Critical Roles of the Cysteine–Glutathione Axis in the Production of γ-Glutamyl Peptides in the Nervous System
Abstract
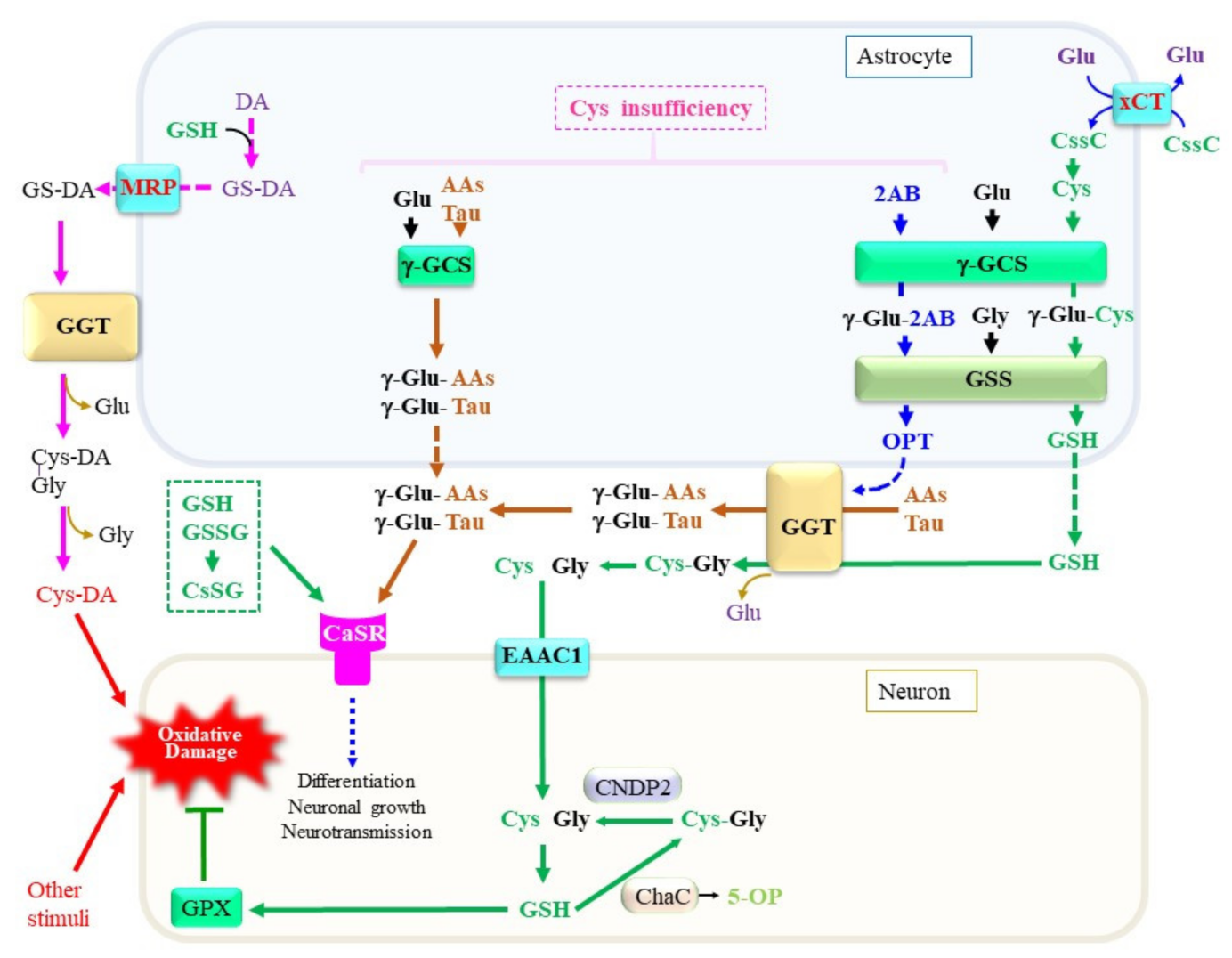
1. Introduction
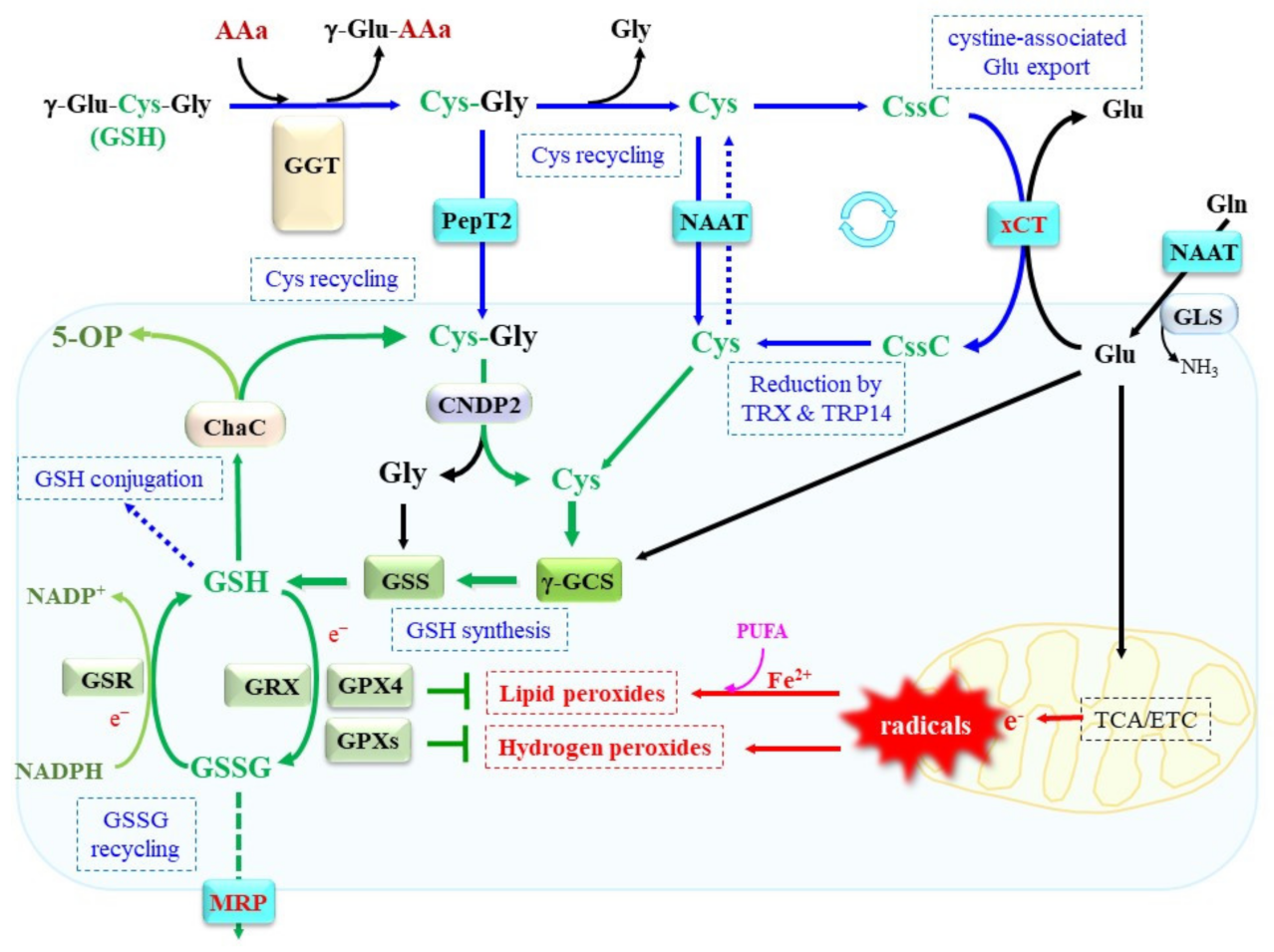
2. Regulation of Cellular GSH Content by Balancing Synthesis, Recycling, and Degradation
2.1. Dynamics of Cys Responsible for Cellular Redox Homeostasis
2.2. Uptake of Extracellular Cys and Cystine
2.3. GSH Synthesis and Consequence of Its Defect
2.4. Consumption of GSH and Reductive Recycling of GSSG
2.5. GGT Responsible for γ-Glutamyl Peptide Metabolism on the Cell Surface
2.6. γ-Glutamylcyclotransferase Responsible for Intracellular GSH Degradation
2.7. Dipeptidase for Recruit of Cys from GSH Degradation Product
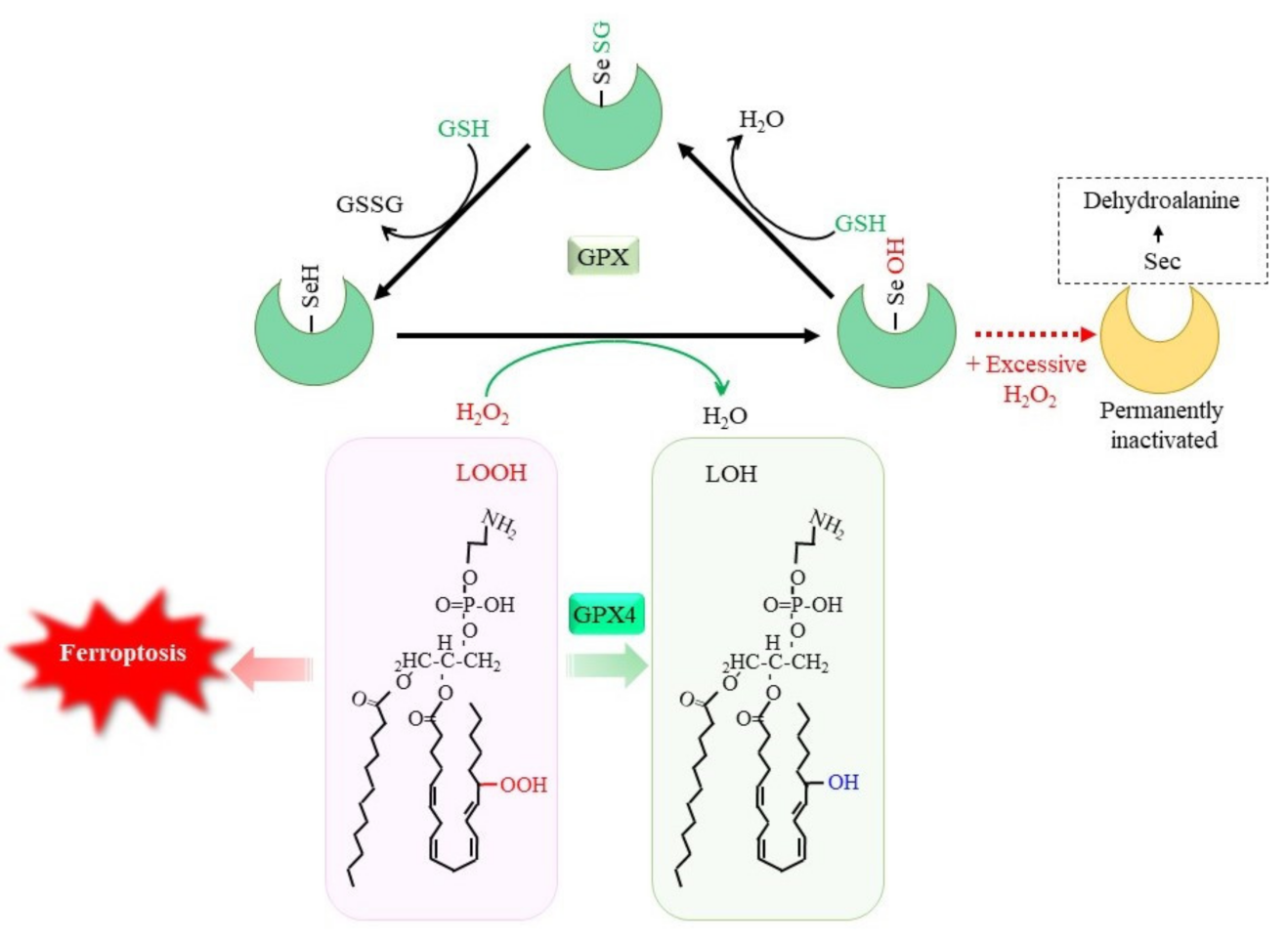
3. GSH Protects Cells against Stress through Multiple Pathways
3.1. Reductive Detoxification of Peroxides via GPX
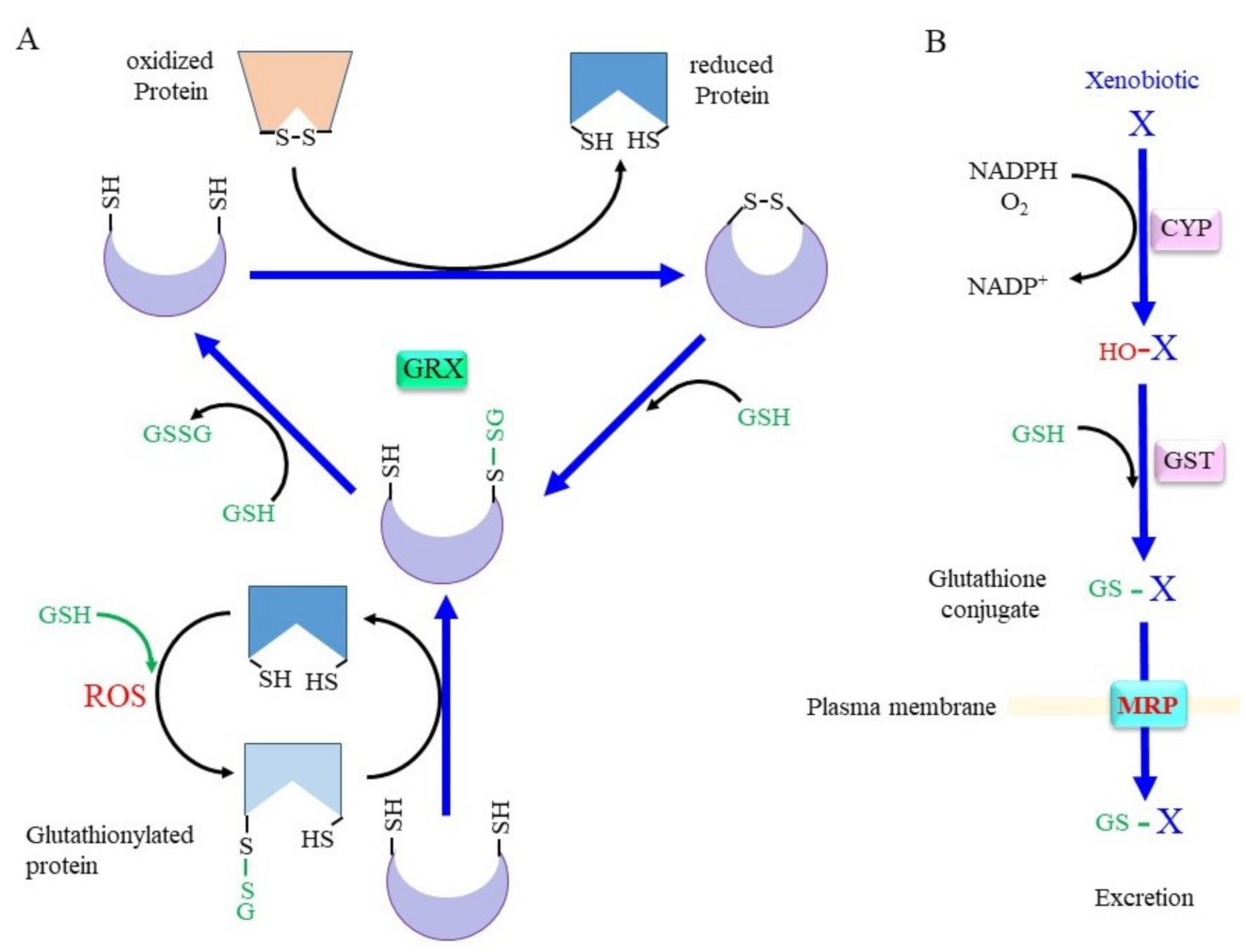
3.2. Rescuing Proteins from Oxidative Modification through GRX
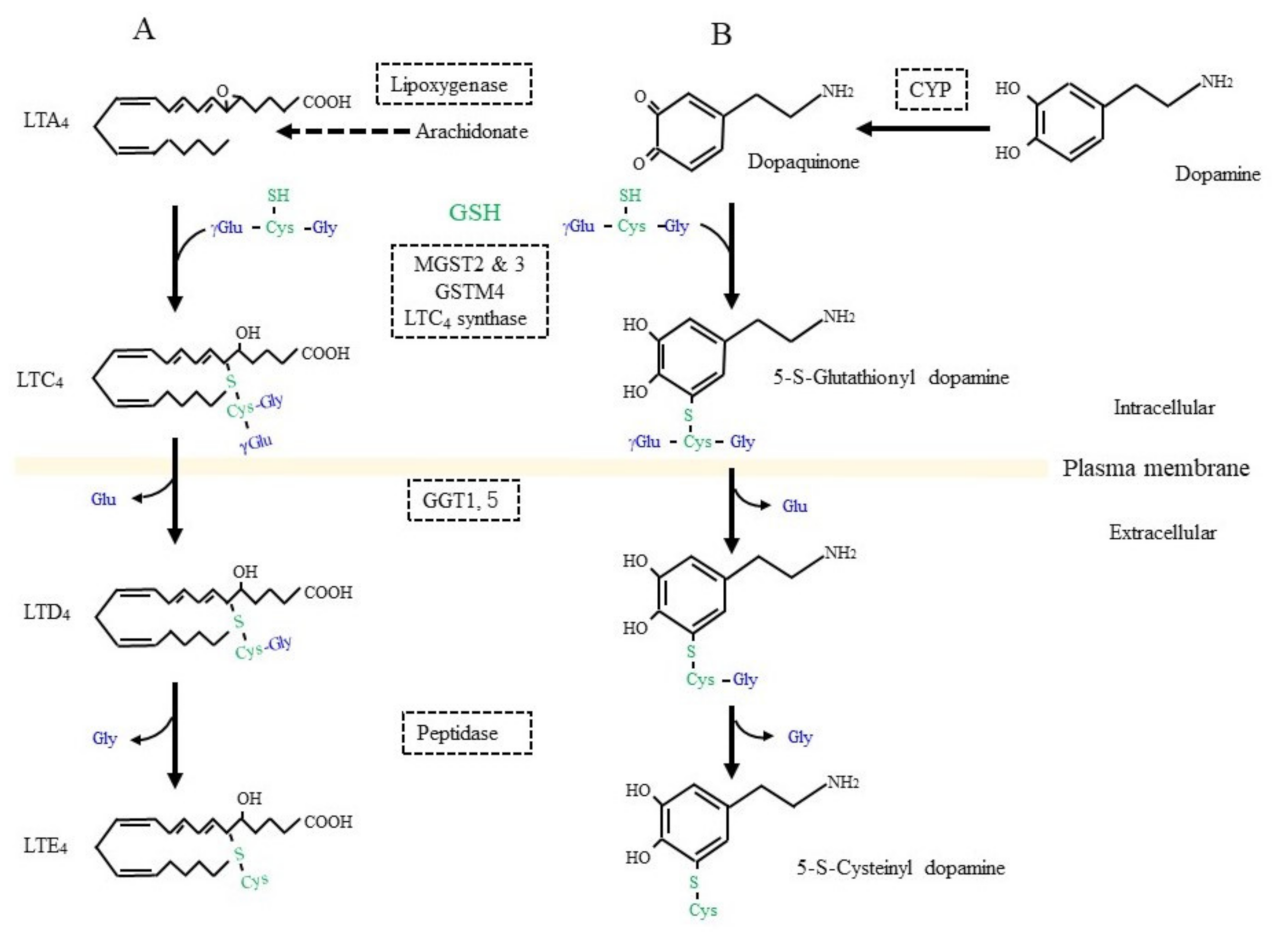
3.3. Glutathione Conjugation in the Detoxification of Xenobiotics and in the Production of Bioreactive Compounds
3.4. GSNO in Nitric Oxide Signal Transduction
3.5. GSH Status Associated with Neuronal Diseases
3.6. Production of a Variety of γ-Glutamyl Peptides by Means of γ-GCS and GGT
3.7. Calcium-Sensing Receptor as a Target of γ-Glutamyl Peptides
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkhitko, A.A.; Jouandin, P.; Mohr, S.E.; Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 2019, 18, 13034. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.A.; Pérez-Sala, D. Mammalian Sulfur Amino Acid Metabolism: A Nexus Between Redox Regulation, Nutrition, Epigenetics, and Detoxification. Met transsulfuration. Antioxid. Redox Signal. 2018, 29, 408–452. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Danekas, A.; Farrell, W.; Garcia, T.; Mehboob, W.; White, M. At the intersection of sulfur redox chemistry, cellular signal transduction and proteostasis: A useful perspective from which to understand and treat neurodegeneration. Free Radic. Biol. Med. 2022, 178, 161–173. [Google Scholar] [CrossRef] [PubMed]
- McBean, G.J. Cysteine, Glutathione, and Thiol Redox Balance in Astrocytes. Antioxidants 2017, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Mazzetti, A.P.; Fiorile, M.C.; Primavera, A.; Lo Bello, M. Glutathione transferases and neurodegenerative diseases. Neurochem. Int. 2015, 82, 10–18. [Google Scholar] [CrossRef]
- McBean, G.J.; Aslan, M.; Griffiths, H.R.; Torrão, R.C. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015, 5, 186–194. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Anderson, G.; Dean, O.; Berk, M.; Galecki, P.; Martin-Subero, M.; Maes, M. The glutathione system: A new drug target in neuroimmune disorders. Mol. Neurobiol. 2014, 50, 1059–1084. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Fujii, J. Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs. Curr. Drug. Metab. 2015, 16, 560–571. [Google Scholar] [CrossRef]
- Ni, G.; Hu, Z.; Wang, Z.; Zhang, M.; Liu, X.; Yang, G.; Yan, Z.; Zhang, Y. Cysteine Donor-Based Brain-Targeting Prodrug: Opportunities and Challenges. Oxid. Med. Cell Longev. 2022, 2022, 4834117. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Chen, Y.; Schneider, S.N.; Nebert, D.W.; Shertzer, H.G. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004, 37, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ito, J.I.; Zhang, X.; Kurahashi, T. Unveiling the roles of the glutathione redox system in vivo by analyzing genetically modified mice. J. Clin. Biochem. Nutr. 2011, 49, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Thakur, A.; Kaur, J.; Zulkifli, M. Glutathione transporters. Biochim. Biophys. Acta 2013, 1830, 3154–3164. [Google Scholar] [CrossRef]
- Dwivedi, D.; Megha, K.; Mishra, R.; Mandal, P.K. Glutathione in Brain: Overview of Its Conformations, Functions, Biochemical Characteristics, Quantitation and Potential Therapeutic Role in Brain Disorders. Neurochem. Res. 2020, 45, 1461–1480. [Google Scholar] [CrossRef]
- Haddad, M.; Hervé, V.; Ben Khedher, M.R.; Rabanel, J.M.; Ramassamy, C. Glutathione: An Old and Small Molecule with Great Functions and New Applications in the Brain and in Alzheimer’s Disease. Antioxid. Redox Signal. 2021, 35, 270–292. [Google Scholar] [CrossRef]
- Ramírez-Guerrero, S.; Guardo-Maya, S.; Medina-Rincón, G.J.; Orrego-González, E.E.; Cabezas-Pérez, R.; González-Reyes, R.E. Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship. Front. Mol. Neurosci. 2022, 15, 937789. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Geerts, C.; Furtos, A.; Waters, P.; Cyr, D.; Wang, S.; Mitchell, G.A. The multiple facets of acetyl-CoA metabolism: Energetics, biosynthesis, regulation, acylation and inborn errors. Mol. Genet. Metab. 2023, 138, 106966. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Evande, R.; Kabil, O.; Ojha, S.; Taoka, S. Reaction mechanism and regulation of cystathionine beta-synthase. Biochim. Biophys. Acta 2003, 1647, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Myszkowska, J.; Derevenkov, I.; Makarov, S.V.; Spiekerkoetter, U.; Hannibal, L. Biosynthesis, Quantification and Genetic Diseases of the Smallest Signaling Thiol Metabolite: Hydrogen Sulfide. Antioxidants 2021, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Zou, C.G. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: A PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef]
- Lee, J.; Kang, E.S.; Kobayashi, S.; Homma, T.; Sato, H.; Seo, H.G.; Fujii, J. The viability of primary hepatocytes is maintained under a low cysteine-glutathione redox state with a marked elevation in ophthalmic acid production. Exp. Cell Res. 2017, 361, 178–191. [Google Scholar] [CrossRef] [PubMed]
- McBean, G.J. The transsulfuration pathway: A source of cysteine for glutathione in astrocytes. Amino Acids 2012, 42, 199–205. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Kanai, Y.; Hediger, M.A. The glutamate and neutral amino acid transporter family: Physiological and pharmacological implications. Eur. J. Pharmacol. 2003, 479, 237–247. [Google Scholar] [CrossRef]
- Errasti-Murugarren, E.; Palacín, M. Heteromeric Amino Acid Transporters in Brain: From Physiology to Pathology. Neurochem. Res. 2022, 47, 23–36. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Glutathione in Cellular Redox Homeostasis: Association with the Excitatory Amino Acid Carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system xc-: Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tamba, M.; Okuno, S.; Sato, K.; Keino-Masu, K.; Masu, M.; Bannai, S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J. Neurosci. 2002, 22, 8028–8033. [Google Scholar] [CrossRef] [PubMed]
- Ottestad-Hansen, S.; Hu, Q.X.; Follin-Arbelet, V.V.; Bentea, E.; Sato, H.; Massie, A.; Zhou, Y.; Danbolt, N.C. The cystine-glutamate exchanger (xCT, Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain. Glia 2018, 66, 951–970. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sato, M.; Kasakoshi, T.; Tsutsui, T.; Sugimoto, M.; Osaki, M.; Okada, F.; Igarashi, K.; Hiratake, J.; Homma, T.; et al. Cystathionine is a novel substrate of cystine/glutamate transporter: Implications for immune function. J. Biol. Chem. 2015, 290, 8778–8788. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nomura, S.; Maebara, K.; Sato, K.; Tamba, M.; Bannai, S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem. Biophys. Res. Commun. 2004, 325, 109–116. [Google Scholar] [CrossRef]
- Ishii, T.; Mann, G.E. Redox status in mammalian cells and stem cells during culture in vitro: Critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014, 2, 786–794. [Google Scholar] [CrossRef]
- Pader, I.; Sengupta, R.; Cebula, M.; Xu, J.; Lundberg, J.O.; Holmgren, A.; Johansson, K.; Arnér, E.S. Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc. Natl. Acad. Sci. USA 2014, 111, 6964–6969. [Google Scholar] [CrossRef]
- Sato, H.; Shiiya, A.; Kimata, M.; Maebara, K.; Tamba, M.; Sakakura, Y.; Makino, N.; Sugiyama, F.; Yagami, K.; Moriguchi, T.; et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005, 280, 37423–37429. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Williams, L.E.; Featherstone, D.E. Regulation of hippocampal synaptic strength by glial xCT. J. Neurosci. 2014, 34, 16093–16102. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P.; Methner, A. Regulation of xCT expression and system x (c) (-) function in neuronal cells. Amino Acids 2012, 42, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Haseena, P.A.; Diwakar, L.; Ravindranath, V. Protein Glutathionylation and Glutaredoxin: Role in Neurodegenerative Diseases. Antioxidants 2022, 11, 2334. [Google Scholar] [CrossRef]
- Massie, A.; Boillée, S.; Hewett, S.; Knackstedt, L.; Lewerenz, J. Main path and byways: Non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission. J. Neurochem. 2015, 135, 1062–1079. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Lin, C.H.; Lane, H.Y. Cystine/Glutamate Antiporter in Schizophrenia: From Molecular Mechanism to Novel Biomarker and Treatment. Int. J. Mol. Sci. 2021, 22, 9718. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Santoyo, A.O.; Ruiz-Rodríguez, V.M.; Mares-Barbosa, T.B.; Patrón-Soberano, A.; Howe, A.G.; Portales-Pérez, D.P.; Miquelajáuregui-Graf, A.; Estrada-Sánchez, A.M. Revealing the contribution of astrocytes to glutamatergic neuronal transmission. Front. Cell Neurosci. 2023, 16, 1037641. [Google Scholar] [CrossRef]
- Domercq, M.; Sánchez-Gómez, M.V.; Sherwin, C.; Etxebarria, E.; Fern, R.; Matute, C. System xc- and glutamate transporter inhibition mediates microglial toxicity to oligodendrocytes. J. Immunol. 2007, 178, 6549–6556. [Google Scholar] [CrossRef]
- Mesci, P.; Zaïdi, S.; Lobsiger, C.S.; Millecamps, S.; Escartin, C.; Seilhean, D.; Sato, H.; Mallat, M.; Boillée, S. System xC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice. Brain 2015, 138, 53–68. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Savaskan, N.E.; Heckel, A.; Hahnen, E.; Engelhorn, T.; Doerfler, A.; Ganslandt, O.; Nimsky, C.; Buchfelder, M.; Eyüpoglu, I.Y. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat. Med. 2008, 14, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, K.; Okazaki, S.; Ohmura, M.; Ishikawa, M.; Sampetrean, O.; Onishi, N.; Wakimoto, H.; Yoshikawa, M.; Seishima, R.; Iwasaki, Y.; et al. The EGF Receptor Promotes the Malignant Potential of Glioma by Regulating Amino Acid Transport System xc(-). Cancer Res. 2016, 76, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Huberfeld, G.; Vecht, C.J. Seizures and gliomas--towards a single therapeutic approach. Nat. Rev. Neurol. 2016, 12, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, G.; Fang, X.; Bai, S.; Yuan, G.; Pan, Y. Ferroptosis and Its Potential Role in Glioma: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants 2022, 11, 2123. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457. [Google Scholar] [CrossRef]
- Bröer, S. Amino Acid Transporters as Targets for Cancer Therapy: Why, Where, When, and How. Int. J. Mol. Sci. 2020, 26, 6156. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Yoshimura, S.H.; Katoh, H. High cell density increases glioblastoma cell viability under glucose deprivation via degradation of the cystine/glutamate transporter xCT (SLC7A11). J. Biol. Chem. 2020, 295, 6936–6945. [Google Scholar] [CrossRef]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Ma, W.Q.; Sun, X.J.; Zhu, Y.; Liu, N.F. Metformin attenuates hyperlipidaemia-associated vascular calcification through anti-ferroptotic effects. Free Radic. Biol. Med. 2021, 165, 229–242. [Google Scholar] [CrossRef]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Njålsson, R. Glutathione synthetase deficiency. Cell Mol. Life Sci. 2005, 62, 1938–1945. [Google Scholar] [CrossRef]
- Konrad, P.N.; Richards, F., 2nd; Valentine, W.N.; Paglia, D.E. γ-Glutamyl-cysteine synthetase deficiency. A cause of hereditary hemolytic anemia. N. Engl. J. Med. 1972, 286, 557–561. [Google Scholar] [CrossRef]
- Mañú Pereira, M.; Gelbart, T.; Ristoff, E.; Crain, K.C.; Bergua, J.M.; López Lafuente, A.; Kalko, S.G.; García Mateos, E.; Beutler, E.; Vives Corrons, J.L. Chronic non-spherocytic hemolytic anemia associated with severe neurological disease due to gamma-glutamylcysteine synthetase deficiency in a patient of Moroccan origin. Haematologica 2007, 92, e102–e105. [Google Scholar] [CrossRef]
- Almusafri, F.; Elamin, H.E.; Khalaf, T.E.; Ali, A.; Ben-Omran, T.; El-Hattab, A.W. Clinical and molecular characterization of 6 children with glutamate-cysteine ligase deficiency causing hemolytic anemia. Blood Cells Mol. Dis. 2017, 65, 73–77. [Google Scholar] [CrossRef]
- Xia, H.; Ye, J.; Wang, L.; Zhu, J.; He, Z. A case of severe glutathione synthetase deficiency with novel GSS mutations. Braz. J. Med. Biol. Res. 2018, 51, e6853. [Google Scholar] [CrossRef]
- Guney Varal, I.; Dogan, P.; Gorukmez, O.; Dorum, S.; Akdag, A. Glutathione synthetase deficiency: A novel mutation with femur agenesis. Fetal Pediatr. Pathol. 2020, 39, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ristoff, E.; Hebert, C.; Njålsson, R.; Norgren, S.; Rooyackers, O.; Larsson, A. Glutathione synthetase deficiency: Is gamma-glutamylcysteine accumulation a way to cope with oxidative stress in cells with insufficient levels of glutathione? J. Inherit. Metab. Dis. 2002, 25, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Osei-Frimpong, J.; Kala, G.; Kala, S.V.; Barrios, R.J.; Habib, G.M.; Lukin, D.J.; Danney, C.M.; Matzuk, M.M.; Lieberman, M.W. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA 2000, 97, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Dieter, M.Z.; Yang, Y.; Shertzer, H.G.; Nebert, D.W. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: Embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem. Biophys. Res. Commun. 2000, 279, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Miller, M.L.; Shen, D.; Shertzer, H.G.; Stringer, K.F.; Wang, B.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 2007, 45, 1118–1128. [Google Scholar] [CrossRef]
- Kurniawan, H.; Franchina, D.G.; Guerra, L.; Bonetti, L.; Baguet, L.S.; Grusdat, M.; Schlicker, L.; Hunewald, O.; Dostert, C.; Merz, M.P.; et al. Glutathione Restricts Serine Metabolism to Preserve Regulatory T Cell Function. Cell Metab. 2020, 31, 920–936.e7. [Google Scholar] [CrossRef]
- Su, H.; Huang, J.; Weng, S.; Zhang, B.; Zhang, T.; Xu, Y. Glutathione synthesis primes monocytes metabolic and epigenetic pathway for β-glucan-trained immunity. Redox Biol. 2021, 48, 102206. [Google Scholar] [CrossRef]
- Hashimoto, S.; Matsuba, Y.; Takahashi, M.; Kamano, N.; Watamura, N.; Sasaguri, H.; Takado, Y.; Yoshihara, Y.; Saito, T.; Saido, T.C. Neuronal glutathione loss leads to neurodegeneration involving gasdermin activation. Sci. Rep. 2023, 3, 109. [Google Scholar] [CrossRef]
- Yang, Y.; Dieter, M.Z.; Chen, Y.; Shertzer, H.G.; Nebert, D.W.; Dalton, T.P. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(-/-) knockout mouse. Novel model system for a severely compromised oxidative stress response. J. Biol. Chem. 2002, 277, 49446–49452. [Google Scholar] [CrossRef]
- Kann, S.; Estes, C.; Reichard, J.F.; Huang, M.Y.; Sartor, M.A.; Schwemberger, S.; Chen, Y.; Dalton, T.P.; Shertzer, H.G.; Xia, Y.; et al. Butylhydroquinone protects cells genetically deficient in glutathione biosynthesis from arsenite-induced apoptosis without significantly changing their prooxidant status. Toxicol. Sci. 2005, 87, 365–384. [Google Scholar] [CrossRef]
- McConnachie, L.A.; Mohar, I.; Hudson, F.N.; Ware, C.B.; Ladiges, W.C.; Fernandez, C.; Chatterton-Kirchmeier, S.; White, C.C.; Pierce, R.H.; Kavanagh, T.J. Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol. Sci. 2007, 99, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Botta, D.; Shi, S.; White, C.C.; Dabrowski, M.J.; Keener, C.L.; Srinouanprachanh, S.L.; Farin, F.M.; Ware, C.B.; Ladiges, W.C.; Pierce, R.H.; et al. Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J. Biol. Chem. 2006, 281, 28865–28875. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Johansson, E.; Fan, Y.; Shertzer, H.G.; Vasiliou, V.; Nebert, D.W.; Dalton, T.P. Early onset senescence occurs when fibroblasts lack the glutamate-cysteine ligase modifier subunit. Free Radic. Biol. Med. 2009, 47, 410–418. [Google Scholar] [CrossRef]
- Corcoba, A.; Gruetter, R.; Do, K.Q.; Duarte, J.M.N. Social isolation stress and chronic glutathione deficiency have a common effect on the glutamine-to-glutamate ratio and myo-inositol concentration in the mouse frontal cortex. J. Neurochem. 2017, 142, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Manna, S.K.; Golla, S.; Krausz, K.W.; Cai, Y.; Garcia-Milian, R.; Chakraborty, T.; Chakraborty, J.; Chatterjee, R.; Thompson, D.C.; et al. Glutathione deficiency-elicited reprogramming of hepatic metabolism protects against alcohol-induced steatosis. Free Radic. Biol. Med. 2019, 143, 127–139. [Google Scholar] [CrossRef]
- Cole, S.P.; Deeley, R.G. Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol. Sci. 2006, 27, 438–446. [Google Scholar] [CrossRef]
- Dean, M.; Allikmets, R. Complete characterization of the human ABC gene family. J. Bioenerg. Biomembr. 2001, 33, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspects Med. 2009, 30, 13–28. [Google Scholar] [CrossRef]
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Sun, Q.A.; Kirnarsky, L.; Sherman, S.; Gladyshev, V.N. Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systems. Proc. Natl. Acad. Sci. USA 2001, 98, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Novoselov, S.V.; Sun, Q.A.; Moustafa, M.E.; Zhou, Y.; Oko, R.; Hatfield, D.L.; Gladyshev, V.N. Mammalian selenoprotein thioredoxin-glutathione reductase. Roles in disulfide bond formation and sperm maturation. J. Biol. Chem. 2005, 280, 26491–26498. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, M.V.; Su, D.; Gladyshev, V.N. CUG start codon generates thioredoxin/glutathione reductase isoforms in mouse testes. J. Biol. Chem. 2010, 285, 4595–4602. [Google Scholar] [CrossRef] [PubMed]
- Vyas, B.; Bhowmik, R.; Akhter, M.; Ahmad, F.J. Identification, analysis of deleterious SNPs of the human GSR gene and their effects on the structure and functions of associated proteins and other diseases. Sci. Rep. 2022, 12, 5474. [Google Scholar] [CrossRef]
- Pretsch, W. Glutathione reductase activity deficiency in homozygous Gr1a1Neu mice does not cause haemolytic anaemia. Genet. Res. 1999, 73, 1–5. [Google Scholar] [CrossRef]
- Rogers, L.K.; Tamura, T.; Rogers, B.J.; Welty, S.E.; Hansen, T.N.; Smith, C.V. Analyses of glutathione reductase hypomorphic mice indicate a genetic knockout. Toxicol. Sci. 2004, 82, 367–373. [Google Scholar] [CrossRef]
- Rogers, L.K.; Bates, C.M.; Welty, S.E.; Smith, C.V. Diquat induces renal proximal tubule injury in glutathione reductase-deficient mice. Toxicol. Appl. Pharmacol. 2006, 217, 289–298. [Google Scholar] [CrossRef]
- Tipple, T.E.; Welty, S.E.; Rogers, L.K.; Hansen, T.N.; Choi, Y.E.; Kehrer, J.P.; Smith, C.V. Thioredoxin-related mechanisms in hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2007, 37, 405–413. [Google Scholar] [CrossRef]
- Robbins, M.E.; Cho, H.Y.; Hansen, J.M.; Luchsinger, J.R.; Locy, M.L.; Velten, M.; Kleeberger, S.R.; Rogers, L.K.; Tipple, T.E. Glutathione reductase deficiency alters lung development and hyperoxic responses in neonatal mice. Redox Biol. 2021, 38, 101797. [Google Scholar] [CrossRef]
- Eriksson, S.; Prigge, J.R.; Talago, E.A.; Arnér, E.S.; Schmidt, E.E. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat. Commun. 2015, 6, 6479. [Google Scholar] [CrossRef]
- Bourbouloux, A.; Shahi, P.; Chakladar, A.; Delrot, S.; Bachhawat, A.K. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 13259–13265. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Kaur, A. Glutathione Degradation. Antioxid. Redox Signal. 2017, 27, 1200–1216. [Google Scholar] [CrossRef]
- Heisterkamp, N.; Groffen, J.; Warburton, D.; Sneddon, T.P. The human γ-glutamyltransferase gene family. Hum. Genet. 2008, 123, 321–332. [Google Scholar] [CrossRef]
- Ikeda, Y.; Taniguchi, N. Gene expression of gamma-glutamyltranspeptidase. Methods Enzymol. 2005, 401, 408–425. [Google Scholar] [CrossRef]
- Corti, A.; Belcastro, E.; Dominici, S.; Maellaro, E.; Pompella, A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an ‘antioxidant’ enzyme. Free Radic. Biol. Med. 2020, 160, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Frey, I.M.; Rubio-Aliaga, I.; Siewert, A.; Sailer, D.; Drobyshev, A.; Beckers, J.; de Angelis, M.H.; Aubert, J.; Bar Hen, A.; Fiehn, O.; et al. Profiling at mRNA, protein, and metabolite levels reveals alterations in renal amino acid handling and glutathione metabolism in kidney tissue of Pept2-/- mice. Physiol. Genomics 2007, 28, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Lu, K. Substrates of the human oligopeptide transporter hPEPT2. Biosci. Trends 2015, 9, 207–213. [Google Scholar] [CrossRef]
- Kang, Y.P.; Mockabee-Macias, A.; Jiang, C.; Falzone, A.; Prieto-Farigua, N.; Stone, E.; Harris, I.S.; DeNicola, G.M. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 2021, 33, 174–189.e7. [Google Scholar] [CrossRef]
- Cambier, D.; Rutin, J.; Alliot, F.; Pessac, B. Expression of gamma-glutamyl transpeptidase in mouse perivascular astrocytes and in a protoplasmic-like astroglial cell clone. Brain Res. 2000, 852, 191–197. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Chan, F.K.L.; Ji, J.; Yu, J.; Liang, J.Q. Gamma-glutamyltransferase 7 suppresses gastric cancer by cooperating with RAB7 to induce mitophagy. Oncogene 2022, 41, 3485–3497. [Google Scholar] [CrossRef]
- Bui, T.T.; Nitta, R.T.; Kahn, S.A.; Razavi, S.M.; Agarwal, M.; Aujla, P.; Gholamin, S.; Recht, L.; Li, G. γ-Glutamyl transferase 7 is a novel regulator of glioblastoma growth. BMC Cancer 2015, 15, 225. [Google Scholar] [CrossRef] [PubMed]
- Hayashima, K.; Katoh, H. Expression of gamma-glutamyltransferase 1 in glioblastoma cells confers resistance to cystine deprivation-induced ferroptosis. J. Biol. Chem. 2022, 298, 101703. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.C.; Stern, J.; Ersser, R.; Patrick, A.D. Glutathionuria: Gamma-glutamyl transpeptidase deficiency. J. Inherit. Metab. Dis. 1980, 2, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Tsuji, T.; Kunieda, T. Phenotypic characterization of Ggt1(dwg/dwg) mice, a mouse model for hereditary γ-glutamyltransferase deficiency. Exp. Anim. 2013, 62, 151–157. [Google Scholar] [CrossRef]
- Harding, C.O.; Williams, P.; Wagner, E.; Chang, D.S.; Wild, K.; Colwell, R.E.; Wolff, J.A. Mice with genetic gamma-glutamyl transpeptidase deficiency exhibit glutathionuria, severe growth failure, reduced life spans, and infertility. J. Biol. Chem. 1997, 272, 12560–12567. [Google Scholar] [CrossRef]
- Held, P.; Harding, C.O. L-2-oxothiazolidine-4-carboxylate supplementation in murine gamma-GT deficiency. Free Radic. Biol. Med. 2003, 34, 1482–1487. [Google Scholar] [CrossRef]
- Lieberman, M.W.; Wiseman, A.L.; Shi, Z.Z.; Carter, B.Z.; Barrios, R.; Ou, C.N.; Chévez-Barrios, P.; Wang, Y.; Habib, G.M.; Goodman, J.C.; et al. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 7923–7926. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Han, B.; Habib, G.M.; Matzuk, M.M.; Lieberman, M.W. Disruption of gamma-glutamyl leukotrienase results in disruption of leukotriene D(4) synthesis in vivo and attenuation of the acute inflammatory response. Mol. Cell. Biol. 2001, 21, 5389–5395. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S.; Jainarayanan, A.K.; Dubey, P. Heart failure and the glutathione cycle: An integrated view. Biochem. J. 2020, 477, 3123–3130. [Google Scholar] [CrossRef]
- Kaur, A.; Gautam, R.; Srivastava, R.; Chandel, A.; Kumar, A.; Karthikeyan, S.; Bachhawat, A.K. ChaC2, an Enzyme for Slow Turnover of Cytosolic Glutathione. J. Biol. Chem. 2017, 92, 638–651. [Google Scholar] [CrossRef]
- Mungrue, I.N.; Pagnon, J.; Kohannim, O.; Gargalovic, P.S.; Lusis, A.J. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J. Immunol. 2009, 182, 466–476. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 2014, 3, e02523. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Wang, S.F.; Hsu, C.Y.; Yin, P.H.; Yeh, T.S.; Lee, H.C.; Tseng, L.M. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 2017, 8, 114588–114602. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zheng, X.; Mao, S.; Zhang, Q.; Hu, G.; Wei, Y. DJ-1 inhibits glutathione degradation by downregulating CHAC1 expression in astrocytes. Neurosci. Res. 2022, 184, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Yang, S.C.; Hsu, S.C.; Chang, F.P.; Lin, Y.T.; Chen, S.F.; Cheng, C.L.; Hsiao, M.; Lu, F.L.; Lu, J. CHAC2 is essential for self-renewal and glutathione maintenance in human embryonic stem cells. Free Radic. Biol. Med. 2017, 113, 439–451. [Google Scholar] [CrossRef]
- Liu, S.; Fei, W.; Shi, Q.; Li, Q.; Kuang, Y.; Wang, C.; He, C.; Hu, X. CHAC2, downregulated in gastric and colorectal cancers, acted as a tumor suppressor inducing apoptosis and autophagy through unfolded protein response. Cell Death Dis. 2017, 8, e3009. [Google Scholar] [CrossRef]
- Chand, S.; Mehta, V.; Sharma, R.K.; Anvikar, A.R.; Chander, H. Cancer informatics analysis indicates high CHAC2 associated with unfavorable prognosis in breast cancer. Front. Oncol. 2022, 12, 1058931. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, J.; Qiao, Y. A metabolism-associated gene signature for prognosis prediction of hepatocellular carcinoma. Front. Mol. Biosci. 2022, 9, 988323. [Google Scholar] [CrossRef]
- Dieck, S.T.; Heuer, H.; Ehrchen, J.; Otto, C.; Bauer, K. The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative beta-Ala-Lys-Nepsilon-AMCA in astrocytes. Glia 1999, 25, 10–20. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Döring, F.; Nickolaus, M.; Daniel, H.; Fischer, A. Expression of PEPT2 peptide transporter mRNA and protein in glial cells of rat dorsal root ganglia. Neurosci. Lett. 2001, 304, 181–184. [Google Scholar] [CrossRef]
- Kobayashi, S.; Homma, T.; Okumura, N.; Han, J.; Nagaoka, K.; Sato, H.; Konno, H.; Yamada, S.; Takao, T.; Fujii, J. Carnosine dipeptidase II (CNDP2) protects cells under cysteine insufficiency by hydrolyzing glutathione-related peptides. Free Radic. Biol. Med. 2021, 174, 12–27. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, their substrates and diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chan, D.W.; Zhu, Y.; Li, J.J.; Ng, I.O.; Wan, D.; Gu, J. Identification of carboxypeptidase of glutamate like-B as a candidate suppressor in cell growth and metastasis in human hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 6617–6625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miao, L.; Xin, X.; Zhang, J.; Yang, S.; Miao, M.; Kong, X.; Jiao, B. Underexpressed CNDP2 participates in gastric cancer growth inhibition through activating the MAPK signaling pathway. Mol. Med. 2014, 20, 17–28. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, Z.; Yu, H.; Yu, M.; Yuan, K.; Yan, T.; Miao, M.; Shi, H. Up-regulation of CNDP2 facilitates the proliferation of colon cancer. BMC Gastroenterol. 2014, 14, 96. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Yang, H.Q.; Yang, S.Q.; Wang, Y.; Chen, X.J.; Lu, H.S.; Zhao, L.P. CNDP2 Acts as an Activator for Human Ovarian Cancer Growth and Metastasis via the PI3K/AKT Pathway. Technol. Cancer Res. Treat. 2019, 18, 1533033819874773. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, M.; Wang, X.; Ding, S.; Lv, J.; Gao, D.; Wang, Z.; Niu, Z. Ubenimex attenuates acquired sorafenib resistance in renal cell carcinoma by inhibiting Akt signaling in a lipophagy associated mechanism. Oncotarget 2016, 7, 79141–79153. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Liu, S.; Zhao, Q.; Liang, X.; Ma, Z.; Gupta, P.K.; Zhao, M.; Wang, A. Autophagy flux inhibition, G2/M cell cycle arrest and apoptosis induction by ubenimex in glioma cell lines. Oncotarget 2017, 8, 107730–107743. [Google Scholar] [CrossRef]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid. Redox Signal. 2020, 32, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.G.; Hayes, J.D. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 2011, 43, 92–137. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Lee, S.; Lee, G.T.; Woo, H.A.; Choi, E.J.; Rhee, S.G. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid. Redox Signal. 2010, 12, 1235–1246. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Fisher, A.B.; Vasquez-Medina, J.P.; Dodia, C.; Sorokina, E.M.; Tao, J.Q.; Feinstein, S.I. Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes. Redox Biol. 2018, 14, 41–46. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione peroxidase 4: A new player in neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef]
- Brigelius, R.; Muckel, C.; Akerboom, T.P.; Sies, H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem. Pharmacol. 1983, 32, 2529–2534. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Colombo, R.; Milzani, A. S-glutathionylation in protein redox regulation. Free Radic. Biol. Med. 2007, 43, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc. Natl. Acad. Sci. USA 1976, 73, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.T.; Branco, V.; Vale, F.F.; Coppo, L. Glutaredoxin: Discovery, redox defense and much more. Redox Biol. 2021, 43, 101975. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. Redox control of neural function: Background, mechanisms, and significance. Antioxid. Redox Signal. 2006, 8, 1941–1970. [Google Scholar] [CrossRef] [PubMed]
- Sabens Liedhegner, E.A.; Gao, X.H.; Mieyal, J.J. Mechanisms of altered redox regulation in neurodegenerative diseases--focus on S--glutathionylation. Antioxid. Redox Signal. 2012, 16, 543–566. [Google Scholar] [CrossRef]
- Kenchappa, R.S.; Diwakar, L.; Boyd, M.R.; Ravindranath, V. Thioltransferase (glutaredoxin) mediates recovery of motor neurons from excitotoxic mitochondrial injury. J. Neurosci. 2002, 22, 8402–8410. [Google Scholar] [CrossRef]
- Diwakar, L.; Kenchappa, R.S.; Annepu, J.; Ravindranath, V. Downregulation of glutaredoxin but not glutathione loss leads to mitochondrial dysfunction in female mice CNS: Implications in excitotoxicity. Neurochem. Int. 2007, 51, 37–46. [Google Scholar] [CrossRef]
- Saeed, U.; Durgadoss, L.; Valli, R.K.; Joshi, D.C.; Joshi, P.G.; Ravindranath, V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: Implication in neurodegenerative diseases. PLoS One 2008, 3, e2459. [Google Scholar] [CrossRef]
- Arodin, L.; Miranda-Vizuete, A.; Swoboda, P.; Fernandes, A.P. Protective effects of the thioredoxin and glutaredoxin systems in dopamine-induced cell death. Free Radic. Biol. Med. 2014, 3, 328–336. [Google Scholar] [CrossRef]
- Johnson, W.M.; Golczak, M.; Choe, K.; Curran, P.L.; Miller, O.G.; Yao, C.; Wang, W.; Lin, J.; Milkovic, N.M.; Ray, A.; et al. Regulation of DJ-1 by Glutaredoxin 1 in Vivo: Implications for Parkinson’s Disease. Biochemistry 2016, 55, 4519–4532. [Google Scholar] [CrossRef]
- Romero, J.I.; Hanschmann, E.M.; Gellert, M.; Eitner, S.; Holubiec, M.I.; Blanco-Calvo, E.; Lillig, C.H.; Capani, F. Thioredoxin 1 and glutaredoxin 2 contribute to maintain the phenotype and integrity of neurons following perinatal asphyxia. Biochim. Biophys. Acta 2015, 1850, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, S.; Saeed, U.; Ramakrishnan, S.; Koumar, R.C.; Ravindranath, V. Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res. 2007, 1185, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Ray, A.; Bapat, D.; Diwakar, L.; Kommaddi, R.P.; Schneider, B.L.; Hirsch, E.C.; Ravindranath, V. Glutaredoxin 1 Downregulation in the Substantia Nigra Leads to Dopaminergic Degeneration in Mice. Mov. Disord. 2020, 35, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, D.W.; Shin, M.J.; Yeo, H.J.; Yeo, E.J.; Lee, L.R.; Song, Y.; Kim, D.S.; Han, K.H.; Park, J.; et al. PEP-1-GLRX1 Reduces Dopaminergic Neuronal Cell Loss by Modulating MAPK and Apoptosis Signaling in Parkinson’s Disease. Molecules 2021, 26, 3329. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Andersen, J.K. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: Implications for Parkinson’s disease. Antioxid. Redox Signal. 2009, 11, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Lepka, K.; Volbracht, K.; Bill, E.; Schneider, R.; Rios, N.; Hildebrandt, T.; Ingwersen, J.; Prozorovski, T.; Lillig, C.H.; van Horssen, J.; et al. Iron-sulfur glutaredoxin 2 protects oligodendrocytes against damage induced by nitric oxide release from activated microglia. Glia 2017, 65, 1521–1534. [Google Scholar] [CrossRef]
- Gorelenkova Miller, O.; Behring, J.B.; Siedlak, S.L.; Jiang, S.; Matsui, R.; Bachschmid, M.M.; Zhu, X.; Mieyal, J.J. Upregulation of Glutaredoxin-1 Activates Microglia and Promotes Neurodegeneration: Implications for Parkinson’s Disease. Antioxid. Redox Signal. 2016, 25, 967–982. [Google Scholar] [CrossRef]
- Johnson, W.M.; Yao, C.; Siedlak, S.L.; Wang, W.; Zhu, X.; Caldwell, G.A.; Wilson-Delfosse, A.L.; Mieyal, J.J.; Chen, S.G. Glutaredoxin deficiency exacerbates neurodegeneration in C. elegans models of Parkinson’s disease. Hum. Mol. Genet. 2015, 24, 1322–1335. [Google Scholar] [CrossRef]
- Kommaddi, R.P.; Tomar, D.S.; Karunakaran, S.; Bapat, D.; Nanguneri, S.; Ray, A.; Schneider, B.L.; Nair, D.; Ravindranath, V. Glutaredoxin1 Diminishes Amyloid Beta-Mediated Oxidation of F-Actin and Reverses Cognitive Deficits in an Alzheimer’s Disease Mouse Model. Antioxid Redox. Signal. 2019, 31, 1321–1338. [Google Scholar] [CrossRef]
- Akterin, S.l; Cowburn, R.F.; Miranda-Vizuete, A.; Jiménez, A.; Bogdanovic, N.; Winblad, B.; Cedazo-Minguez, A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell. Death Differ. 2006, 13, 1454–1465. [Google Scholar] [CrossRef]
- Ferri, A.; Fiorenzo, P.; Nencini, M.; Cozzolino, M.; Pesaresi, M.G.; Valle, C.; Sepe, S.; Moreno, S.; Carrì, M.T. Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity. Hum. Mol. Genet. 2010, 19, 4529–4542. [Google Scholar] [CrossRef]
- Sau, A.; Pellizzari Tregno, F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Brandmann, M.; Hohnholt, M.C.; Blumrich, E.M. Glutathione-Dependent Detoxification Processes in Astrocytes. Neurochem. Res. 2015, 40, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G. The use of glutathione transferase-knockout mice as pharmacological and toxicological models. Expert Opin. Drug Metab. Toxicol. 2007, 3, 421–433. [Google Scholar] [CrossRef]
- Henderson, C.J.; Wolf, C.R. Knockout and transgenic mice in glutathione transferase research. Drug Metab. Rev. 2011, 43, 152–164. [Google Scholar] [CrossRef]
- Smeyne, M.; Smeyne, R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013, 62, 13–25. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Austen, K.F. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv. Immunol. 2019, 142, 65–84. [Google Scholar] [CrossRef]
- Gelosa, P.; Colazzo, F.; Tremoli, E.; Sironi, L.; Castiglioni, L. Cysteinyl Leukotrienes as Potential Pharmacological Targets for Cerebral Diseases. Mediators Inflamm. 2017, 2017, 3454212. [Google Scholar] [CrossRef]
- Lam, B.K. Leukotriene C(4) synthase. Prostaglandins Leukot. Essent. Fatty Acids. 2003, 69, 111–116. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhang, S.; Li, C.; Zhang, L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: Implications for cerebral ischemia and neurodegenerative diseases. Neurobiol. Aging 2020, 87, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Hausladen, A.; Wang, Y.J.; Zhang, G.F.; Stomberski, C.; Brunengraber, H.; Hess, D.T.; Stamler, J.S. Identification of S-nitroso-CoA reductases that regulate protein S-nitrosylation. Proc. Natl. Acad. Sci. USA 2014, 111, 18572–18577. [Google Scholar] [CrossRef] [PubMed]
- Sircar, E.; Rai, S.R.; Wilson, M.A.; Schlossmacher, M.G.; Sengupta, R. Neurodegeneration: Impact of S-nitrosylated Parkin, DJ-1 and PINK1 on the pathogenesis of Parkinson’s disease. Arch. Biochem. Biophys. 2021, 704, 108869. [Google Scholar] [CrossRef]
- Seth, D.; Hess, D.T.; Hausladen, A.; Wang, L.; Wang, Y.J.; Stamler, J.S. A Multiplex Enzymatic Machinery for Cellular Protein S-nitrosylation. Mol. Cell 2018, 69, 451–464.e6. [Google Scholar] [CrossRef]
- Stomberski, C.T.; Hess, D.T.; Stamler, J.S. Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling. Antioxid. Redox Signal. 2019, 30, 1331–1351. [Google Scholar] [CrossRef]
- Benhar, M.; Forrester, M.T.; Stamler, J.S. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 721–732. [Google Scholar] [CrossRef]
- Jensen, D.E.; Belka, G.K.; Du Bois, G.C. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 1998, 331, 659–668. [Google Scholar] [CrossRef]
- Liu, L.; Hausladen, A.; Zeng, M.; Que, L.; Heitman, J.; Stamler, J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001, 410, 490–494. [Google Scholar] [CrossRef]
- Bateman, R.L.; Rauh, D.; Tavshanjian, B.; Shokat, K.M. Human carbonyl reductase 1 is an S-nitrosoglutathione reductase. J. Biol. Chem. 2008, 283, 35756–35762. [Google Scholar] [CrossRef]
- Rizza, S.; Di Leo, L.; Pecorari, C.; Giglio, P.; Faienza, F.; Montagna, C.; Maiani, E.; Puglia, M.; Bosisio, F.M.; Petersen, T.S.; et al. GSNOR deficiency promotes tumor growth via FAK1 S-nitrosylation. Cell Rep. 2023, 42, 111997. [Google Scholar] [CrossRef]
- Nakamura, T.; Oh, C.K.; Zhang, X.; Lipton, S.A. Protein S-nitrosylation and oxidation contribute to protein misfolding in neurodegeneration. Free Radic. Biol. Med. 2021, 172, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Vrettou, S.; Wirth, B. S-Glutathionylation and S-Nitrosylation in Mitochondria: Focus on Homeostasis and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 15849. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; Park, M.T.M.; Jeon, P.; Limongi, R.; Yang, K.; Sawa, A.; Théberge, J. Is There a Glutathione Centered Redox Dysregulation Subtype of Schizophrenia? Antioxidants 2021, 10, 1703. [Google Scholar] [CrossRef]
- Peeples, E.S.; Genaro-Mattos, T.C. Ferroptosis: A Promising Therapeutic Target for Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2022, 23, 7420. [Google Scholar] [CrossRef] [PubMed]
- Gleitze, S.; Paula-Lima, A.; Núñez, M.T.; Hidalgo, C. The calcium-iron connection in ferroptosis-mediated neuronal death. Free Radic. Biol. Med. 2021, 175, 28–41. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, X.; Wu, M.; Cao, X.; Bao, W.; Zhu, L.Q. Ferroptosis, a Potential Therapeutic Target in Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 9, 704298. [Google Scholar] [CrossRef]
- Jakaria, M.; Belaidi, A.A.; Bush, A.I.; Ayton, S. Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J. Neurochem. 2021, 159, 804–825. [Google Scholar] [CrossRef]
- Crapper-McLachlan., D.R.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.; Bernardi, J.; Costa, L.; Fiuza, T.; Brandão, R.; Ribeiro, M.F.; Amaral, J.D.; Rodrigues, C.M.P.; Pereira, M.E. N-acetylcysteine treatment attenuates the cognitive impairment and synaptic plasticity loss induced by streptozotocin. Chem. Biol. Interact. 2017, 272, 37–46. [Google Scholar] [CrossRef] [PubMed]
- More, J.; Galusso, N.; Veloso, P.; Montecinos, L.; Finkelstein, J.P.; Sanchez, G.; Bull, R.; Valdés, J.L.; Hidalgo, C.; Paula-Lima, A. N-Acetylcysteine Prevents the Spatial Memory Deficits and the Redox-Dependent RyR2 Decrease Displayed by an Alzheimer’s Disease Rat Model. Front. Aging Neurosci. 2018, 10, 399. [Google Scholar] [CrossRef]
- Stapper, Z.A.; Jahn, T.R. Changes in Glutathione Redox Potential Are Linked to Aβ42-Induced Neurotoxicity. Cell Rep. 2018, 24, 1696–1703. [Google Scholar] [CrossRef]
- Guiney, S.J.; Adlard, P.A.; Bush, A.I.; Finkelstein, D.I.; Ayton, S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem. Int. 2017, 104, 34–48. [Google Scholar] [CrossRef]
- Lin, K.J.; Chen, S.D.; Lin, K.L.; Liou, C.W.; Lan, M.Y.; Chuang, Y.C.; Wang, P.W.; Lee, J.J.; Wang, F.S.; Lin, H.Y.; et al. Iron Brain Menace: The Involvement of Ferroptosis in Parkinson Disease. Cells 2022, 11, 3829. [Google Scholar] [CrossRef]
- Thapa, K.; Khan, H.; Kanojia, N.; Singh, T.G.; Kaur, A.; Kaur, G. Therapeutic Insights on Ferroptosis in Parkinson’s disease. Eur. J. Pharmacol. 2022, 930, 175133. [Google Scholar] [CrossRef]
- Bisaglia, M.; Soriano, M.E.; Arduini, I.; Mammi, S.; Bubacco, L. Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: Implications for mitochondrial dysfunction in Parkinson disease. Biochim. Biophys. Acta 2010, 1802, 699–706. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Asanuma, M.; Miyazaki, I. Glutathione and Related Molecules in Parkinsonism. Int. J. Mol. Sci. 2021, 22, 8689. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Ramírez, I.; Saniger, J.M.; Rivas-Arancibia, S. 5-S-cysteinyl-dopamine, a neurotoxic endogenous metabolite of dopamine: Implications for Parkinson’s disease. Neurochem. Int. 2019, 129, 104514. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; Bruno, M.K.; Hennig, G.E.; Horton, R.A.; Roberts, J.C.; Cohen, S.D. Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity in CD-1 mice: I. Enhancement of acetaminophen nephrotoxicity by acetaminophen-cysteine. Toxicol. Appl. Pharmacol. 2005, 202, 151–159. [Google Scholar] [CrossRef]
- Kim, K. Glutathione in the Nervous System as a Potential Therapeutic Target to Control the Development and Progression of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Valle, C.; Carrì, M.T. Cysteine Modifications in the Pathogenesis of ALS. Front. Mol. Neurosci. 2017, 10, 5. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, X.; Wang, X.; Shi, X.; Ma, L.; Zhang, Y.; Zhou, T.; Zhao, C.; Zhang, X.; Fan, B.; et al. Edaravone Modulates Neuronal GPX4/ACSL4/5-LOX to Promote Recovery After Spinal Cord Injury. Front. Cell Dev. Biol. 2022, 10, 849854. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tanaka, M.; Yuki, S.; Hirai, M.; Yamamoto, Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J. Clin. Biochem. Nutr. 2018, 62, 20–38. [Google Scholar] [CrossRef]
- Spasić, S.; Nikolić-Kokić, A.; Miletić, S.; Oreščanin-Dušić, Z.; Spasić, M.B.; Blagojević, D.; Stević, Z. Edaravone May Prevent Ferroptosis in ALS. Curr. Drug Targets 2020, 21, 776–780. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I.; Dominy, J.E., Jr.; Simmons, C.R.; Hirschberger, L.L. Cysteine dioxygenase: A robust system for regulation of cellular cysteine levels. Amino Acids 2009, 37, 55–63. [Google Scholar] [CrossRef]
- Wright, C.E.; Tallan, H.H.; Lin, Y.Y.; Gaull, G.E. Taurine: Biological update. Annu. Rev. Biochem. 1986, 55, 427–453. [Google Scholar] [CrossRef]
- Chen, Q.; Konrad, C.; Sandhu, D.; Roychoudhury, D.; Schwartz, B.I.; Cheng, R.R.; Bredvik, K.; Kawamata, H.; Calder, E.L.; Studer, L.; et al. Accelerated transsulfuration metabolically defines a discrete subclass of amyotrophic lateral sclerosis patients. Neurobiol. Dis. 2020, 144, 105025. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Török, K.; Feuer, L.; Gulyás, J.; Somogyi, J. γ-Glutamyltransferase in the brain and its role in formation of γ-L-glutamyl-taurine. Prog. Clin. Biol. Res. 1985, 179, 115–125. [Google Scholar] [PubMed]
- Kobayashi, S.; Tokairin, Y.; Miyakoshi, T.; Saito, T.; Nagaoka, T.; Ikeda, Y.; Fujii, J.; Konno, H. Quantitative analysis of γ-glutamylpeptides by liquid chromatography-mass spectrometry and application for γ-glutamyltransferase assays. Anal. Biochem. 2019, 578, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ikeda, Y.; Shigeno, Y.; Konno, H.; Fujii, J. γ-Glutamylcysteine synthetase and γ-glutamyl transferase as differential enzymatic sources of γ-glutamylpeptides in mice. Amino Acids 2020, 52, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Varga, V.; Janáky, R.; Marnela, K.M.; Saransaari, P.; Oja, S.S. Interactions of gamma-L-glutamyltaurine with excitatory aminoacidergic neurotransmission. Neurochem. Res. 1994, 19, 243–248. [Google Scholar] [CrossRef]
- Uemura, S.; Ienaga, K.; Higashiura, K.; Kimura, H. Gamma-glutamyltaurine has potent and long-lasting antiepileptic action as demonstrated by intra-amygdaloid injection in amygdala-kindled rats. Brain Res. 1992, 594, 347–350. [Google Scholar] [CrossRef]
- Bittner, S.; Win, T.; Gupta, R. γ-L-glutamyltaurine. Amino Acids 2005, 28, 343–356. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S. Ferroptosis caused by cysteine insufficiency and oxidative insult. Free Radic. Res. 2020, 54, 969–980. [Google Scholar] [CrossRef]
- Orlowski, M.; Wilk, S. Intermediates of the gamma-glutamyl cycle in mouse tissues. Influence of administration of amino acids on pyrrolidone carboxylate and gamma-glutamyl amino acids. Eur. J. Biochem. 1975, 53, 581–590. [Google Scholar]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef]
- Kobayashi, S.; Lee, J.; Takao, T.; Fujii, J. Increased ophthalmic acid production is supported by amino acid catabolism under fasting conditions in mice. Biochem. Biophys. Res. Commun. 2017, 491, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Sugimoto, M.; Honma, M.; Mori, M.; Igarashi, K.; Kashikura, K.; Ikeda, S.; Hirayama, A.; Yamamoto, T.; Yoshida, H.; et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J. Hepatol. 2011, 55, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef]
- Homma, T.; Kobayashi, S.; Fujii, J. Methionine Deprivation Reveals the Pivotal Roles of Cell Cycle Progression in Ferroptosis That Is Induced by Cysteine Starvation. Cells 2022, 11, 1603. [Google Scholar] [CrossRef]
- Maclean, K.N.; Jiang, H.; Aivazidis, S.; Kim, E.; Shearn, C.T.; Harris, P.S.; Petersen, D.R.; Allen, R.H.; Stabler, S.P.; Roede, J.R. Taurine treatment prevents derangement of the hepatic γ-glutamyl cycle and methylglyoxal metabolism in a mouse model of classical homocystinuria: Regulatory crosstalk between thiol and sulfinic acid metabolism. FASEB J. 2018, 32, 1265–1280. [Google Scholar] [CrossRef]
- Li, H.Q.; Xia, S.N.; Xu, S.Y.; Liu, P.Y.; Gu, Y.; Bao, X.Y.; Xu, Y.; Cao, X. γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress. Oxid. Med. Cell Longev. 2021, 2021, 2961079. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta 2015, 1852, 792–804. [Google Scholar] [CrossRef]
- Hewson, C.K.; Capraro, A.; Wong, S.L.; Pandzic, E.; Zhong, L.; Fernando, B.S.M.; Awatade, N.T.; Hart-Smith, G.; Whan, R.M.; Thomas, S.R.; et al. Novel Antioxidant Therapy with the Immediate Precursor to Glutathione, γ-Glutamylcysteine (GGC), Ameliorates LPS-Induced Cellular Stress in In Vitro 3D-Differentiated Airway Model from Primary Cystic Fibrosis Human Bronchial Cells. Antioxidants 2020, 9, 1204. [Google Scholar] [CrossRef]
- Bi, A.; Wang, Y.; Chen, L.; Yin, Z.; Luo, L. γ-Glutamylcysteine attenuates amyloid-β oligomers-induced neuroinflammation in microglia via blocking NF-κB signaling pathway. Chem. Biol. Interact. 2022, 363, 110019. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, X.; Bi, X.; Lu, S.; Liu, X.; Yang, C.; Shi, Y.; Luo, L.; Yin, Z. γ-Glutamylcysteine rescues mice from TNBS-driven inflammatory bowel disease through regulating macrophages polarization. Inflamm. Res. 2023, 72, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Li, B.; Yao, H.; Zarka, M.; Welch, J.; Sachdev, P.; Bridge, W.; Braidy, N. Supplementation with γ-glutamylcysteine (γ-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem. Int. 2021, 144, 104931. [Google Scholar] [CrossRef]
- Zhang, R.; Lei, J.; Chen, L.; Wang, Y.; Yang, G.; Yin, Z.; Luo, L. γ-Glutamylcysteine Exerts Neuroprotection Effects against Cerebral Ischemia/Reperfusion Injury through Inhibiting Lipid Peroxidation and Ferroptosis. Antioxidants 2022, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.; Bridge, W. Glutamate cysteine ligase and the age-related decline in cellular glutathione: The therapeutic potential of γ-glutamylcysteine. Arch. Biochem. Biophys. 2016, 593, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zarka, M.H.; Bridge, W.J. Oral administration of γ-glutamylcysteine increases intracellular glutathione levels above homeostasis in a randomised human trial pilot study. Redox Biol. 2017, 11, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Fernandez-Fernandez, S.; Bobo-Jimenez, V.; Escobar, J.; Sastre, J.; Almeida, A.; Bolaños, J.P. γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat. Commun. 2012, 3, 718. [Google Scholar] [CrossRef]
- Wang, M.; Yao, Y.; Kuang, D.; Hampson, D.R. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 2006, 281, 8864–8870. [Google Scholar] [CrossRef]
- Goralski, T.; Ram, J.L. Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives. Int. J. Mol. Sci. 2022, 23, 717. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Tfelt-Hansen, J.; Chattopadhyay, N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J. Neurosci Res. 2010, 88, 2073–2082. [Google Scholar] [CrossRef]
- Brennan, S.C.; Davies, T.S.; Schepelmann, M.; Riccardi, D. Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing: Control of taste modulation and intestinal hormone secretion. Br. J. Nutr. 2014, 111, S16–S22. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. J Comprehensive Review of γ-Glutamyl Peptides (γ-GPs) and Their Effect on Inflammation Concerning Cardiovascular Health. Agric. Food Chem. 2022, 70, 7851–7870. [Google Scholar] [CrossRef] [PubMed]
- Giudice, M.L.; Mihalik, B.; Dinnyés, A.; Kobolák, J. The Nervous System Relevance of the Calcium Sensing Receptor in Health and Disease. Molecules 2019, 24, 2546. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Brandi, M.L. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13, 1059369. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Armato, U.; Gardenal, E.; Gui, L.; Dal Prà, I. Amyloid β-Exposed Human Astrocytes Overproduce Phospho-Tau and Overrelease It within Exosomes, Effects Suppressed by Calcilytic NPS 2143-Further Implications for Alzheimer’s Therapy. Front. Neurosci. 2017, 11, 217. [Google Scholar] [CrossRef]
- Dal Prà, I.; Armato, U.; Chiarini, A. Family C G-Protein-Coupled Receptors in Alzheimer’s Disease and Therapeutic Implications. Front. Pharmacol. 2019, 10, 1282. [Google Scholar] [CrossRef]

| Disease | Description on GSH and Related Subjects | Ref. |
|---|---|---|
| Alzheimer’s disease (AD) | ||
| Overviewing roles of glutathione function | [19] | |
| Possible involvement of ferroptosis | [199,200,201] | |
| Protection by glutaredoxin | [169,170] | |
| Investigation of protective roles of vitamin E | [202] | |
| Protection by N-Acetylcysteine | [204] | |
| Roles of the calcium-sensing receptor (CaSR) | [255,256] | |
| Parkinson’s disease (PD) | ||
| Reviewing roles of glutathione function | [177,185,211] | |
| Possible involvement of ferroptosis | [206,207,208] | |
| Toxic roles of dopamine | [209,210,212] | |
| Roles of DJ-1 in association with glutaredoxin | [124,160] | |
| MAPK and apoptosis signaling | [164] | |
| Roles of glutaredoxin | [160,165,167,168] | |
| Amyotrophic lateral sclerosis (ALS) | ||
| Protection by glutathione | [214] | |
| Cysteine modifications in the pathogenesis | [215,221] | |
| Protection by edaravone | [217,218] | |
| Involvement of ferroptosis | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, J.; Osaki, T.; Soma, Y.; Matsuda, Y. Critical Roles of the Cysteine–Glutathione Axis in the Production of γ-Glutamyl Peptides in the Nervous System. Int. J. Mol. Sci. 2023, 24, 8044. https://doi.org/10.3390/ijms24098044
Fujii J, Osaki T, Soma Y, Matsuda Y. Critical Roles of the Cysteine–Glutathione Axis in the Production of γ-Glutamyl Peptides in the Nervous System. International Journal of Molecular Sciences. 2023; 24(9):8044. https://doi.org/10.3390/ijms24098044
Chicago/Turabian StyleFujii, Junichi, Tsukasa Osaki, Yuya Soma, and Yumi Matsuda. 2023. "Critical Roles of the Cysteine–Glutathione Axis in the Production of γ-Glutamyl Peptides in the Nervous System" International Journal of Molecular Sciences 24, no. 9: 8044. https://doi.org/10.3390/ijms24098044
APA StyleFujii, J., Osaki, T., Soma, Y., & Matsuda, Y. (2023). Critical Roles of the Cysteine–Glutathione Axis in the Production of γ-Glutamyl Peptides in the Nervous System. International Journal of Molecular Sciences, 24(9), 8044. https://doi.org/10.3390/ijms24098044





