Cadmium Absorption in Various Genotypes of Rice under Cadmium Stress
Abstract
1. Introduction
2. Results
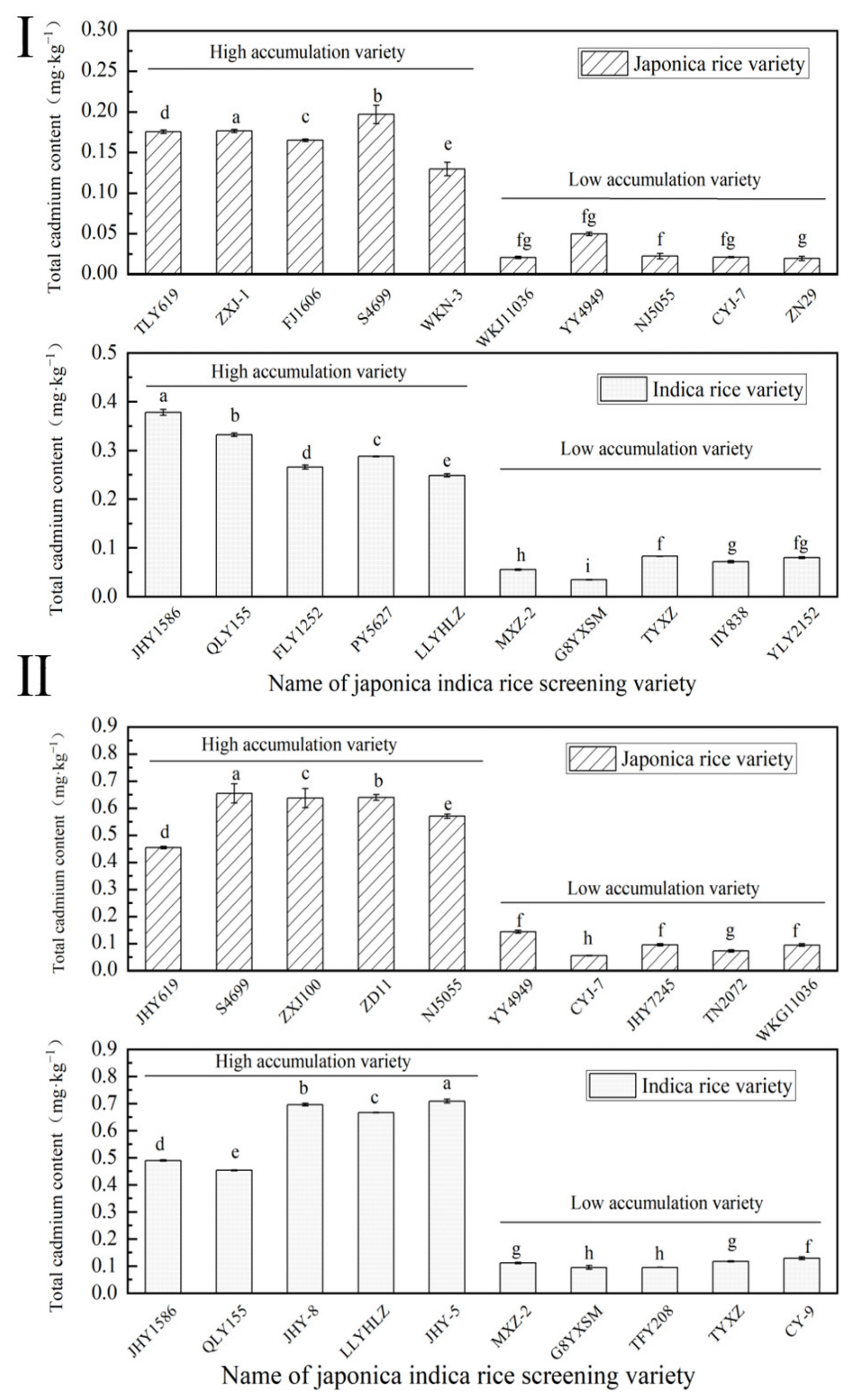
2.1. Rice Variety Screening
2.1.1. Screening Results of Rice Varieties in 2021
2.1.2. Screening Results of Rice Varieties in 2022
2.2. Cd Kinetic Uptake in Various Organs of Rice
2.3. Malondialdehyde (MDA) Content in Rice
2.4. Rice Antioxidant Enzyme System
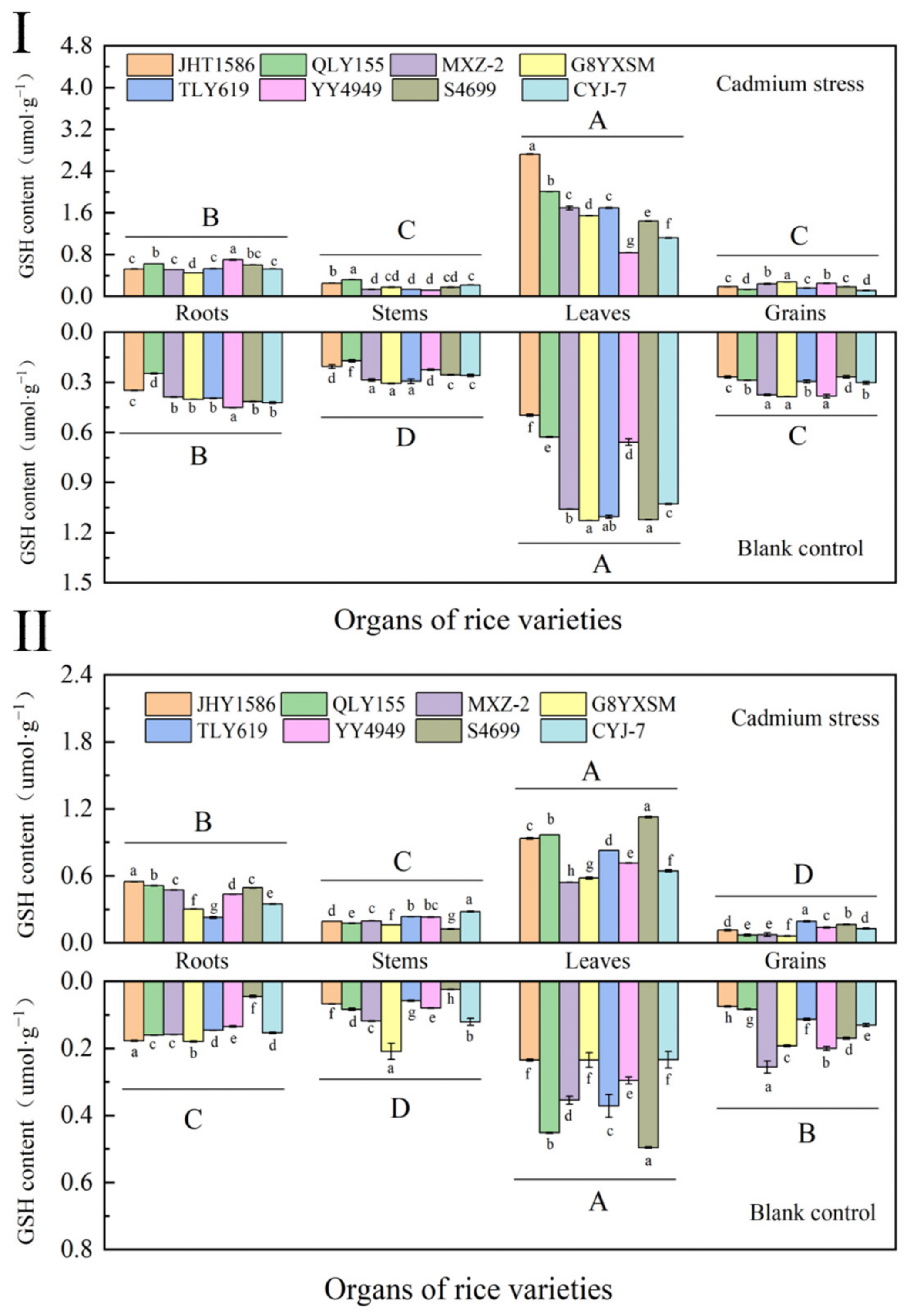
2.4.1. Determination of Reduced Glutathione (GSH) in Rice at Various Periods
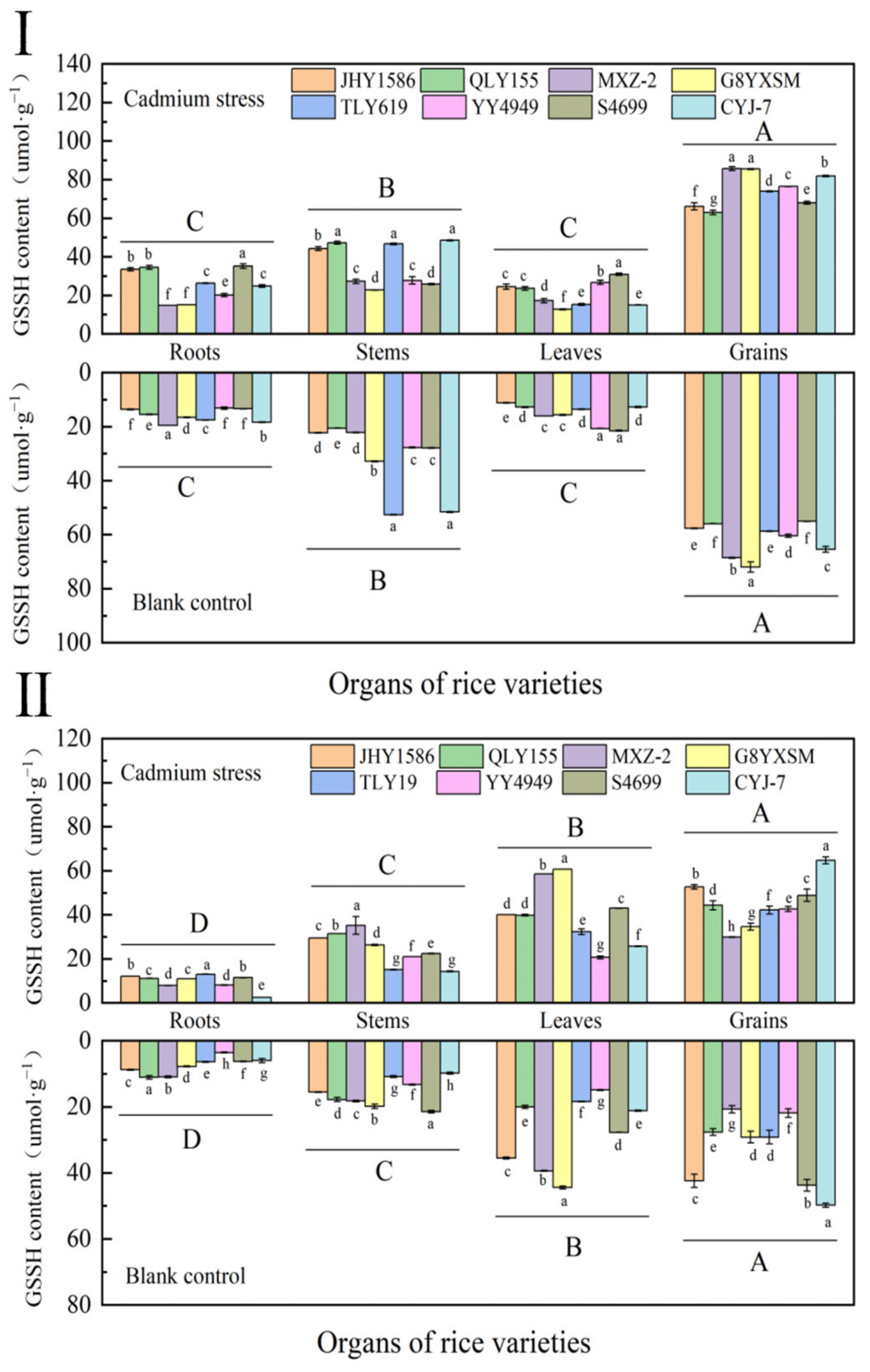
2.4.2. Determination of Oxidized Glutathione (GSSH) in Rice at Various Periods
2.4.3. Determination of Rice Peroxidase (POD) in Various Periods
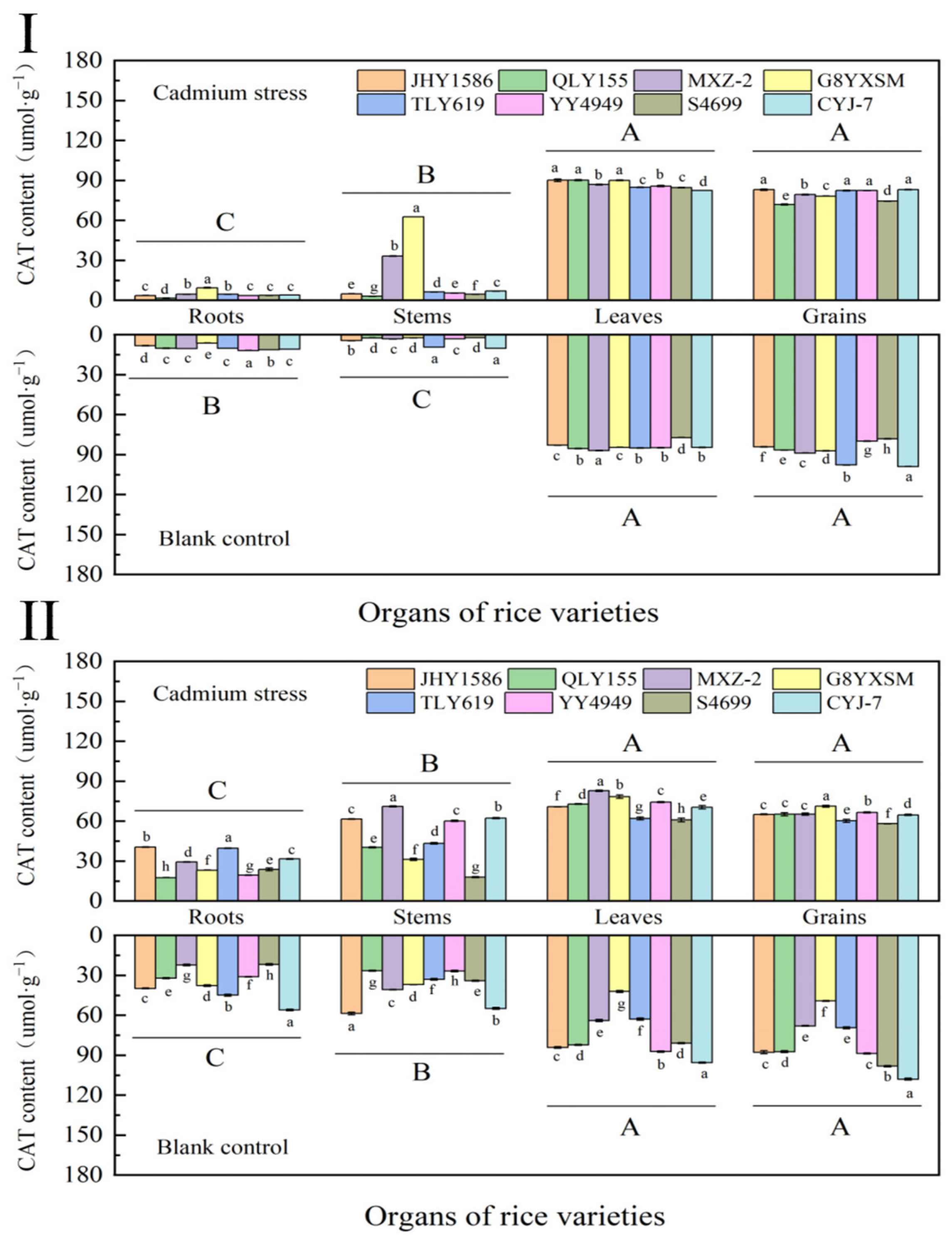
2.4.4. Determination of Rice Catalase (CAT) in Various Periods
2.4.5. Results of Superoxide Dismutase (SOD) Measurements in Rice at Various Periods
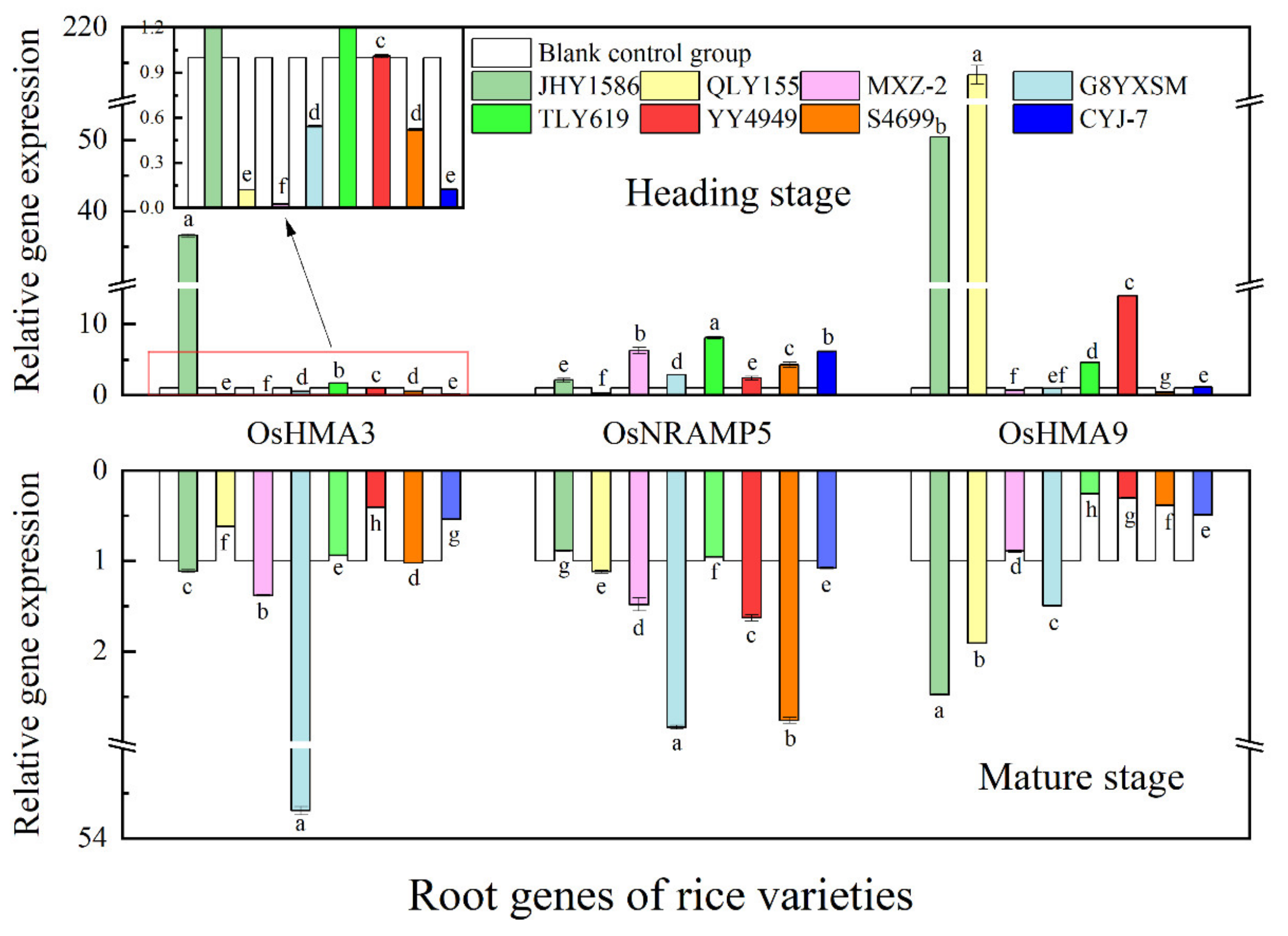
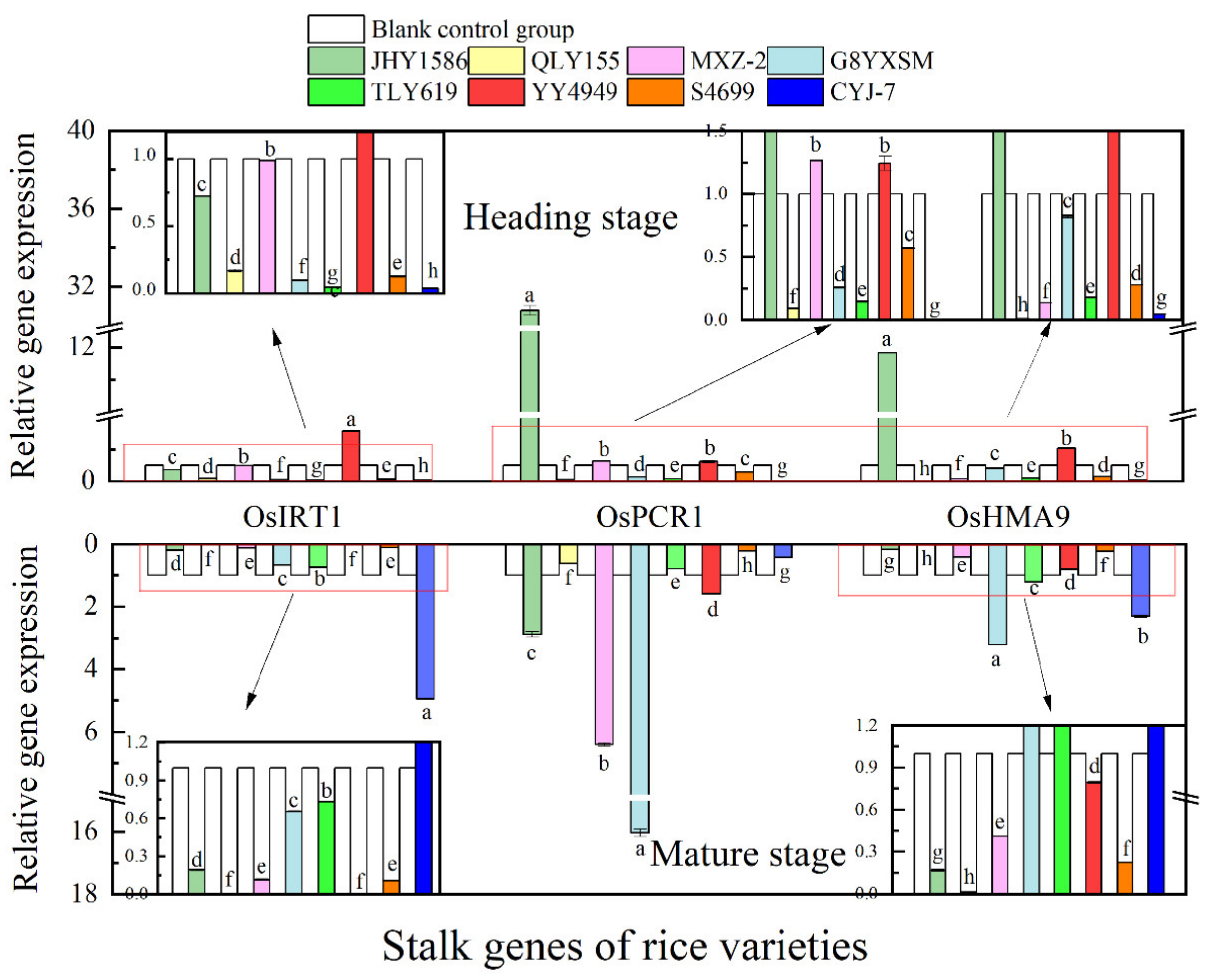
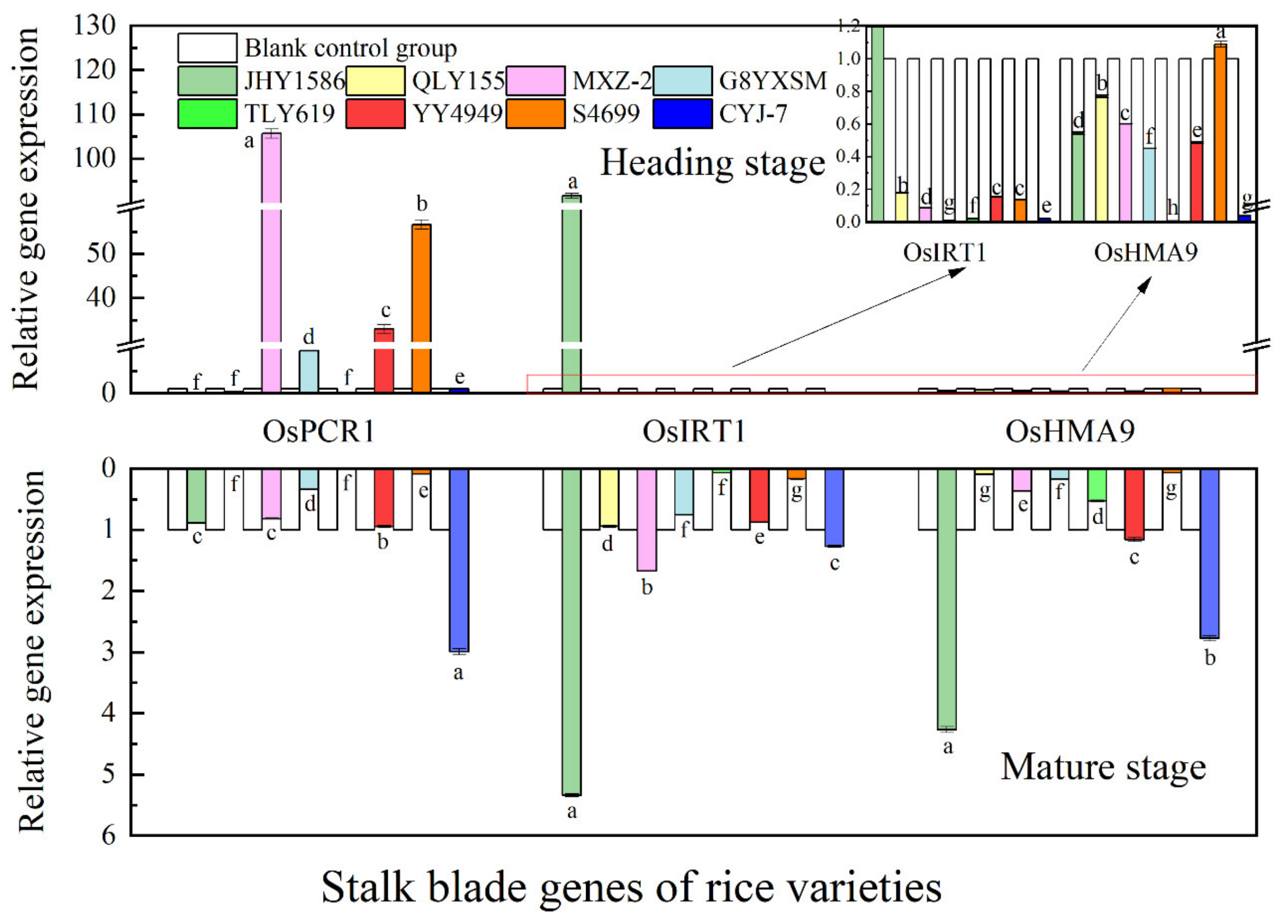
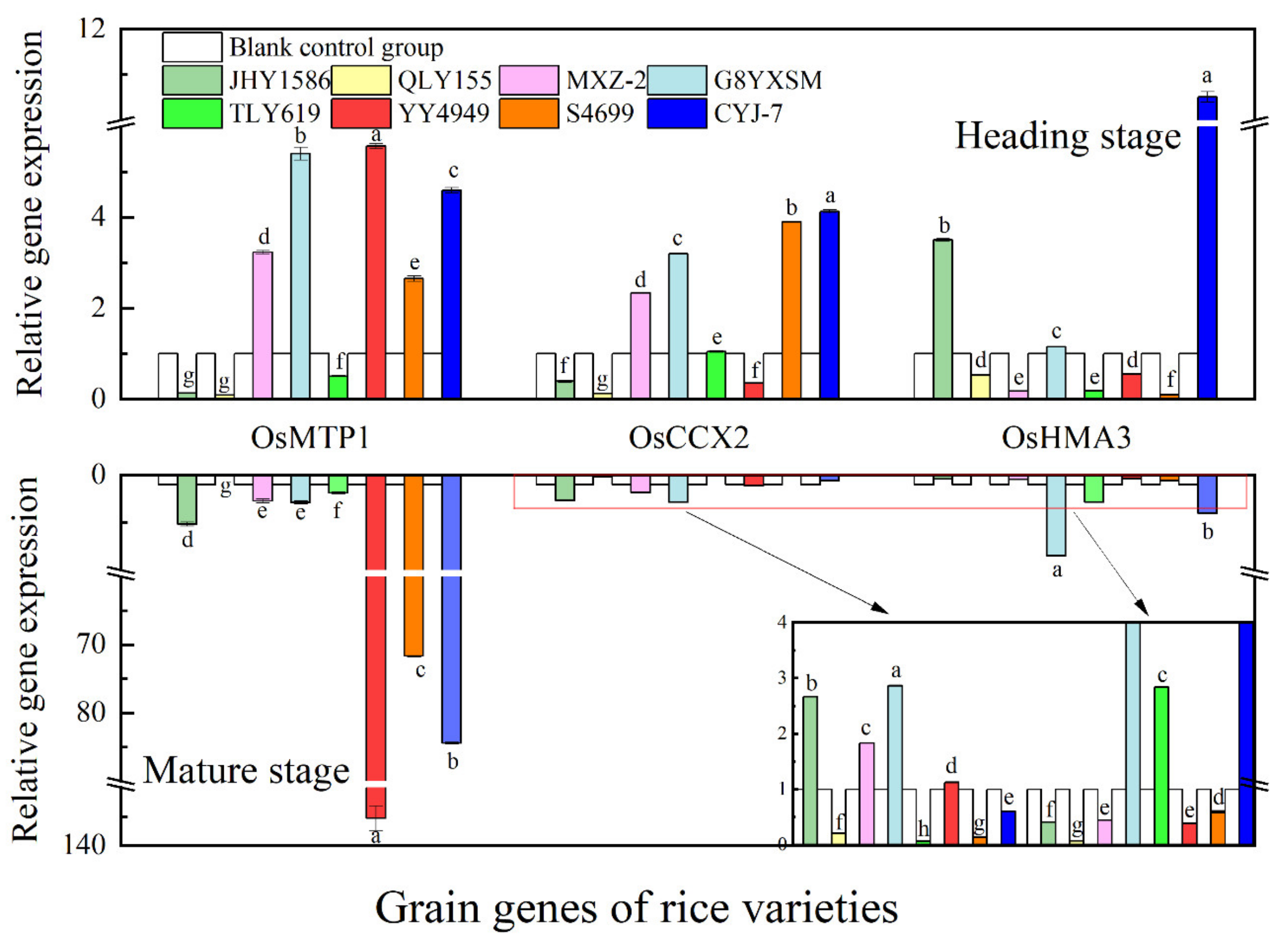
2.5. Analysis of rice gene expression by RT-qPCR
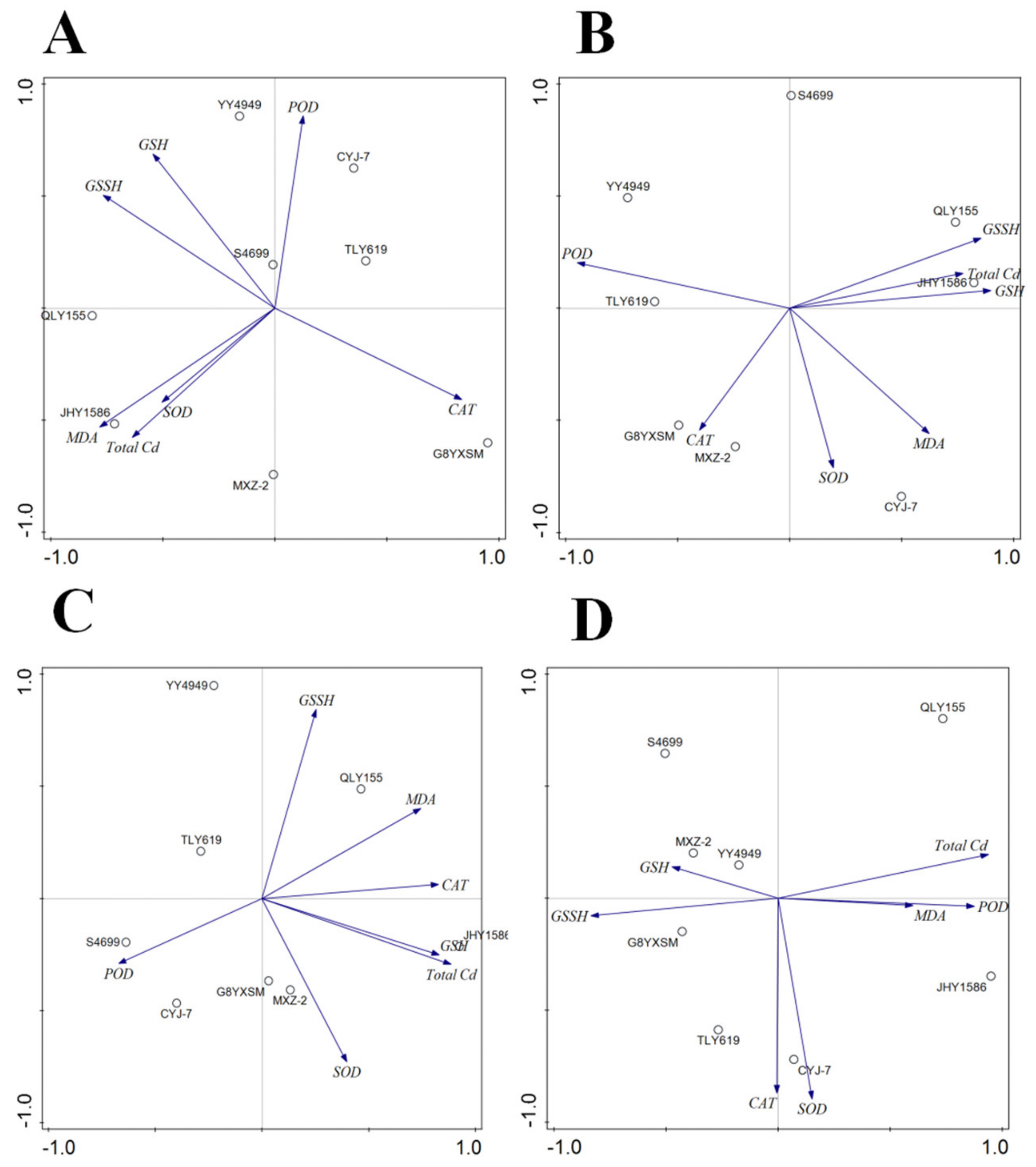
2.6. Correlation Analysis
2.6.1. Correlation Analysis of Total Cd and Enzymatic Activities in Various Organs of Rice at Different Periods
2.6.2. Correlation Analysis between Total Cd and Enzyme Activities of Rice Varieties under Different Factors
3. Discussion
3.1. Low-Cd Rice Variety Screening
3.2. Response of Enzyme Contents to Cadmium Absorption and Transport
3.3. Changes in Expression of Cd Transporter Genes in Rice in Response to Cd Stress
4. Materials and Methods
4.1. Experimental Material Cultivation and Screening
4.2. Determination of Total Cadmium Content and Antioxidant Enzyme Contents
4.3. Gene Expression Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemens, S.; Ma, J.F. Toxicheavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar]
- Wang, R.Y.; Sang, P.T.; Guo, Y.H.; Jin, P.; Cheng, Y.L.; Yu, H.; Xie, Y.F.; Yao, W.R.; Qian, H. Cadmium in food: Source, distribution and removal. Food Chem. 2022, 405, 134666. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, Environmental Exposure, And Health Outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Guo, H.; Li, R.Y.; Li, L.Q.; Pan, G.X.; Chang, A.; Joseph, S. Low uptake affinity cultivars with biochar to tackle Cd-tainted rice—A field study over four rice seasons in Hunan, China. Sci. Total Environ. 2016, 541, 1489–4898. [Google Scholar] [CrossRef] [PubMed]
- Mariyo, K.; Satoru, I.; Kazumi, I.; Koichi, C.; Hiroaki, H.; Shuichi, Y.; Tadakatsu, Y. Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2010, 56, 839–847. [Google Scholar]
- Song, Y.; Wang, Y.; Mao, W.F.; Sui, X.X.; Yong, L.; Yang, D.J.; Jiang, G.G.; Zhang, L.; Gong, Y.Y. Dietary cadmium exposure asessentt among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar]
- Gohari, G.; Farhadi, H.; Panahirad, S.; Zareei, E.; Labib, P.; Jafari, H.; Mahdavinia, G.; Hassanpouraghdam, M.B.; Ioannou, A.; Kulak, M.; et al. Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int. J. Biol. Macromol. 2022, 224, 893–907. [Google Scholar]
- Rasheed, R.; Ashraf, M.A.; Ahmad, S.J.N.; Parveen, N.; Hussain, I.; Bashir, R. Taurine regulates ROS metabolism, osmotic adjustment, and nutrient uptake to lessen the effects of alkaline stress on Trifolium alexandrinum L. plants. S. Afr. J. Bot. 2022, 148, 482–498. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar]
- Naseer, H.; Shaukat, K.; Zahra, N.; Hafeez, M.B.; Raza, A.; Nizar, M.; Qazi, M.A.; Ali, Q.; AAl-Huqail, A.; Siddiqui, M.H.; et al. Appraisal of foliar spray of iron and salicylic acid under artificial magnetism on morpho-physiological attributes of pea (Pisum sativum L.) plants. PLoS ONE 2022, 17, e0265654. [Google Scholar]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecule 2023, 28, 1447. [Google Scholar]
- Cai, Y.; Lin, L.; Cheng, W.; Wu, F. Genotypic dependent effect of exogenous glutathione on Cd-induced changes in cadmium and mineral uptake and accumulation in rice grainlings (Oryza sativa). Plant Soil Environ. 2010, 64, 516–525. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Foyer, C.H.; Theodoulou, F.L.; Delrot, S. The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci. 2001, 6, 486–492. [Google Scholar]
- Thanwisai, L.; Janket, A.; Tran, H.T.K.; Siripornadulsil, W.; Siripornadulsil, S. Low Cd-accumulating rice grain production through inoculation of germinating rice grains with a plant growth-promoting endophytic bacterium. Ecotoxicol. Environ. Saf. 2023, 251, 114535. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Alfonso, M.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef] [PubMed]
- Takio, N.; Yadav, M.; Yadav, H.S. Catalase-mediated remediation of environmental pollutants and potential application—A review. Biocatal. Biotransformation 2021, 39, 389–407. [Google Scholar] [CrossRef]
- Jiang, W.; Ye, Q.; Wu, Z.; Zhang, Q.Y.; Wang, L.H.; Liu, J.L.; Hu, X.F.; Guo, D.D.; Wang, X.Q.; Zhang, Z.L.; et al. Analysis of CAT Gene Family and Functional Identification of OsCAT3 in Rice. Genes 2023, 14, 138. [Google Scholar] [CrossRef]
- Fang, J.; Zeng, D.; Xu, T. Preparation of an Environmentally Friendly Rice Seed Coating Agent and Study of Its Mechanism of Action in Seedlings. Sustainability 2023, 15, 869. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Bashir, K.; Senoura, T.; Sugimoto, K.; Ono, K.; Suzui, N.; Kawachi, N.; Ishii, S. From laboratory to field: OsNRAMP5-knockdown rice is a promising candidate for Cd phytoremediation in paddy fields. PLoS ONE 2014, 9, e98816. [Google Scholar]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zeng, M.; Wang, J.; Zeng, Z.; Dai, J.; Xie, Z.; Yang, Y.; Tian, L.; Chen, L.; Li, D. A node-expressed transporter OsCCX2 is involved in grain cadmium accumulation of rice. Front. Plant Sci. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Uraguchi, S.; Kajikawa, M. A rice PHD-finger protein OsTITANIA, is a growth regulator that functions through elevating expression of transporter genes for multiple metals. Plant J. 2018, 96, 997–1006. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Fang, W.; Yuan, J.G.; Yang, Z.Y. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci. Total Environ. 2006, 370, 302–309. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Qi, X.; Huang, L.; Huang, L.; Ye, Z.H. Identification of rice cultivars with low brown rice mixed cadmium and lead contents and their interactions with the micronutrients iron, zinc, nickel and manganese. J. Environ. Sci. 2012, 24, 1790–1798. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Chen, Y.; Jing, H.N.; Wu, P.; Yang, W.T. Selection of rice and maize varieties with low cadmium accumulation and derivation of soil environmental thresholds in karst. Ecotoxicol. Environ. Saf. 2022, 247, 114244. [Google Scholar] [CrossRef]
- Sun, L.; Wang, R.; Tang, W.; Chen, Y.C.; Zhou, J.Q.; Ma, H.R.; Li, S.; Deng, H.B.; Han, L.; Chen, Y.B.; et al. Robust identification of low-Cd rice varieties by boosting the genotypic effect of grain Cd accumulation in combination with marker-assisted selection. J. Hazard. Mater. 2021, 424, 127703. [Google Scholar] [CrossRef]
- Ueno, D.; Koyama, E.; Yamaji, E.; Feng, M.J. Physiological, genetic and molecular characterization of a high-Cd-accumulating rice cultivar, Jarjan. J. Exp. Bot. 2011, 62, 2265–2272. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice grainlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, A.; Ansary, M.M.U.; Watanabe, A.; Fujita, M.; Tran, L.S.P. Hydrogen sulfide modulates cadmium induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef]
- Chen, P.; Yu, T.; Zhou, H. Effects of Se on growth and some physiological characteristics of rice grainling under Cd stress. Guangxi Zhiwu 2002, 22, 277–282. [Google Scholar]
- Shao, G.; Hassan, M.J.; Zhang, X. Effects of Cadmium Stress on Plant Growth and Antioxidative Enzyme System in Different Rice Genotype. Chin. J. Rice Sci. 2004, 18, 239. [Google Scholar]
- Hsu, Y.T.; Kao, C.H. Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul. 2004, 42, 227–238. [Google Scholar] [CrossRef]
- Kanu, A.S.; Ashraf, U.; Mo, Z.; Sabir, S.U.R.; Baggie, I.; Charley, C.S.; Tang, X.R. Calcium amendment improved the performance of fragrant rice and reduced metal uptake under cadmium toxicity. Environ. Sci. Pollut. Res. Int. 2019, 26, 24748–24757. [Google Scholar] [CrossRef] [PubMed]
- Afzal, J.; Saleem, M.H.; Batool, F.; Sabir, S.U.R.; Baggie, I.; Charley, C.S.; Tang, X.R. Role of Ferrous Sulfate (FeSO4) in Resistance to Cadmium Stress in Two Rice (Oryza sativa L.) Genotypes. Biomolecules 2020, 10, 1693. [Google Scholar] [CrossRef]
- Huybrechts, M.; Hendrix, S.; Kyndt, T.; Demeestere, K.; Vandamme, D.; Cuypers, A. Short-term effects of cadmium on leaf growth and nutrient transport in rice plants. Plant Sci. 2021, 313, 111054. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Jubayer, A.M.; Hesham, F.; Alharby Masayuki, F. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Cao, F.; Cai, Y.; Liu, L.; Zhang, M.; He, X.Y.; Zhang, G.P.; Wu, F.B. Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul. 2015, 75, 715–723. [Google Scholar] [CrossRef]
- Belouchi, A.; Kwan, T.; Gros, P. Cloning and characterization of the OsNramp family from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions. Plant Mol. Biol. 1997, 33, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.; Li, Y.X.; Fu, Y.F. Mutation at Different Sites of Metal Transporter Gene OsNramp5 Affects Cd Accumulation and Related Agronomic Traits in Rice (Oryza sativa L.). Front. Plant Sci. 2019, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.M.; Zhang, L.P.; Chen, C.Y.; He, H.J.; Wang, W.P.; Zeng, X.F.; Shao, Y.; et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, L.J.; Hu, J.T.; Zhang, X.; Lu, K.; Dong, H.X.; Wang, D.J.; Zhao, F.J.; Huang, C.F.; et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.D.; Huang, S.; Yamaji, N.; Zhang, W.W.; Ma, J.F.; Zhao, F.J. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Ecol. Environ. Conserv. 2020, 43, 2476–2491. [Google Scholar] [CrossRef]
- Zhao, J.L.; Yang, W.; Zhang, S.H.; Yang, T.F.; Liu, Q.; Dong, J.F.; Fu, H.; Mao, X.X.; Liu, B. Genome-wide association study and candidate gene analysis of rice cadmium accumulation in grain in a diverse rice collection. Rice 2018, 11, 61. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.; Dong, D.; Jia, X.M.; McCouch, S.R.; Kochian, L.V. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 6503–6508. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Dong, H.; Zhang, Y.Y.; Lv, K.; Wang, D.J.; Lian, X.M. OsNRAMP3 is a vascular bundles-specific manganese transporter that is responsible for manganese distribution in rice. PLoS ONE 2013, 8, e83990. [Google Scholar] [CrossRef]
- Chen, G.; Du, R.; Wang, X. Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding. Int. J. Mol. Sci. 2023, 24, 1247. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.J.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Tezuka, K.; Miyadate, H.; Katou, K.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.J.; Takahashi, H.; et al. A single recessive gene controls cadmium translocation in the cadmium hyper accumulating rice cultivar Cho-Ko-Koku. Theor. Angew. Genet. 2010, 120, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 2009, 32, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.X.; Wang, Z.F.; Wang, Q.H.; Wang, M.M.; Bao, Y.M.; Huang, J.; Zhang, H.S. Characterization of a vacuolar zinc transporter OZT1 in rice (Oryza sativa L.). Mol. Biol. Rep. 2013, 40, 1201–1210. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]





| Time/ Organ | Index | Total Cadmium | MDA | GSH | GSSH | POD | CAT | SOD |
|---|---|---|---|---|---|---|---|---|
| MS Root | Cd | 1.0000 | 0.83 ** | −0.2100 | 0.2400 | −0.4400 | −0.3300 | 0.2700 |
| MDA | 1.0000 | 0.0000 | 0.4300 | −0.5100 | −0.3600 | 0.4700 | ||
| GSH | 1.0000 | 0.74 * | 0.3000 | −0.68 * | 0.1100 | |||
| GSSH | 1.0000 | 0.3100 | −0.72 * | −0.0500 | ||||
| POD | 1.0000 | −0.3400 | −0.4600 | |||||
| CAT | 1.0000 | −0.3800 | ||||||
| SOD | 1.0000 | |||||||
| MS Stalk | Cd | 1.0000 | 0.2700 | 0.89 ** | 0.75 * | −0.96 ** | −0.6300 | −0.4900 |
| MDA | 1.0000 | 0.3900 | 0.5000 | −0.98 ** | −0.3700 | 0.4900 | ||
| GSH | 1.0000 | 0.9100 ** | −0.98 ** | −0.82 * | −0.0800 | |||
| GSSH | 1.0000 | −0.96 ** | −0.96 ** | −0.1100 | ||||
| POD | 1.0000 | 0.1800 | −0.6500 | |||||
| CAT | 1.0000 | −0.1500 | ||||||
| SOD | 1.0000 | |||||||
| MS Leaf | Cd | 1.0000 | 0.5900 | 0.90 ** | 0.0100 | −0.3100 | 0.67 * | 0.5500 |
| MDA | 1.0000 | 0.4200 | 0.0300 | −0.4900 | 0.6200 | 0.1100 | ||
| GSH | 1.0000 | 0.0600 | −0.2400 | 0.69 * | 0.3200 | |||
| GSSH | 1.0000 | −0.0900 | −0.3200 | −0.5800 | ||||
| POD | 1.0000 | −0.6200 | −0.1800 | |||||
| CAT | 1.0000 | 0.3500 | ||||||
| SOD | 1.0000 | |||||||
| MS grain | Cd | 1.0000 | 0.3300 | 0.68 * | 0.5300 | −0.74 * | −0.1800 | −0.0900 |
| MDA | 1.0000 | 0.3600 | 0.3900 | −0.6600 | −0.0500 | 0.3600 | ||
| GSH | 1.0000 | 0.73 * | −0.90 ** | −0.2700 | 0.1100 | |||
| GSSH | 1.0000 | −0.71 * | −0.6300 | 0.0400 | ||||
| POD | 1.0000 | 0.1000 | −0.2200 | |||||
| CAT | 1.0000 | −0.0600 | ||||||
| SOD | 1.0000 |
| Time/ Organ | Index | Total Cadmium | MDA | GSH | GSSH | POD | CAT | SOD |
|---|---|---|---|---|---|---|---|---|
| MS Root | Cd | 1.0000 | 0.83 ** | −0.76 * | −0.3200 | −0.2000 | −0.3000 | 0.86 ** |
| MDA | 1.0000 | −0.85 ** | −0.3800 | 0.2100 | −0.4000 | 0.90 ** | ||
| GSH | 1.0000 | 0.0100 | −0.2600 | 0.1400 | −0.80 ** | |||
| GSSH | 1.0000 | −0.0700 | 0.0200 | −0.3800 | ||||
| POD | 1.0000 | 0.4600 | 0.1800 | |||||
| CAT | 1.0000 | −0.2800 | ||||||
| SOD | 1.0000 | |||||||
| MS Stalk | Cd | 1.0000 | −0.0800 | −0.4200 | −0.76 * | 0.0900 | −0.3300 | −0.1400 |
| MDA | 1.0000 | −0.4700 | −0.0600 | −0.3700 | 0.3700 | 0.94 ** | ||
| GSH | 1.0000 | 0.5200 | 0.5900 | 0.0700 | −0.4000 | |||
| GSSH | 1.0000 | −0.3100 | 0.3000 | −0.1600 | ||||
| POD | 1.0000 | −0.1100 | −0.2500 | |||||
| CAT | 1.0000 | 0.2700 | ||||||
| SOD | 1.0000 | |||||||
| MS Leaf | Cd | 1.0000 | −0.5200 | −0.1700 | 0.1700 | 0.1300 | 0.1900 | −0.2100 |
| MDA | 1.0000 | 0.3900 | 0.4000 | −0.2800 | −0.6200 | −0.3500 | ||
| GSH | 1.0000 | 0.2600 | 0.5300 | 0.3600 | −0.2900 | |||
| GSSH | 1.0000 | 0.0400 | −0.4300 | −0.5100 | ||||
| POD | 1.0000 | 0.69 * | 0.1200 | |||||
| CAT | 1.0000 | 0.0300 | ||||||
| SOD | 1.0000 | |||||||
| MS grain | Cd | 1.0000 | −0.1700 | −0.88 ** | −0.82 ** | 0.3400 | 0.1700 | 0.2300 |
| MDA | 1.0000 | −0.1600 | 0.1000 | −0.4300 | 0.2500 | 0.6100 | ||
| GSH | 1.0000 | 0.82 ** | −0.2100 | −0.3400 | −0.3100 | |||
| GSSH | 1.0000 | 0.0300 | −0.0700 | −0.3500 | ||||
| POD | 1.0000 | −0.1500 | −0.4700 | |||||
| CAT | 1.0000 | −0.1400 | ||||||
| SOD | 1.0000 |
| Factor | DF | Total Cadmium | MDA | GSH | GSSH | POD | CAT | SOD |
|---|---|---|---|---|---|---|---|---|
| Stage (S) | 1 | NS | NS | NS | NS | NS | NS | NS |
| Cultivar (C) | 7 | 8.8362 *** | 7.7332 *** | 6.5727 *** | 4.0454 *** | 5.1296 *** | 6.0583 *** | 6.3067 *** |
| Organ (T) | 3 | 60.2580 *** | 61.4730 ** | 60.2580 *** | 49.6807 *** | 62.8730 *** | 3.9253 *** | 63.6275 *** |
| Stage × Cultivar (S × C) | 7 | 3.4184 ** | 2.2034 * | 3.4184 ** | 5.6438 *** | 4.8394 *** | 63.1775 * | 3.6649 ** |
| Stage × organ (S × T) | 3 | 8.8900 *** | 6.9457 ** | 8.8900 *** | 16.6708 *** | NS | 10.6520 *** | 8.2789 *** |
| Cultivar × organ (C × T) | 21 | NS | NS | NS | NS | 1.8119 * | NS | NS |
| Stage × Cultivar × organ (S × C × T) | 21 | NS | NS | NS | NS | NS | NS | NS |
| Name | Method Linkage |
|---|---|
| MDA | http://www.cominbio.com/a/shijihe/shenghuashiji/yanghuayukangyanghuaxilie/2018/0708/109.html (accessed on 15 March 2023) |
| GSSH | http://www.cominbio.com/a/shijihe/shenghuashiji/guguanggantaixilie/2014/1111/428.html (accessed on 15 March 2023) |
| GSH | http://www.cominbio.com/a/shijihe/shenghuashiji/guguanggantaixilie/2014/1111/427.html (accessed on 15 March 2023) |
| POD | http://www.cominbio.com/a/shijihe/shenghuashiji/yanghuayukangyanghuaxilie/2018/0708/153.html (accessed on 15 March 2023) |
| CAT | http://www.cominbio.com/a/shijihe/shenghuashiji/yanghuayukangyanghuaxilie/2019/0703/1843.html (accessed on 15 March 2023) |
| SOD | http://www.cominbio.com/a/shijihe/shenghuashiji/yanghuayukangyanghuaxilie/2018/0708/155.html (accessed on 15 March 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, K.; Li, J.; Yang, Y.; Li, Z.; Wu, W. Cadmium Absorption in Various Genotypes of Rice under Cadmium Stress. Int. J. Mol. Sci. 2023, 24, 8019. https://doi.org/10.3390/ijms24098019
Feng K, Li J, Yang Y, Li Z, Wu W. Cadmium Absorption in Various Genotypes of Rice under Cadmium Stress. International Journal of Molecular Sciences. 2023; 24(9):8019. https://doi.org/10.3390/ijms24098019
Chicago/Turabian StyleFeng, Kaixuan, Jiangxia Li, Yachun Yang, Zhong Li, and Wenge Wu. 2023. "Cadmium Absorption in Various Genotypes of Rice under Cadmium Stress" International Journal of Molecular Sciences 24, no. 9: 8019. https://doi.org/10.3390/ijms24098019
APA StyleFeng, K., Li, J., Yang, Y., Li, Z., & Wu, W. (2023). Cadmium Absorption in Various Genotypes of Rice under Cadmium Stress. International Journal of Molecular Sciences, 24(9), 8019. https://doi.org/10.3390/ijms24098019





