miR-155) led to a sensitivity of 94%. |
| miR-99a miR-145 miR-151-3p miR-205 miR-210 miR-361-5p | [31] | miR-16 | Serum | 28 | 21 | 7 | miR-145 was found to be underexpressed in the cases compared to the controls. miR-210 was found to be overexpressed in the cases. Underexpression of miR-99 and overexpression of miR-155-3p. In invasive breast cancer, the underexpression of miR-145 and overexpression of miR-210 were observed. In metastatic breast cancer, the underexpression of miR-205 and overexpression of miR-361-5p were observed. |
| miR-1246 miR-1307-3p miR-4634 miR-6861-5p miR-6875-5p | [32] | miR-149-3p | Serum | 4116 | 1280 | 2836 | A combination of five miRNAs was able to discriminate cases from controls with 97.3% sensitivity, 82.9% specificity, and 89.7% precision for the breast cancer cohort. In addition, for early-stage breast cancer, the sensitivity was 98%. |
| miR-133a miR-155 p53 CEA CA-15.3 | [33] | SNORD68 | Serum | 80 | 60 | 20 | While miR-155 was significantly overexpressed in the cases, miR-133a was significantly underexpressed. Both CEA and CA-15.3 were found to be significantly elevated in the serum of the cases. A possible relationship was found between miR-133a and tumour stage (underexpression—higher stage) and between miR-155 and lymph node metastasis (overexpression—lymph nodes involved). |
| miR-21 MMP-1 | [34] | miR-16 | Urine | 48 | 22 | 26 | miR-21 was found to be significantly underexpressed in the cases, in contrast to the matrix metalloproteiase-1 (MMP-1), which was found to be significantly overexpressed. Combined, sensitivity reached 95% and specificity was 79%. |
| Let-7b-5p miR-122-5p miR-146b-5p miR-210-3p, 215-5p | [35] | miR-39 | Serum | 514 | 257 | 257 | Eleven miRNAs were significantly deregulated. These were analyzed in seventy-two samples and only five were found to be consistently overexpressed in the cases. The diagnostic capacity of five miRNAs yielded a ROC curve of 0.978, 94% sensitivity, and 88.9% specificity. |
| miR-21 miR-155 let-7c PTEN | [36] | RNU44 | Serum | 93 | 45 | 48 | The expression of miR-21 was significantly higher in the cases compared to the controls. On the contrary, miR-155, let-7c, and PTEN were found to be significantly underexpressed in the cases. |
| miR-29b-2 miR-155 miR-197 miR-205 | [37] | miR-16 | Serum | 130 | 100 | 30 | The expression of miR-155 was significantly higher in the cases versus the controls, while miR-205 was significantly underexpressed. |
| miR-16 miR-21 miR-29c miR-145 miR-191 miR-210 miR-222 | [38] | miR-222-3p | Serum | 68 | 35 | 33 | Only three miRNAs were significantly overexpressed in the cases (miR-145, -191, and -21). Individually, the ROC curves of miR-145 and -191 were 0.931 and 0.904, respectively, and in combination, this rose to 0.984, allowing for the accurate differentiation of cases versus controls. |
| Let-7c miR-21 miR-34a miR-92a miR-155 miR-222 CEA CA 15-3 CA 125 | [39] | miR-16 | Serum | 83 | 55 | 28 | The cases were shown to have a higher expression of miRNAs. The expression of miR-34a was significantly lower in the cases, unlike CEA and CA 15-3. The combination of CEA or CA 15-3 together with miR-34a yielded ROC curves of 0.844 and 0.800, respectively. The specificity of the combination of miR-34a and CA 15-3 was 95%. When the cohort of patients with breast cancer was compared with benign diseases, both sensitivity and specificity and the ROC curve showed better results. |
| miR-23a miR-29b miR-130 miR-144 miR-148a miR-152 miR-182 | [40] | U6 | Serum and tissue biopsy | 202 | 106 | 96 | The expressions of miR-23a-3p, -130a-5p, -144-3p, -148a-3p, and 152-3p were lower in the plasma of the cases compared to the controls. miR-130a-5p, -144-3p, and 152-3p were also found to be underexpressed in the tissue of the cases. The expression of miR-23a-3p, -144-3p, and 152-3p was related to ER and PR + status, in addition to showing significant differences in stage, especially in the early stages. |
| miR-16 miR-25 miR-222 miR-324-3p | [41] | miR-39 | Serum | 152 | 76 | 76 | Four miRNAs were found to be significantly overexpressed in cases versus controls. The AUC was 0.928 for the miRNA profile, with a sensitivity and specificity of 0.921 and 0.934, respectively. Validation of the cohort was considered. |
| miR-21 miR-24 miR-202 miR-206 miR-219B miR-223 miR-373 miR-1246 miR-6875 | [42] | miR-16 | Serum | 136 | 80 | 56 | The combination of two or more miRNAs of the proposed profile reported more precise results than the use of a single miRNA individually. The combination with the highest precision was that of miR-1246, -206, -24, and -373, obtaining a 98% sensitivity, 96% specificity and 97% precision. Combinations such as that made up of miR-1246, -206 and -24 achieved 100% sensitivity, 93% specificity, and 97% precision. Validation of the cohort was considered. |
| miR-15a miR-18a miR-107 miR-133a miR-139-5p miR-143 miR-145 miR-365 miR-425 | [43] | miR-10b miR-30a | Serum | 72 | 48 | 24 | Nine miRNAs were found to be deregulated in the cases versus the controls. Subsequently, a panel composed of nine miRNAs validated in 111 serum samples was developed, yielding an AUC of 0.665. The authors also reflected the possibility of analyzing the risk of suffering from breast cancer ER + with this miRNA profile. |
| miR-155 | [44] | RNU6B | Serum | 50 | 30 | 20 | miR-155 was found to be significantly overexpressed in the cases versus the controls (p < 0.001). In the cases, the expression of this miRNA varied significantly depending on the tumor stage. |
| miRNA | References | Dysregulation | Sample Size (Total) | Cases (Total) | Control (Total) | Relative Use |
|---|---|---|---|---|---|---|
| Let-7a | [30,45] | Upregulated | 338 | 231 | 107 | Diagnosis and prognosis |
| miR-10b | [23,24,30,46,47] | No significative | 359 | 270 | 89 | Diagnosis and prognosis |
| miR-16 | [26,30,38,41,46] | No significative | 536 | 253 | 183 | Normalization |
| miR-19a | [48] | Upregulated | 84 | 63 | 21 | Diagnosis and prognosis |
| miR-21 | [15,16,24,25,26,27,28,29,30,34,36,38,39,42,49,50,51] | Upregulated Downregulated (Urine [49]) | 1791 | 1083 | 708 | Diagnosis and prognosis |
| miR-24 | [42,48] | Upregulated | 84 | 63 | 21 | Diagnosis and prognosis |
| miR-34a | [23,39,46,47] | Upregulated Downregulated [39,46] | 563 | 406 | 157 | Diagnosis and prognosis |
| miR-92a | [28,39] | Downregulated | 251 | 203 | 48 | Diagnosis |
| miR-93 | [47] | Upregulated | 192 | 152 | 40 | Diagnosis (BM TN) |
| miR-99a-5p | [17] | Upregulated (Serum) Downregulated (Tissue biopsy) | 174 | 89 | 75 | Diagnosis |
| miR-106a | [25] | Upregulated | 108 | 68 | 40 | Diagnosis |
| miR-125b | [24,49] | Upregulated Downregulated (urine [49]) | 119 | 85 | 34 | Diagnosis |
| miR-126 | [25] | Downregulated | 108 | 68 | 40 | Diagnosis |
| miR-133a | [33,43] | Downregulated | 191 | 120 | 71 | Diagnosis and prognosis |
| miR-141 | [23] | Upregulated | 118 | 89 | 29 | Diagnosis |
| miR-145 | [24,26,30,31,38,43] | Downregulated | 644 | 380 | 264 | Diagnosis |
| miR-146a | [27] | Upregulated | 22 | 14 | 8 | Diagnosis |
| miR-155 | [18,19,20,23,24,25,30,33,36,37,39,44,46,47,48,49] | Upregulated | 1637 | 1154 | 483 | Diagnosis and prognosis |
| miR-199a | [25] | Downregulated | 108 | 68 | 40 | Diagnosis |
| miR-181b | [46,48] | Upregulated | 254 | 183 | 71 | Diagnosis and prognosis |
| miR-191 | [24,38] | Upregulated | 139 | 96 | 43 | Diagnosis |
| miR-195 | [30,45] | Upregulated | 338 | 231 | 107 | Diagnosis and prognosis |
| miR-199a | [25] | Downregulated | 108 | 68 | 40 | Diagnosis |
| miR-202 | [21] | Upregulated | 60 | 30 | 30 | Diagnosis and predictor |
| miR-205 | [31,37] | Upregulated | 218 | 151 | 67 | Diagnosis and prognosis |
| miR-210 | [31,35] | Upregulated | 542 | 278 | 264 | Diagnosis |
| miR-222 | [39,41] | Upregulated | 235 | 131 | 104 | Diagnosis |
| miR-335 | [25] | Downregulated | 108 | 68 | 40 | Diagnosis |
| miR-373 | [42,47] | Upregulated | 328 | 232 | 96 | Diagnosis (HER2+) |
| miR-451 | [26,49] | Upregulated Downregulated (Urine [49]) | 268 | 144 | 124 | Diagnosis |
| miR-1204 | [32] | Upregulated | 182 | 144 | 38 | Diagnosis |
| miR-1307 | [32] | Upregulated | 4116 | 1280 | 2836 | Diagnosis |
| miR-1246 | [29,32,42] | Upregulated | 4284 | 1376 | 1308 | Diagnosis |
| miR-4634 | [32] | Upregulated | 4116 | 1280 | 2836 | Diagnosis |
| miR-6861-5p | [32] | Upregulated | 4116 | 1280 | 2836 | Diagnosis |
| miR-6875-5p | [32,42] | Upregulated | 4252 | 1360 | 2892 | Diagnosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Palacios, A.; Rojas Carvajal, A.M.; Núñez-Negrillo, A.M.; Cortés-Martín, J.; Sánchez-García, J.C.; Aguilar-Cordero, M.J. MicroRNA Dysregulation in Early Breast Cancer Diagnosis: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 8270. https://doi.org/10.3390/ijms24098270
Garrido-Palacios A, Rojas Carvajal AM, Núñez-Negrillo AM, Cortés-Martín J, Sánchez-García JC, Aguilar-Cordero MJ. MicroRNA Dysregulation in Early Breast Cancer Diagnosis: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(9):8270. https://doi.org/10.3390/ijms24098270
Chicago/Turabian StyleGarrido-Palacios, Alejandro, Ana María Rojas Carvajal, Ana María Núñez-Negrillo, Jonathan Cortés-Martín, Juan Carlos Sánchez-García, and María José Aguilar-Cordero. 2023. "MicroRNA Dysregulation in Early Breast Cancer Diagnosis: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 9: 8270. https://doi.org/10.3390/ijms24098270
APA StyleGarrido-Palacios, A., Rojas Carvajal, A. M., Núñez-Negrillo, A. M., Cortés-Martín, J., Sánchez-García, J. C., & Aguilar-Cordero, M. J. (2023). MicroRNA Dysregulation in Early Breast Cancer Diagnosis: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(9), 8270. https://doi.org/10.3390/ijms24098270