The Role of Exercise in Maintaining Mitochondrial Proteostasis in Parkinson’s Disease
Abstract
1. Introduction
2. Mitochondria and Mitochondrial Proteostasis
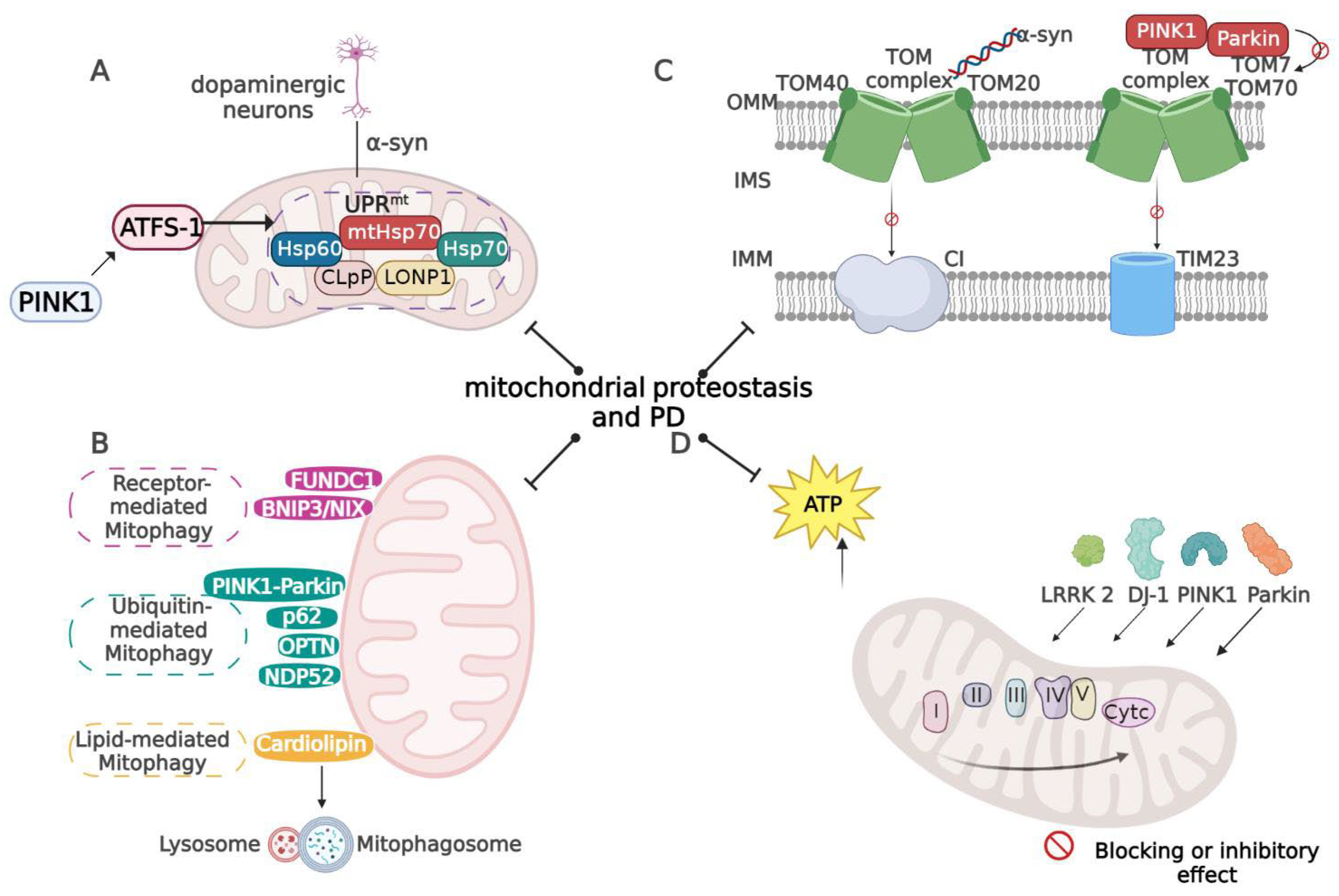
3. Mitochondrial Proteostasis in PD
3.1. UPRmt in PD
3.2. Mitophagy in PD
3.3. Mitochondrial Protein Import in PD
3.4. Mitochondrial Oxidative Stress in PD
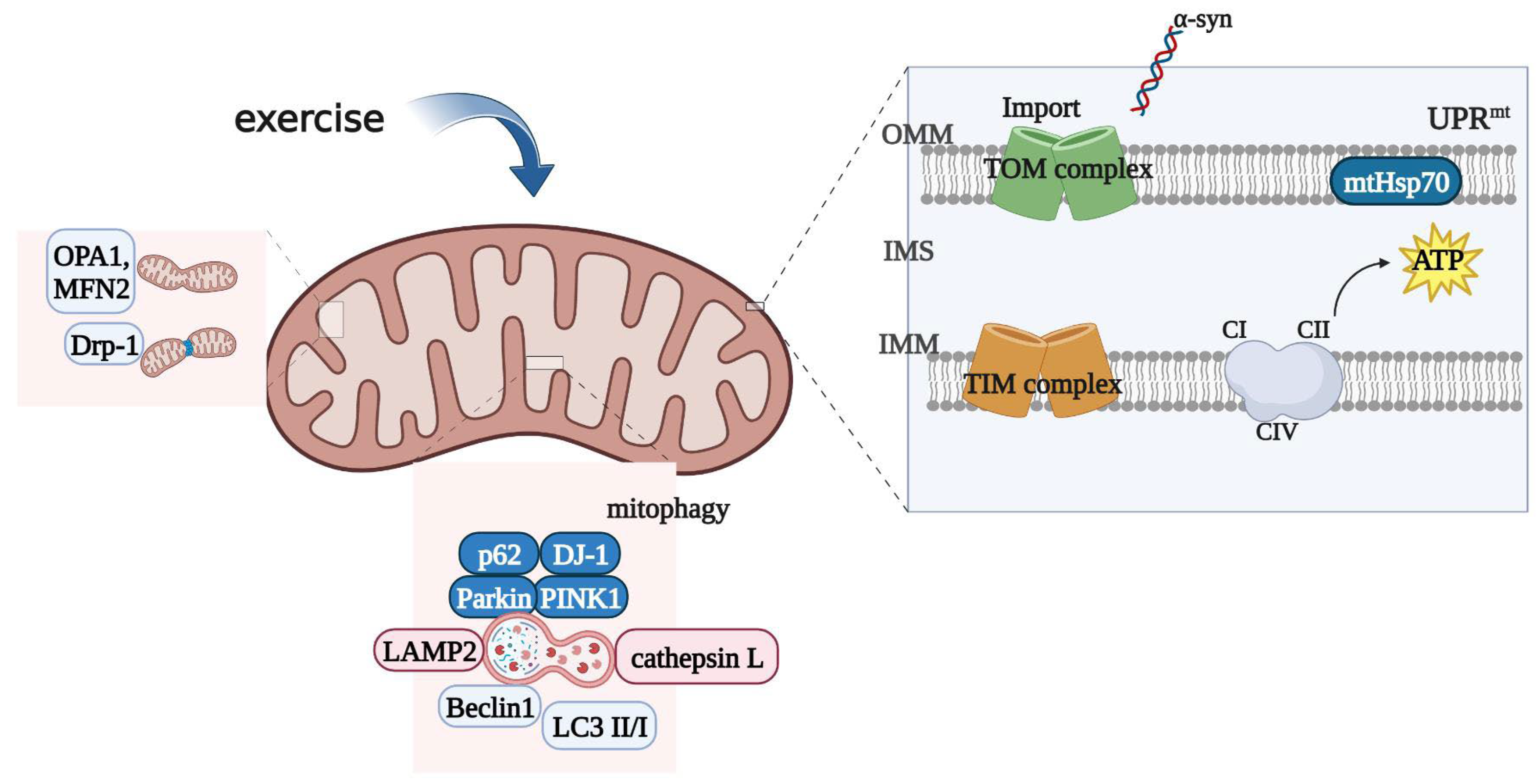
4. Exercise and Mitochondrial Proteostasis
4.1. Exercise and UPRmt
4.2. Exercise and Mitophagy
4.3. Exercise and Mitochondrial Protein Import
4.4. Exercise and Mitochondrial Oxidative Stress
5. Exercise Improves PD by Regulating Mitochondrial Proteostasis
5.1. Exercise Improves PD by Regulating UPRmt
5.2. Exercise Improves PD by Modulating Mitophagy
5.3. Exercise Improves PD by Modulating Mitochondrial Protein Import
5.4. Exercise Improves PD by Modulating Mitochondrial OS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Vijiaratnam, N.; Simuni, T.; Bandmann, O.; Morris, H.R.; Foltynie, T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 2021, 20, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.L.; Chen, Y.; Zhang, C.H.; Wang, Y.X.; Fernandez-Funez, P. Genetics of Parkinson’s disease and related disorders. J Med. Genet 2018, 55, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Robak, L.A.; Yu, M.; Cykowski, M.; Shulman, J.M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. 2023, 18, 95–121. [Google Scholar] [CrossRef]
- Wright, R. Mitochondrial dysfunction and Parkinson’s disease. Nat. Neurosci. 2022, 25, 2. [Google Scholar] [CrossRef]
- Grunewald, A.; Kumar, K.R.; Sue, C.M. New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 2019, 177, 73–93. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Q.L.; He, L.; Chen, L.X. Mitochondrial unfolded protein response: An emerging pathway in human diseases. Free Radical. Bio. Med. 2021, 163, 125–134. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Romano, R.; Coelho, H.J.; Bucci, C.; Marzetti, E. Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson’s Disease: Roads to Biomarker Discovery. Biomolecules 2021, 11, 1508. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Thomas, R.A.; Ragheb, M.A.; Fallahi, A.; Fon, E.A. Mitochondrial Quality Control in Health and in Parkinson’s Disease. Physiol. Rev. 2022, 102, 1721–1755. [Google Scholar] [CrossRef]
- Huang, C.; Deng, K.; Wu, M. Mitochondrial cristae in health and disease. Int. J. Biol. Macromol. 2023, 235, 123755. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.T.; Zhang, S.K.; Wang, H.; Zhou, W.S.; Ren, F.F.; Liang, H.M.; Wu, D.D.; Ji, X.Y.; Hashimoto, M.; et al. Efficacy and evaluation of therapeutic exercises on adults with Parkinson’s disease: A systematic review and network meta-analysis. BMC Geriatr. 2022, 22, 813. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Lau, C.K.Y.; Choi, K.C.; Chan, H.Y.L. Effects of Mindfulness Yoga vs. Stretching and Resistance Training Exercises on Anxiety and Depression for People with Parkinson Disease a Randomized Clinical Trial. JAMA Neurol. 2019, 76, 755–763. [Google Scholar] [CrossRef]
- Schenkman, M.; Moore, C.G.; Kohrt, W.M.; Hall, D.A.; Delitto, A.; Comella, C.L.; Josbeno, D.A.; Christiansen, C.L.; Berman, B.D.; Kluger, B.M.; et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 219–226. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Roger, A.J.; Munoz-Gomez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef]
- Schulte, U.; den Brave, F.; Haupt, A.; Gupta, A.; Song, J.; Muller, C.S.; Engelke, J.; Mishra, S.; Martensson, C.; Ellenrieder, L.; et al. Mitochondrial complexome reveals quality-control pathways of protein import. Nature 2023, 614, 153–159. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic. Acids. Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef]
- Song, J.; Herrmann, J.M.; Becker, T. Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell Biol. 2021, 22, 54–70. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.R.; Minczuk, M. Mitochondrial transcription and translation: Overview. Essays Biochem. 2018, 62, 309–320. [Google Scholar] [PubMed]
- Ruan, L.; Zhou, C.; Jin, E.; Kucharavy, A.; Zhang, Y.; Wen, Z.; Florens, L.; Li, R. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017, 543, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Inigo, J.R.; Chandra, D. The mitochondrial unfolded protein response (UPR(mt)): Shielding against toxicity to mitochondria in cancer. J. Hematol. Oncol. 2022, 15, 98. [Google Scholar] [CrossRef]
- Metzger, M.B.; Scales, J.L.; Dunklebarger, M.F.; Loncarek, J.; Weissman, A.M. A protein quality control pathway at the mitochondrial outer membrane. eLife 2020, 9, e51065. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, X.; Fu, N.; Chen, L. Mitochondrial unfolded protein response: A novel pathway in metabolism and immunity. Pharmacol. Res. 2021, 168, 105603. [Google Scholar] [CrossRef]
- Anderson, N.S.; Haynes, C.M. Folding the Mitochondrial UPR into the Integrated Stress Response. Trends Cell Biol. 2020, 30, 428–439. [Google Scholar] [CrossRef]
- Inigo, J.R.; Kumar, R.; Chandra, D. Targeting the mitochondrial unfolded protein response in cancer: Opportunities and challenges. Trends Cancer 2021, 7, 1050–1053. [Google Scholar] [CrossRef]
- Melber, A.; Haynes, C.M. UPR(mt) regulation and output: A stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018, 28, 281–295. [Google Scholar] [CrossRef]
- Cooper, J.F.; Machiela, E.; Dues, D.J.; Spielbauer, K.K.; Senchuk, M.M.; Van Raamsdonk, J.M. Activation of the mitochondrial unfolded protein response promotes longevity and dopamine neuron survival in Parkinson’s disease models. Sci. Rep. 2017, 7, 16441. [Google Scholar] [CrossRef]
- Liu, M.; Yu, S.; Wang, J.; Qiao, J.; Liu, Y.; Wang, S.; Zhao, Y. Ginseng protein protects against mitochondrial dysfunction and neurodegeneration by inducing mitochondrial unfolded protein response in Drosophila melanogaster PINK1 model of Parkinson’s disease. J. Ethnopharmacol. 2020, 247, 112213. [Google Scholar] [CrossRef]
- Pellegrino, M.W.; Nargund, A.M.; Kirienko, N.V.; Gillis, R.; Fiorese, C.J.; Haynes, C.M. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 2014, 516, 414–417. [Google Scholar] [CrossRef]
- Martinez, B.A.; Petersen, D.A.; Gaeta, A.L.; Stanley, S.P.; Caldwell, G.A.; Caldwell, K.A. Dysregulation of the Mitochondrial Unfolded Protein Response Induces Non-Apoptotic Dopaminergic Neurodegeneration in C. elegans Models of Parkinson’s Disease. J. Neurosci. 2017, 37, 11085–11100. [Google Scholar]
- Choong, C.J.; Okuno, T.; Ikenaka, K.; Baba, K.; Hayakawa, H.; Koike, M.; Yokota, M.; Doi, J.; Kakuda, K.; Takeuchi, T.; et al. Alternative mitochondrial quality control mediated by extracellular release. Autophagy 2021, 17, 2962–2974. [Google Scholar] [CrossRef]
- Lou, G.; Palikaras, K.; Lautrup, S.; Scheibye-Knudsen, M.; Tavernarakis, N.; Fang, E.F. Mitophagy and Neuroprotection. Trends Mol. Med. 2020, 26, 8–20. [Google Scholar] [CrossRef]
- Malpartida, A.B.; Williamson, M.; Narendra, D.P.; Wade-Martins, R.; Ryan, B.J. Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef]
- Chu, C.T. Mechanisms of selective autophagy and mitophagy: Implications for neurodegenerative diseases. Neurobiol. Dis. 2019, 122, 23–34. [Google Scholar] [CrossRef]
- Guzman, J.N.; Ilijic, E.; Yang, B.; Sanchez-Padilla, J.; Wokosin, D.; Galtieri, D.; Kondapalli, J.; Schumacker, P.T.; Surmeier, D.J. Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J. Clin. Investig. 2018, 128, 2266–2280. [Google Scholar] [CrossRef]
- Verma, M.; Callio, J.; Otero, P.A.; Sekler, I.; Wills, Z.P.; Chu, C.T. Mitochondrial Calcium Dysregulation Contributes to Dendrite Degeneration Mediated by PD/LBD-Associated LRRK2 Mutants. J. Neurosci. 2017, 37, 11151–11165. [Google Scholar] [CrossRef]
- Majcher, V.; Goode, A.; James, V.; Layfield, R. Autophagy receptor defects and ALS-FTLD. Mol. Cell Neurosci. 2015, 66, 43–52. [Google Scholar] [CrossRef]
- Lizama, B.N.; Chu, C.T. Neuronal autophagy and mitophagy in Parkinson’s disease. Mol. Aspects Med. 2021, 82, 100972. [Google Scholar] [CrossRef] [PubMed]
- Bras, J.; Guerreiro, R.; Hardy, J. SnapShot: Genetics of Parkinson’s Disease. Cell 2015, 160, 570–U239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Dai, L.; Li, D.Y. Mitophagy in neurological disorders. J. Neuroinflamm. 2021, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xue, W.; Kong, J.; Chen, Y.; Qiu, X.; An, X.; Li, Y.; Wang, H.; An, J. Ultrafine black carbon caused mitochondrial oxidative stress, mitochondrial dysfunction and mitophagy in SH-SY5Y cells. Sci. Total Environ. 2022, 813, 151899. [Google Scholar] [CrossRef] [PubMed]
- Shaltouki, A.; Hsieh, C.H.; Kim, M.J.; Wang, X.N. Alpha-synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson’s models. Acta Neuropathol. 2018, 136, 607–620. [Google Scholar] [CrossRef]
- Ryan, T.; Bamm, V.V.; Stykel, M.G.; Coackley, C.L.; Humphries, K.M.; Jamieson-Williams, R.; Ambasudhan, R.; Mosser, D.D.; Lipton, S.A.; Harauz, G.; et al. Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 2018, 9, 817. [Google Scholar] [CrossRef]
- Imberechts, D.; Kinnart, I.; Wauters, F.; Terbeek, J.; Manders, L.; Wierda, K.; Eggermont, K.; Madeiro, R.F.; Sue, C.; Verfaillie, C.; et al. DJ-1 is an essential downstream mediator in PINK1/parkin-dependent mitophagy. Brain 2022, 145, 4368–4384. [Google Scholar] [CrossRef]
- Watanabe, R.; Buschauer, R.; Bohning, J.; Audagnotto, M.; Lasker, K.; Lu, T.W.; Boassa, D.; Taylor, S.; Villa, E. The In Situ Structure of Parkinson’s Disease-Linked LRRK2. Cell 2020, 182, 1508. [Google Scholar] [CrossRef]
- Hauser, D.N.; Mamais, A.; Conti, M.M.; Primiani, C.T.; Kumaran, R.; Dillman, A.A.; Langston, R.G.; Beilina, A.; Garcia, J.H.; Diaz-Ruiz, A.; et al. Hexokinases link DJ-1 to the PINK1/parkin pathway. Mol. Neurodegener. 2017, 12, 70. [Google Scholar] [CrossRef]
- Wauters, F.; Cornelissen, T.; Imberechts, D.; Martin, S.; Koentjoro, B.; Sue, C.; Vangheluwe, P.; Vandenberghe, W. LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy 2020, 16, 203–222. [Google Scholar] [CrossRef]
- Robinson, D.R.L.; Hock, D.H.; Muellner-Wong, L.; Kugapreethan, R.; Reljic, B.; Surgenor, E.E.; Rodrigues, C.H.M.; Caruana, N.J.; Stroud, D.A. Applying Sodium Carbonate Extraction Mass Spectrometry to Investigate Defects in the Mitochondrial Respiratory Chain. Front Cell Dev. Biol. 2022, 10, 786268. [Google Scholar] [CrossRef]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef]
- Richter-Dennerlein, R.; Oeljeklaus, S.; Lorenzi, I.; Ronsor, C.; Bareth, B.; Schendzielorz, A.B.; Wang, C.; Warscheid, B.; Rehling, P.; Dennerlein, S. Mitochondrial Protein Synthesis Adapts to Influx of Nuclear-Encoded Protein. Cell 2016, 167, 471–483. [Google Scholar] [CrossRef]
- Boos, F.; Labbadia, J.; Herrmann, J.M. How the Mitoprotein-Induced Stress Response Safeguards the Cytosol: A Unified View. Trends Cell Biol. 2020, 30, 241–254. [Google Scholar] [CrossRef]
- Rolland, S.G.; Schneid, S.; Schwarz, M.; Rackles, E.; Fischer, C.; Haeussler, S.; Regmi, S.G.; Yeroslaviz, A.; Habermann, B.; Mokranjac, D.; et al. Compromised Mitochondrial Protein Import Acts as a Signal for UPRmt. Cell Rep. 2019, 28, 1659–1669. [Google Scholar] [CrossRef]
- Youle, R.J. Mitochondria-Striking a balance between host and endosymbiont. Science 2019, 365, 655. [Google Scholar] [CrossRef]
- Priesnitz, C.; Becker, T. Pathways to balance mitochondrial translation and protein import. Gene Dev. 2018, 32, 1285–1296. [Google Scholar] [CrossRef]
- Goyal, S.; Chaturvedi, R.K. Mitochondrial Protein Import Dysfunction in Pathogenesis of Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 1418–1437. [Google Scholar] [CrossRef]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016, 8, 342ra78. [Google Scholar] [CrossRef]
- Franco-Iborra, S.; Cuadros, T.; Parent, A.; Romero-Gimenez, J.; Vila, M.; Perier, C. Defective mitochondrial protein import contributes to complex I-induced mitochondrial dysfunction and neurodegeneration in Parkinson’s disease. Cell Death Dis. 2018, 9, 1122. [Google Scholar] [CrossRef]
- Palmer, C.S.; Anderson, A.J.; Stojanovski, D. Mitochondrial protein import dysfunction: Mitochondrial disease, neurodegenerative disease and cancer. FEBS Lett. 2021, 595, 1107–1131. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Wang, C.; Sideris, D.P.; Bunker, E.; Zhang, Z.; Youle, R.J. Reciprocal Roles of Tom7 and OMA1 during Mitochondrial Import and Activation of PINK1. Mol. Cell 2019, 73, 1028–1043 e5. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Cho, J.Y.; Lee, U.B. Treadmill exercise alleviates motor deficits and improves mitochondrial import machinery in an MPTP-induced mouse model of Parkinson’s disease. Exp. Gerontol. 2017, 89, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389. [Google Scholar] [CrossRef]
- Greene, J.; Segaran, A.; Lord, S. Targeting OXPHOS and the electron transport chain in cancer; Molecular and therapeutic implications. Semin. Cancer Biol. 2022, 86, 851–859. [Google Scholar] [CrossRef]
- Cheng, X.T.; Huang, N.; Sheng, Z.H. Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron 2022, 110, 1899–1923. [Google Scholar] [CrossRef]
- Shen, Y.H.; Wu, Q.; Shi, J.S.; Zhou, S.Y. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef]
- Imbriani, P.; Martella, G.; Bonsi, P.; Pisani, A. Oxidative stress and synaptic dysfunction in rodent models of Parkinson’s disease. Neurobiol. Dis. 2022, 173, 105851. [Google Scholar] [CrossRef]
- Mailloux, R.J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Wang, S.; Yang, D.; Zhang, Y.; Xu, L.; Ma, L.; Zheng, J.; Petersen, R.B.; Zheng, L.; et al. Copper and iron ions accelerate the prion-like propagation of α-synuclein: A vicious cycle in Parkinson’s disease. Int. J. Biol. Macromol. 2020, 163, 562–573. [Google Scholar] [CrossRef]
- Goncalves, A.M.; Pereira-Santos, A.R.; Esteves, A.R.; Cardoso, S.M.; Empadinhas, N. The Mitochondrial Ribosome: A World of Opportunities for Mitochondrial Dysfunction Toward Parkinson’s Disease. Antioxid. Redox Sign. 2021, 34, 694–711. [Google Scholar] [CrossRef]
- Martin-Jimenez, R.; Lurette, O.; Hebert-Chatelain, E. Damage in Mitochondrial DNA Associated with Parkinson’s Disease. DNA Cell Biol. 2020, 39, 1421–1430. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Wan, J.M.; Morse, P.T.; Zurek, M.P.; Turner, A.A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell B 2020, 121, 105704. [Google Scholar] [CrossRef]
- Dar, G.M.; Ahmad, E.; Ali, A.; Mahajan, B.; Ashraf, G.M.; Saluja, S.S. Genetic aberration analysis of mitochondrial respiratory complex I implications in the development of neurological disorders and their clinical significance. Ageing Res. Rev. 2023, 87, 101906. [Google Scholar] [CrossRef]
- Cabral-Costa, J.V.; Kowaltowski, A.J. Neurological disorders and mitochondria. Mol. Aspects Med. 2020, 71, 100826. [Google Scholar] [CrossRef]
- Jimenez-Salvador, I.; Meade, P.; Iglesias, E.; Bayona-Bafaluy, P.; Ruiz-Pesini, E. Developmental origins of Parkinson disease: Improving the rodent models. Ageing Res. Rev. 2023, 86, 101880. [Google Scholar] [CrossRef]
- Penniman, C.M.; Bhardwaj, G.; Nowers, C.J.; Brown, C.U.; Junck, T.L.; Boyer, C.K.; Jena, J.; Fuqua, J.D.; Lira, V.A.; O’Neill, B.T. Loss of FoxOs in muscle increases strength and mitochondrial function during aging. J. Cachexia Sarcopenia Muscle 2023, 14, 243–259. [Google Scholar] [CrossRef]
- Cordeiro, A.V.; Peruca, G.F.; Braga, R.R.; Bricola, R.S.; Lenhare, L.; Silva, V.R.R.; Anaruma, C.P.; Katashima, C.K.; Crisol, B.M.; Barbosa, L.T.; et al. High-intensity exercise training induces mitonuclear imbalance and activates the mitochondrial unfolded protein response in the skeletal muscle of aged mice. Geroscience 2021, 43, 1513–1518. [Google Scholar] [CrossRef]
- Cordeiro, A.V.; Bricola, R.S.; Braga, R.R.; Lenhare, L.; Silva, V.R.R.; Anaruma, C.P.; Katashima, C.K.; Crisol, B.M.; Simabuco, F.M.; Silva, A.S.R.; et al. Aerobic Exercise Training Induces the Mitonuclear Imbalance and UPRmt in the Skeletal Muscle of Aged Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2258–2261. [Google Scholar] [CrossRef]
- Braga, R.R.; Crisol, B.M.; Bricola, R.S.; Sant’ana, M.R.; Nakandakari, S.; Costa, S.O.; Prada, P.O.; da Silva, A.S.R.; Moura, L.P.; Pauli, J.R.; et al. Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPR(mt) in the hypothalamus of mice. Sci. Rep. 2021, 11, 3813. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.M.; Min, S.H.; Lee, C.H.; Kim, J.Y.; Lim, H.S.; Choi, M.J.; Jung, S.B.; Park, J.W.; Kim, S.; Park, C.B.; et al. Mitohormesis in Hypothalamic POMC Neurons Mediates Regular Exercise-Induced High-Turnover Metabolism. Cell Metab. 2021, 33, 334–349 e6. [Google Scholar] [CrossRef] [PubMed]
- Fiorese, C.J.; Schulz, A.M.; Lin, Y.F.; Rosin, N.; Pellegrino, M.W.; Haynes, C.M. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. 2016, 26, 2037–2043. [Google Scholar] [CrossRef]
- Slavin, M.B.; Kumari, R.; Hood, D.A. ATF5 is a regulator of exercise-induced mitochondrial quality control in skeletal muscle. Mol. Metab. 2022, 66, 101623. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Mitophagy: Molecular Mechanisms, New Concepts on Parkin Activation and the Emerging Role of AMPK/ULK1 Axis. Cells 2021, 11, 30. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, X.; Li, B.; Wang, J.; Zhang, C.; Xu, B. Treadmill Exercise Improves PINK1/Parkin-Mediated Mitophagy Activity Against Alzheimer’s Disease Pathologies by Upregulated SIRT1-FOXO1/3 Axis in APP/PS1 Mice. Mol. Neurobiol. 2023, 60, 277–291. [Google Scholar] [CrossRef]
- Mikhail, A.I.; Manta, A.; Ng, S.Y.; Osborne, A.K.; Mattina, S.R.; Mackie, M.R.; Ljubicic, V. A single dose of exercise stimulates skeletal muscle mitochondrial plasticity in myotonic dystrophy type 1. Acta Physiol. 2023, 237, e13943. [Google Scholar] [CrossRef]
- Laker, R.C.; Drake, J.C.; Wilson, R.J.; Lira, V.A.; Lewellen, B.M.; Ryall, K.A.; Fisher, C.C.; Zhang, M.; Saucerman, J.J.; Goodyear, L.J.; et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017, 8, 548. [Google Scholar] [CrossRef]
- Liang, J.; Zeng, Z.; Zhang, Y.; Chen, N. Regulatory role of exercise-induced autophagy for sarcopenia. Exp. Gerontol. 2020, 130, 110789. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zeng, Z.; Wu, L.; Zhang, Y.; Guo, Y.; Lv, J.; Wang, C.; Fan, J.; Chen, N. Lifelong Aerobic Exercise Alleviates Sarcopenia by Activating Autophagy and Inhibiting Protein Degradation via the AMPK/PGC-1α Signaling Pathway. Metabolites 2021, 11, 323. [Google Scholar] [CrossRef]
- Balan, E.; Schwalm, C.; Naslain, D.; Nielens, H.; Francaux, M.; Deldicque, L. Regular Endurance Exercise Promotes Fission, Mitophagy, and Oxidative Phosphorylation in Human Skeletal Muscle Independently of Age. Front Physiol. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, H.; Zhu, A.; Lin, Y.; Zhang, L.; Ye, B.; Cheng, J.; Shen, W.; Jin, L.; Liu, C.; et al. Treadmill exercise attenuates cerebral ischaemic injury in rats by protecting mitochondrial function via enhancement of caveolin-1. Life Sci. 2021, 264, 118634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Sha, H.; Zhang, P.; Jia, J.; Hu, Y.; Zhu, J. Early exercise affects mitochondrial transcription factors expression after cerebral ischemia in rats. Int. J. Mol. Sci. 2012, 13, 1670–1679. [Google Scholar] [CrossRef]
- Hood, D.A.; Tryon, L.D.; Vainshtein, A.; Memme, J.; Chen, C.; Pauly, M.; Crilly, M.J.; Carter, H. Exercise and the Regulation of Mitochondrial Turnover. Prog. Mol. Biol. Transl. Sci. 2015, 135, 99–127. [Google Scholar] [PubMed]
- Zhang, Y.; Oliveira, A.N.; Hood, D.A. The intersection of exercise and aging on mitochondrial protein quality control. Exp. Gerontol. 2020, 131, 110824. [Google Scholar] [CrossRef]
- Richards, B.J.; Slavin, M.; Oliveira, A.N.; Sanfrancesco, V.C.; Hood, D.A. Mitochondrial protein import and UPR(mt) in skeletal muscle remodeling and adaptation. Semin. Cell Dev. Biol. 2023, 143, 28–36. [Google Scholar] [CrossRef]
- Wyckelsma, V.L.; Levinger, I.; McKenna, M.J.; Formosa, L.E.; Ryan, M.T.; Petersen, A.C.; Anderson, M.J.; Murphy, R.M. Preservation of skeletal muscle mitochondrial content in older adults: Relationship between mitochondria, fibre type and high-intensity exercise training. J. Physiol. 2017, 595, 3345–3359. [Google Scholar] [CrossRef]
- Ringholm, S.; Gudiksen, A.; Frey Halling, J.; Qoqaj, A.; Meizner Rasmussen, P.; Prats, C.; Plomgaard, P.; Pilegaard, H. Impact of Aging and Lifelong Exercise Training on Mitochondrial Function and Network Connectivity in Human Skeletal Muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 373–383. [Google Scholar] [CrossRef]
- Marin, C.T.; de Souza Lino, A.D.; Avelar, I.D.S.; Barbosa, M.R.; Scarlato, G.C.G.; Cavalini, D.F.; Tamanini, F.; Alexandrino, A.V.; Vercesi, A.E.; Shiguemoto, G.E. Resistance training prevents dynamics and mitochondrial respiratory dysfunction in vastus lateralis muscle of ovariectomized rats. Exp. Gerontol. 2023, 173, 112081. [Google Scholar] [CrossRef]
- Groennebaek, T.; Sieljacks, P.; Nielsen, R.; Pryds, K.; Jespersen, N.R.; Wang, J.; Carlsen, C.R.; Schmidt, M.R.; de Paoli, F.V.; Miller, B.F.; et al. Effect of Blood Flow Restricted Resistance Exercise and Remote Ischemic Conditioning on Functional Capacity and Myocellular Adaptations in Patients with Heart Failure. Circ. Heart Fail. 2019, 12, e006427. [Google Scholar] [CrossRef]
- Lim, A.Y.; Chen, Y.C.; Hsu, C.C.; Fu, T.C.; Wang, J.S. The Effects of Exercise Training on Mitochondrial Function in Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12559. [Google Scholar] [CrossRef]
- Chan, S.H.; Hung, C.H.; Shih, J.Y.; Chu, P.M.; Cheng, Y.H.; Lin, H.C.; Hsieh, P.L.; Tsai, K.L. Exercise intervention attenuates hyperhomocysteinemia-induced aortic endothelial oxidative injury by regulating SIRT1 through mitigating NADPH oxidase/LOX-1 signaling. Redox Biol. 2018, 14, 116–125. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apro, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970 e6. [Google Scholar] [CrossRef]
- Sanguesa, G.; Batlle, M.; Munoz-Moreno, E.; Soria, G.; Alcarraz, A.; Rubies, C.; Sitja-Roqueta, L.; Solana, E.; Martinez-Heras, E.; Meza-Ramos, A.; et al. Intense long-term training impairs brain health compared with moderate exercise: Experimental evidence and mechanisms. Ann. N. Y. Acad. Sci. 2022, 1518, 282–298. [Google Scholar] [CrossRef]
- Church, F.C. Treatment Options for Motor and Non-Motor Symptoms of Parkinson’s Disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef]
- Johansson, M.E.; Cameron, I.G.M.; Van der Kolk, N.M.; de Vries, N.M.; Klimars, E.; Toni, I.; Bloem, B.R.; Helmich, R.C. Aerobic Exercise Alters Brain Function and Structure in Parkinson’s Disease: A Randomized Controlled Trial. Ann. Neurol. 2022, 91, 203–216. [Google Scholar] [CrossRef]
- Feng, Y.S.; Yang, S.D.; Tan, Z.X.; Wang, M.M.; Xing, Y.; Dong, F.; Zhang, F. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020, 245, 117345. [Google Scholar] [CrossRef]
- Curtis, W.M.; Seeds, W.A.; Mattson, M.P.; Bradshaw, P.C. NADPH and Mitochondrial Quality Control as Targets for a Circadian-Based Fasting and Exercise Therapy for the Treatment of Parkinson’s Disease. Cells 2022, 11, 2416. [Google Scholar] [CrossRef]
- Magana, J.C.; Deus, C.M.; Gine-Garriga, M.; Montane, J.; Pereira, S.P. Exercise-Boosted Mitochondrial Remodeling in Parkinson’s Disease. Biomedicines 2022, 10, 3228. [Google Scholar] [CrossRef]
- Munch, C. The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol. 2018, 16, 81. [Google Scholar] [CrossRef]
- Mesbah Moosavi, Z.S.; Hood, D.A. The unfolded protein response in relation to mitochondrial biogenesis in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2017, 312, C583–C594. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.; Katuri, J.; Liang, T.; Bai, Y. Mitochondrial chaperones in human health and disease. Free Radic. Biol. Med. 2022, 179, 363–374. [Google Scholar] [CrossRef]
- Nhu, N.T.; Cheng, Y.J.; Lee, S.D. Effects of Treadmill Exercise on Neural Mitochondrial Functions in Parkinson’s Disease: A Systematic Review of Animal Studies. Biomedicines 2021, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, C.L.; Miller, B.F.; Lewis, T.L. Exercise and mitochondrial remodeling to prevent age-related neurodegeneration. J. Appl. Physiol. 2023, 134, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Cho, J.Y. Treadmill Exercise Attenuates α-Synuclein Levels by Promoting Mitochondrial Function and Autophagy Possibly via SIRT1 in the Chronic MPTP/P-Induced Mouse Model of Parkinson’s Disease. Neurotox. Res. 2017, 32, 473–486. [Google Scholar] [CrossRef]
- Hwang, D.J.; Koo, J.H.; Kwon, K.C.; Choi, D.H.; Shin, S.D.; Jeong, J.H.; Um, H.S.; Cho, J.Y. Neuroprotective effect of treadmill exercise possibly via regulation of lysosomal degradation molecules in mice with pharmacologically induced Parkinson’s disease. J. Physiol. Sci. 2018, 68, 707–716. [Google Scholar] [CrossRef]
- Gendi, F.; Pei, F.F.; Wang, Y.; Li, H.Y.; Fu, J.; Chang, C. Mitochondrial Proteins Unveil the Mechanism by Which Physical Exercise Ameliorates Memory, Learning and Motor Activity in Hypoxic Ischemic Encephalopathy Rat Model. Int. J. Mol. Sci. 2022, 23, 4235. [Google Scholar] [CrossRef]
- Viana, S.D.; Pita, I.R.; Lemos, C.; Rial, D.; Couceiro, P.; Rodrigues-Santos, P.; Caramelo, F.; Carvalho, F.; Ali, S.F.; Prediger, R.D.; et al. The effects of physical exercise on nonmotor symptoms and on neuroimmune RAGE network in experimental parkinsonism. J. Appl. Physiol. (1985) 2017, 123, 161–171. [Google Scholar] [CrossRef]
- Chuang, C.S.; Chang, J.C.; Cheng, F.C.; Liu, K.H.; Su, H.L.; Liu, C.S. Modulation of mitochondrial dynamics by treadmill training to improve gait and mitochondrial deficiency in a rat model of Parkinson’s disease. Life Sci. 2017, 191, 236–244. [Google Scholar] [CrossRef]
- Ferreira, A.F.F.; Binda, K.H.; Singulani, M.P.; Pereira, C.P.M.; Ferrari, G.D.; Alberici, L.C.; Real, C.C.; Britto, L.R. Physical exercise protects against mitochondria alterations in the 6-hidroxydopamine rat model of Parkinson’s disease. Behav. Brain. Res. 2020, 387, 112607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, Y.; Liu, T.; Xu, Y.; Zhao, X.; Wei, J. The Role of Exercise in Maintaining Mitochondrial Proteostasis in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 7994. https://doi.org/10.3390/ijms24097994
Li J, Xu Y, Liu T, Xu Y, Zhao X, Wei J. The Role of Exercise in Maintaining Mitochondrial Proteostasis in Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(9):7994. https://doi.org/10.3390/ijms24097994
Chicago/Turabian StyleLi, Jingwen, Yanli Xu, Tingting Liu, Yuxiang Xu, Xiantao Zhao, and Jianshe Wei. 2023. "The Role of Exercise in Maintaining Mitochondrial Proteostasis in Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 9: 7994. https://doi.org/10.3390/ijms24097994
APA StyleLi, J., Xu, Y., Liu, T., Xu, Y., Zhao, X., & Wei, J. (2023). The Role of Exercise in Maintaining Mitochondrial Proteostasis in Parkinson’s Disease. International Journal of Molecular Sciences, 24(9), 7994. https://doi.org/10.3390/ijms24097994





