Vachellia farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Results
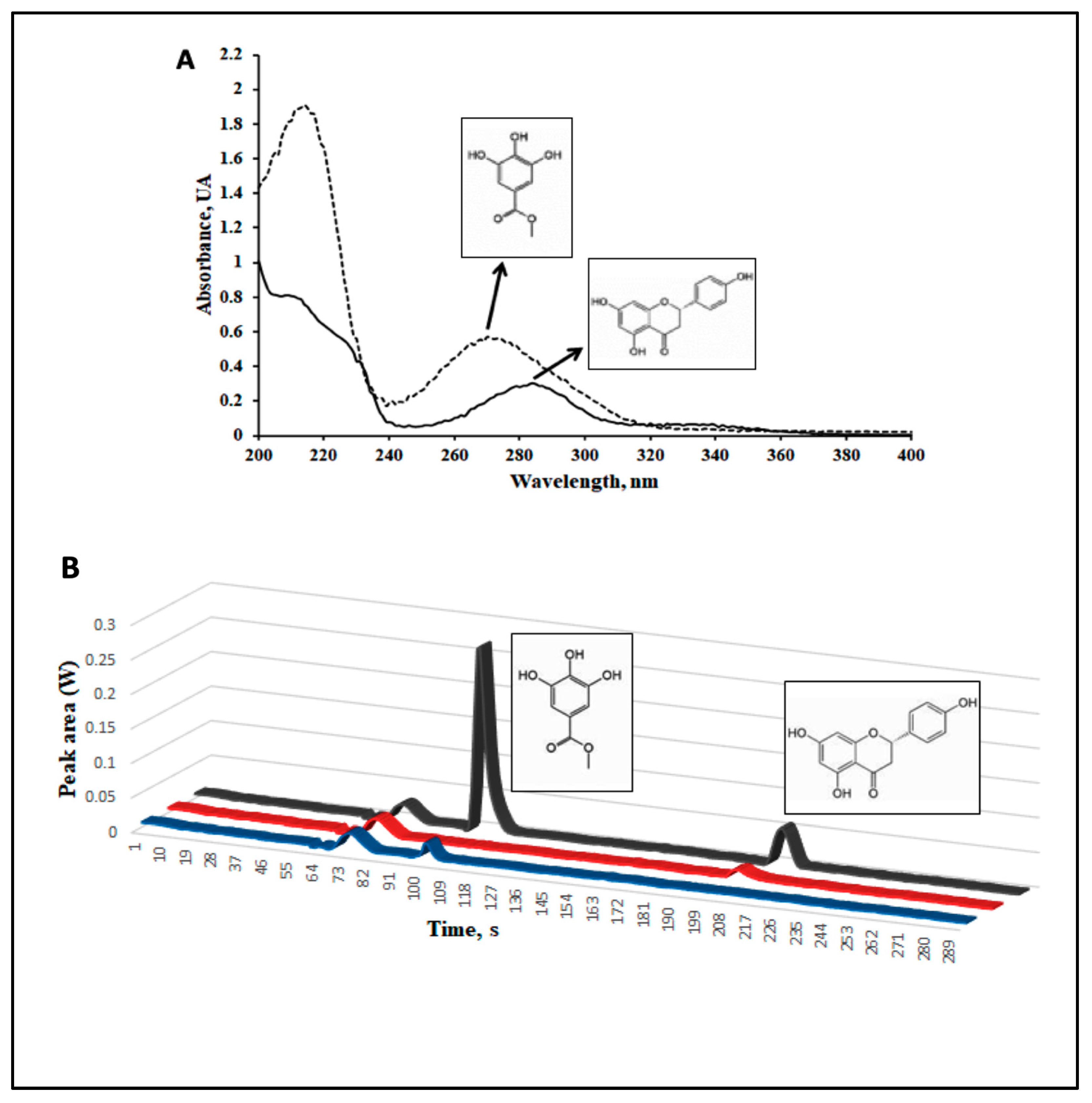
2.1. A Phenolic Extract of Vachellia farnesiana Pods (VFPE) Contains Significant Amounts of the Bioactive Compounds Methyl Gallate and Naringenin
2.2. VFP or a VFPE Prevents Excessive Weight Gain, Fat Mass Accumulation, and Lean Mass Loss in Mice Fed a High-Fat Diet
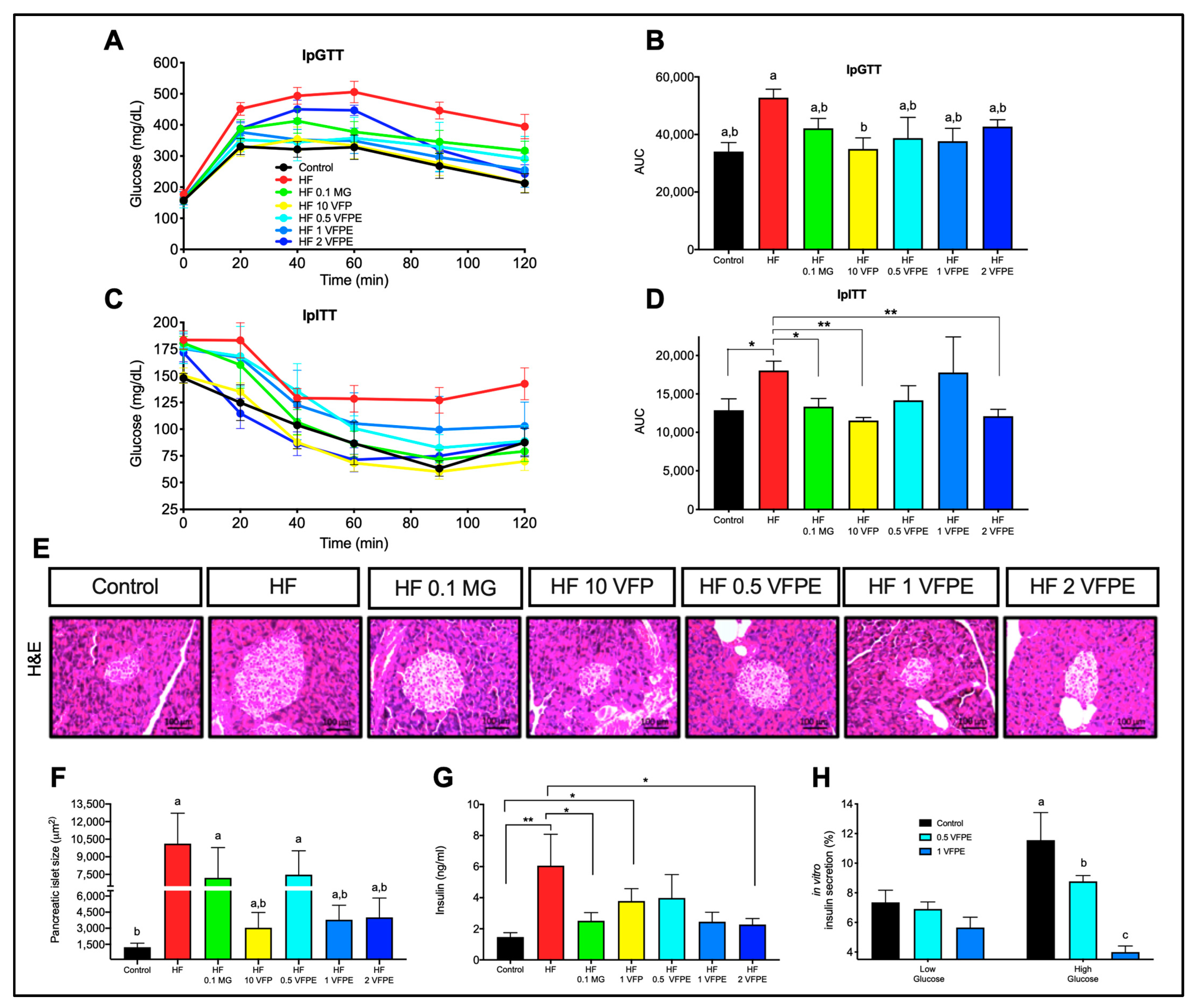
2.3. The Consumption of VFP or VFPE Improves Glucose and Insulin Tolerance, Prevents Pancreatic Islets Hypertrophy in Mice Fed a High-Fat Diet, and Notably, the VFPE Decreases Insulin Secretion in a Dose-Dependent Manner in INS-1E Cells
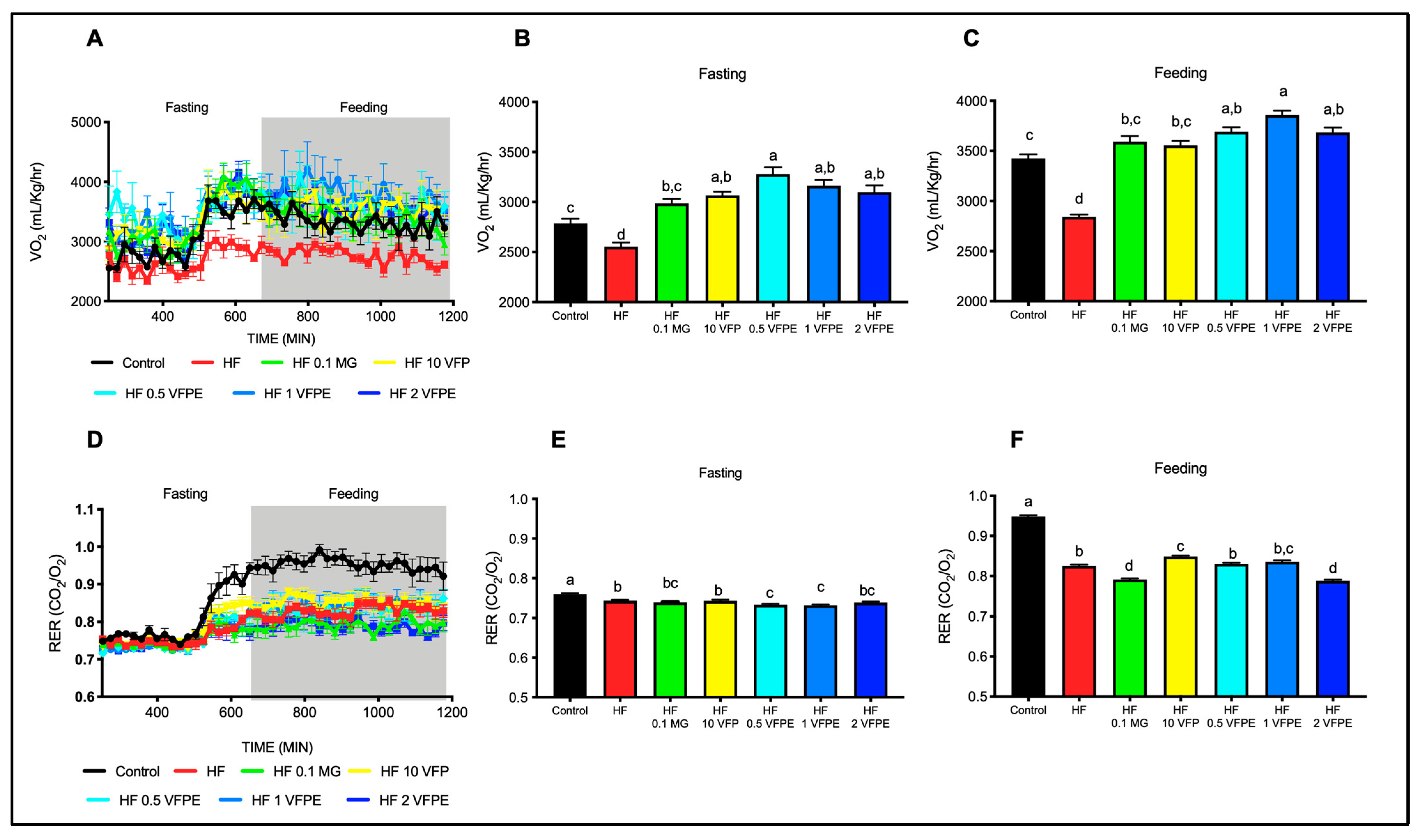
2.4. The Consumption of VFP or VFPE Prevents the Decrease in Energy Expenditure Associated with a High-Fat Diet without Altering Substrate Utilization
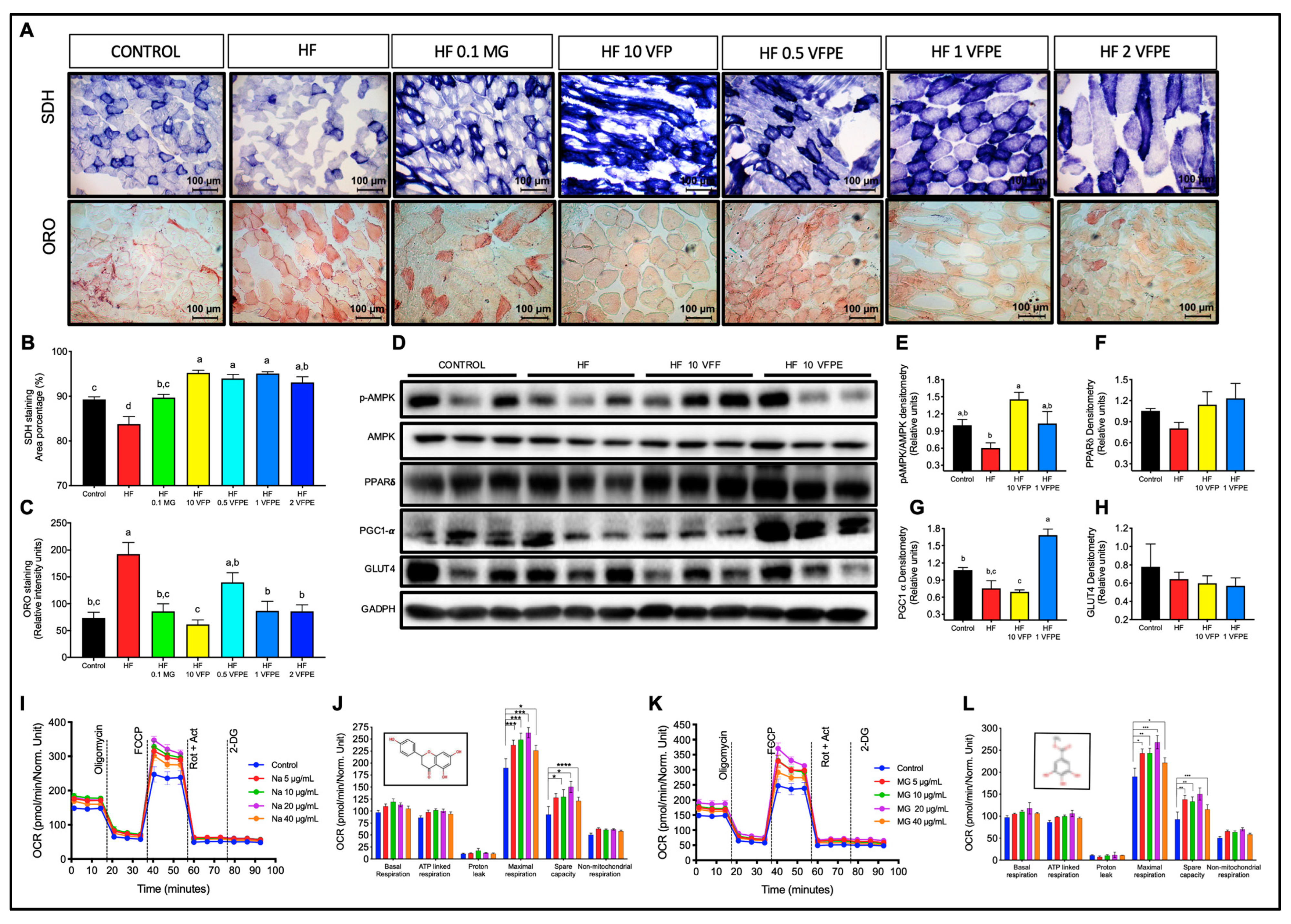
2.5. VFP or VFPE Increases Mitochondrial Abundance in the Skeletal Muscle of Mice Fed with an HFD, and VFPE Augments Mitochondrial Oxidative Metabolism in C2C12 Myotubes
2.6. VFP or VFPE Reduces Hepatic Lipid Content in Mice Associated with Increased Mitochondrial Activity in Primary Hepatocytes Exposed to VFPE
2.7. VFP or VFPE Consumption Prevents Visceral and Subcutaneous Adipocyte Hypertrophy Associated with Increased Thermogenic Activity in Brown Adipose Tissue of Mice Fed a High-Fat Diet
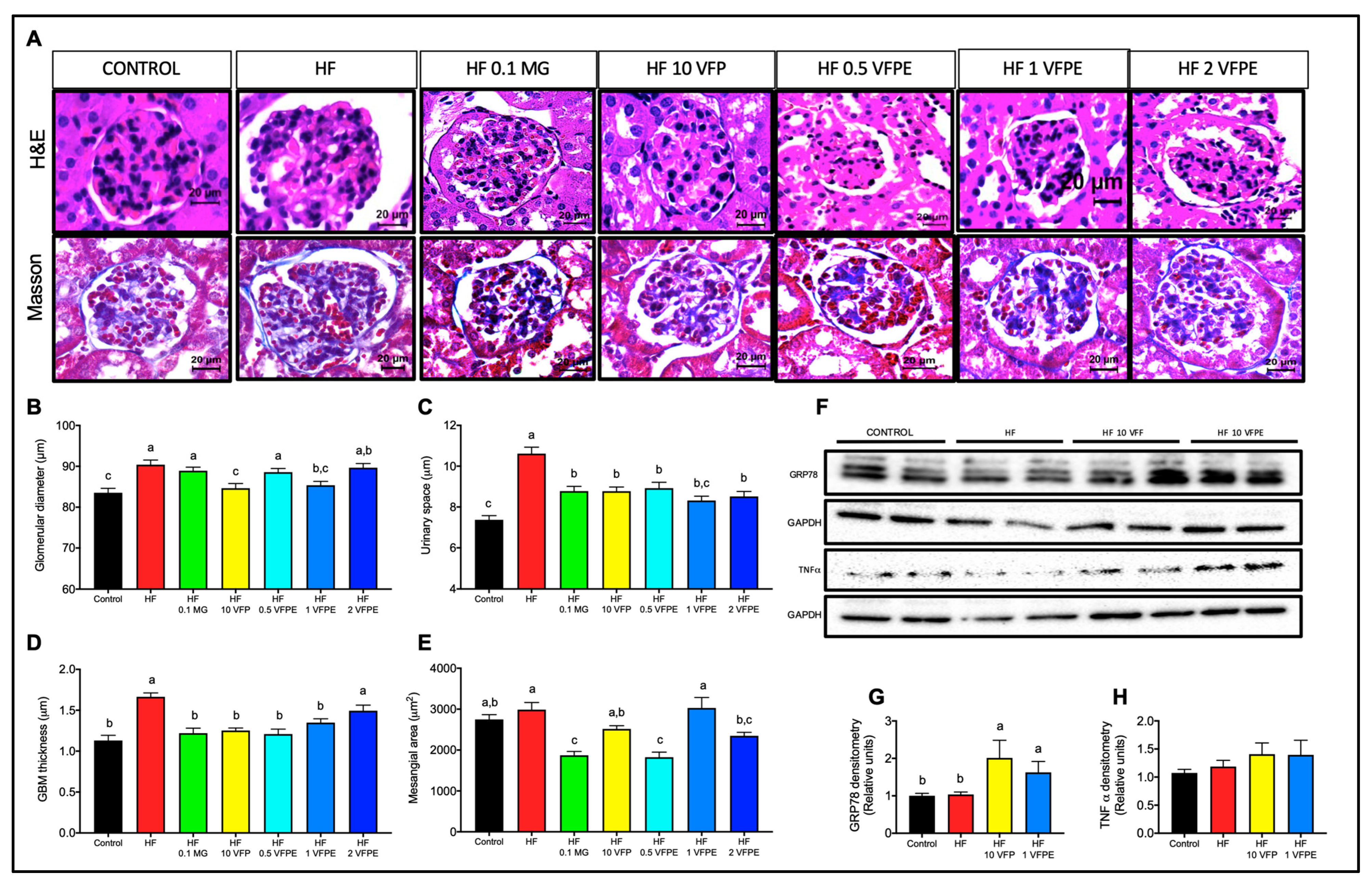
2.8. VFP or VFPE Exert Reno-Protective Effects on Mice Fed a High-Fat Diet
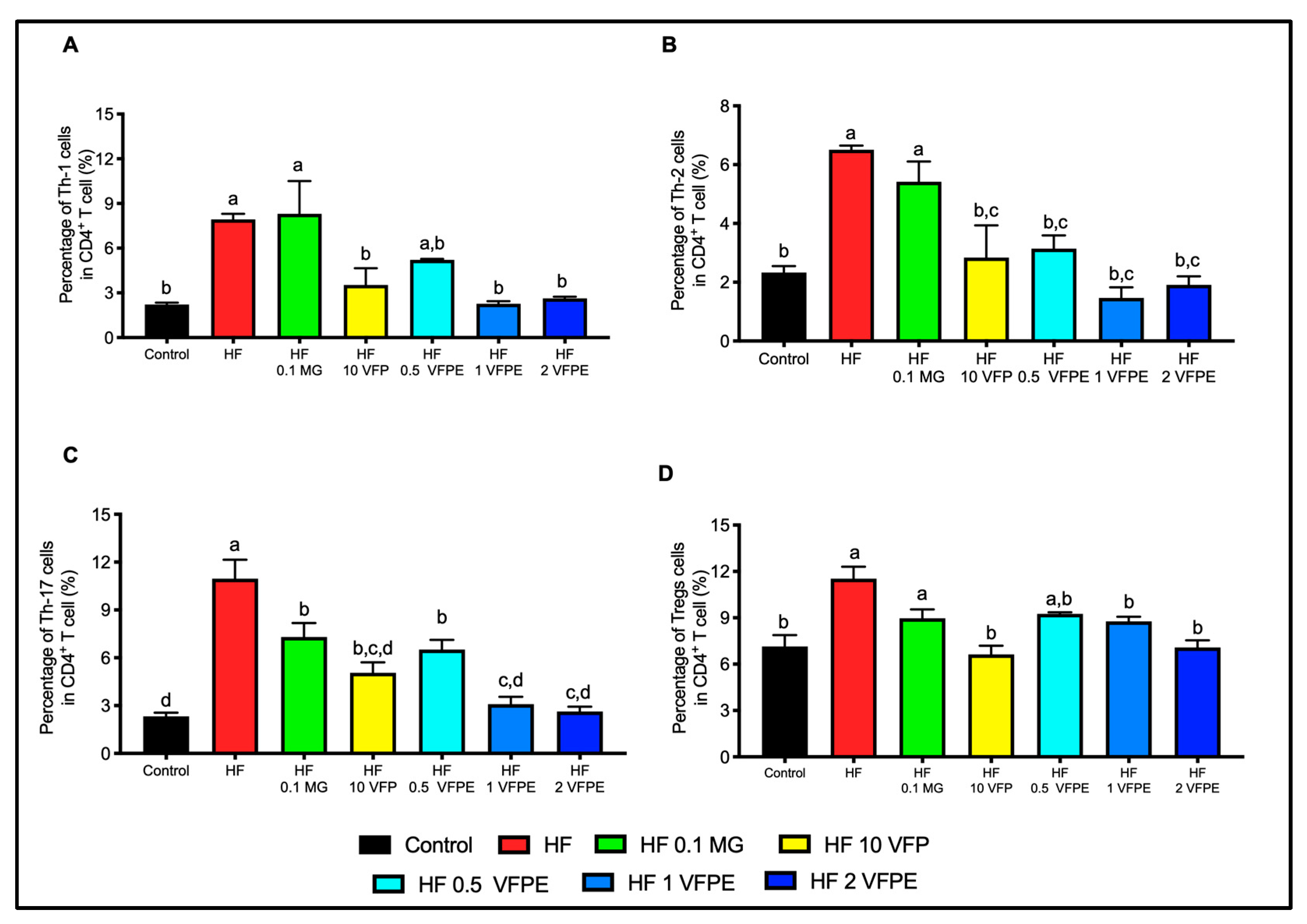
2.9. VFP or VFPE Increases Circulating Anti-Inflammatory Immune Cells
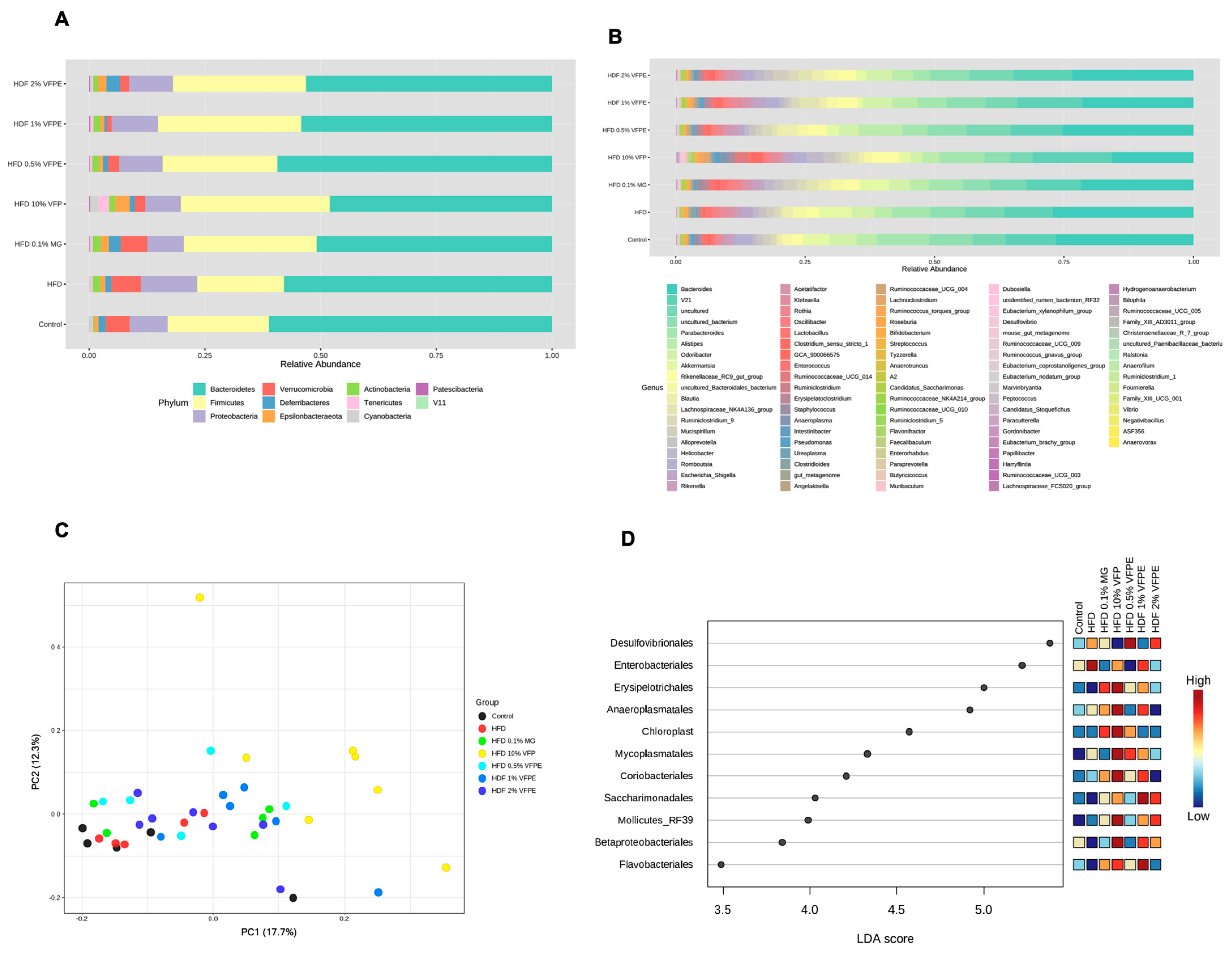
2.10. Effect of VFP or VFPE on Bacterial Taxonomy, Relative Abundance at Phylum and Genus Level, Alpha and Beta Diversity, and Linear Discriminant Analysis (LDA) Score of the 16S rRNA Sequencing of Feces in Mice Fed an HF-Diet
2.11. Dietary Intervention with VFP Decreases Body Weight, Pancreatic Islet Size, and Adipocyte Hypertrophy in Obese Mice
3. Discussion
3.1. HPLC Quantification of Methyl Gallate (MG) and Naringenin (NA) in Vachellia Farnesiana Extract
3.2. VFPE Prevents Excessive Body Weight Gain in Mice Fed a High-Fat Diet by Increasing Whole-Body Oxygen Consumption and Skeletal Muscle Mitochondrial Activity
3.3. VFP or VFPE Reduces Plasma Triglycerides and Hepatic Lipid Content
3.4. Intake of VFP or VFPE Prevents Subcutaneous and Visceral Adipocyte Hypertrophy and Preserves Brown Adipose Tissue Thermogenic Morphology
3.5. Intake of VFP or VFPE Is Not Nephrotoxic
3.6. VFP or VFPE Restored Immune Homeostasis in Mice Fed with a High-Fat Diet, with a Dose-Dependent Effect on the Downregulation of CD4+ Effector T Subsets and Tregs Cells
3.7. Bacterial Taxonomy, Abundance Relative (%) at Phylum and Genus Level of the 16S rRNA Sequencing of Feces in Mice Fed with HF Diet or VFP or VFPE
3.8. VFPE Reverted Visceral and Subcutaneous Adipocyte Hypertrophy and Restored Thermogenic Brown Adipose Tissue Morphology in Obese Mice
4. Materials and Methods
4.1. Vegetal Collection and Pods Extract
4.2. Quantification of Methyl Gallate and Naringenin from VFP
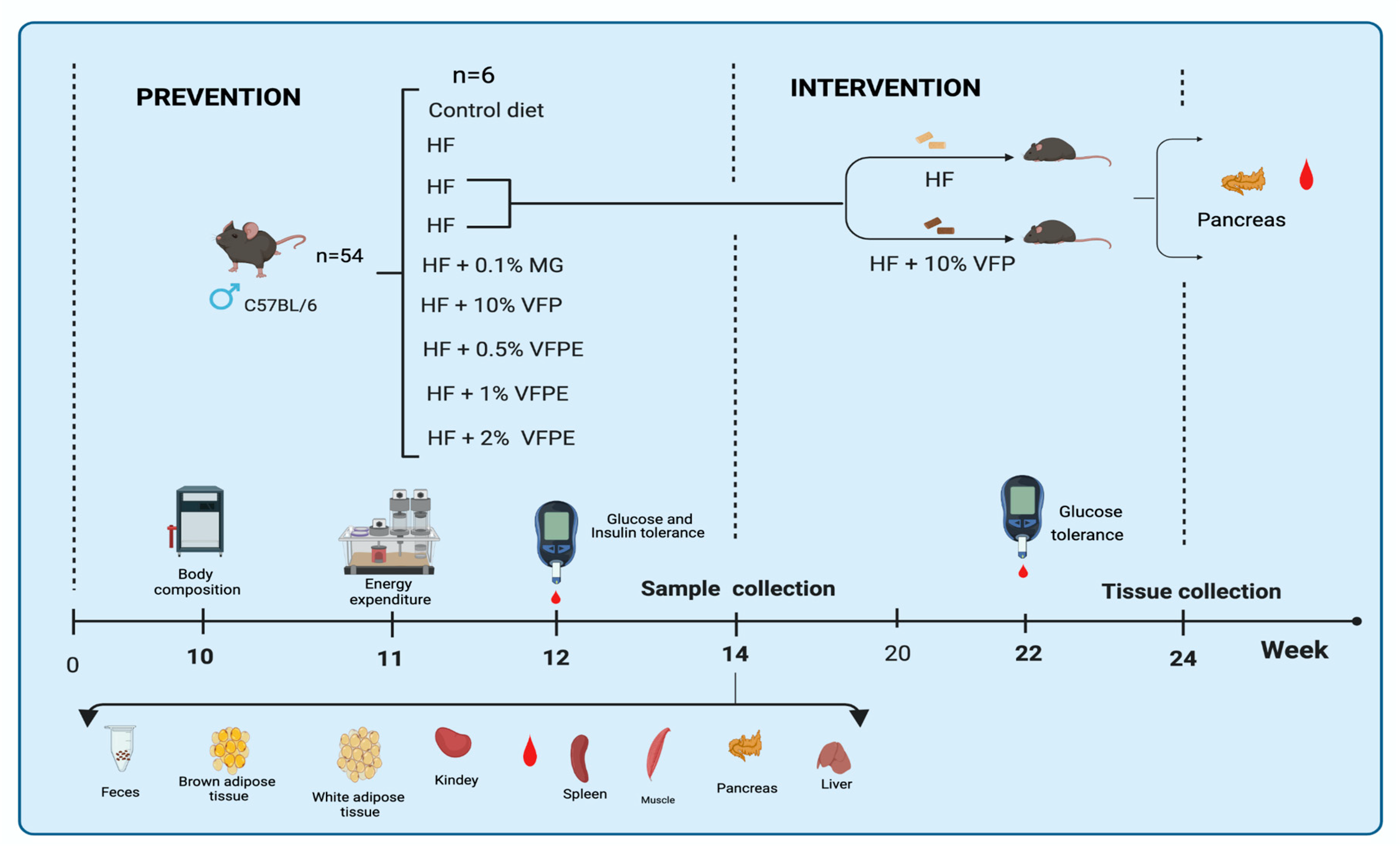
4.3. In Vivo Assay
4.3.1. Animals
4.3.2. Experimental Design and Diets
4.3.3. Evaluation of Body Composition and Energy Expenditure
4.3.4. Evaluation of Glucose and Insulin Tolerance
4.3.5. Histological Analysis of Liver, Pancreas, White and Brown Adipose Tissue
4.3.6. Mitochondria Abundance in Skeletal Muscle
4.3.7. Lipid Content in Liver and Skeletal Muscle
4.3.8. Kidney Histological Analysis
4.3.9. Protein Extraction, SDS/PAGE, and Immunoblotting
4.3.10. Mononuclear Cell Preparations and Flow Cytometric Analysis
4.3.11. Fecal DNA Extraction and 16S rRNA Sequencing
4.3.12. Sequence Analysis
4.4. In Vitro Assays
4.4.1. Extracellular Flux Analysis in Primary Hepatocytes and C2C12 Myotubes
4.4.2. Evaluation of Insulin Secretion in INS-1E Cells
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; Available online: https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf (accessed on 20 July 2022).
- Montgomery, M.K.; De Nardo, W.; Watt, M.J. Impact of Lipotoxicity on Tissue “Cross Talk” and Metabolic Regulation. Physiology 2019, 34, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes End. 2019, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Ziadlou, M.; Karimi, S.; Hosseini-Esfahani, F.; Azizi, F. The association of dietary patterns and adherence to WHO healthy diet with metabolic syndrome in children and adolescents: Tehran lipid and glucose study. BMC Public Health 2019, 19, 1457. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.W.; Einhorn, D. Fatty kidney disease: A new renal and endocrine clinical entity? Describing the role of the kidney in obesity, metabolic syndrome, and Type 2 Diabetes. Endocr. Pract. 2019, 25, 854–858. [Google Scholar] [CrossRef]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and epigenetics action mechanisms of antiobesity medicinal plants and phytochemicals. Evid. Based Complement. Alternat. Med. 2021, 2021, 9995903. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s milk intake prevents obesity, hepatic steatosis and insulin resistance in mice fed a high-fat diet by reducing inflammatory markers and increasing energy expenditure and mitochondrial content in skeletal muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Granados, O.; Torre-Villalvazo, I.; Serna-Saldivar, S.O.; Torres, N.; Palacios-González, B.; Tovar, A.R. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 2014, 112, 886–899. [Google Scholar] [CrossRef]
- Morán-Ramos, S.; Avila-Nava, A.; Tovar, A.R.; Pedraza-Chaverri, J.; López-Romero, P.; Torres, N. Opuntia ficus indica (nopal) attenuates hepatic steatosis and oxidative stress in obese Zucker (fa/fa) rats. Nutr. J. 2012, 142, 1956–1963. [Google Scholar] [CrossRef]
- Torres, A.; Noriega, L.G.; Delgadillo-Puga, C.; Tovar, A.R.; Navarro-Ocaña, A. Caffeoylquinic acid derivatives of purple sweet potato as modulators of mitochondrial function in mouse primary hepatocytes. Molecules 2021, 26, 319. [Google Scholar] [CrossRef]
- Leal-Díaz, A.M.; Noriega, L.G.; Torre-Villalvazo, I.; Torres, N.; Alemán-Escondrillas, G.; López-Romero, P.; Sánchez-Tapia, M.; Aguilar-López, M.; Furuzawa-Carballeda, J.; Velázquez-Villegas, L.A.; et al. Aguamiel concentrate from Agave salmiana and its extracted saponins attenuated obesity and hepatic steatosis and increased Akkermansia muciniphila in C57BL6 mice. Sci. Rep. 2016, 6, 34242. [Google Scholar] [CrossRef]
- Bell, K.L.; Murphy, D.J.; Gardner, M.G. Isolation, via 454 Sequencing, and characterization of microsatellites for Vachellia farnesiana (Fabaceae: Mimosoideae). Appl. Plant Sci. 2013, 1, 1300035. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Cuchillo-Hilario, M. Reviewing the Benefits of Grazing/Browsing Semiarid Rangeland Feed Resources and the Transference of Bioactivity and Pro-Healthy Properties to Goat Milk and Cheese: Obesity, Insulin Resistance, Inflammation and Hepatic Steatosis Prevention. Animals 2021, 11, 2942. [Google Scholar] [CrossRef]
- Hernández-García, E.; García, A.; Alanís, F.G.A.; Rivas-Galindo, V.M.; Delgadillo-Puga, C.; Camacho-Corona, M.D.R. Nuclear magnetic resonance spectroscopy data of isolated compounds from Acacia farnesiana (L.) Willd fruits and two esterified derivatives. Data Brief 2019, 22, 255–268. [Google Scholar] [CrossRef]
- Puga, C.D.; Cuchillo-Hilario, M.; Mendoza, J.G.E.; Campos, O.M.; Jijón, E.M.; Martínez, M.D.; Izazaga, M.A.; Solano, J.L.; Chaverri, J.P. Antioxidant activity and protection against oxidative-induced damage of Acacia shaffneri and Acacia farnesiana pods extracts: In Vitro and in Vivo Assays. BMC Complement. Altern. Med. 2015, 15, 435. [Google Scholar] [CrossRef]
- Claudia, D.P.; Mario, C.-H.; Arturo, N.O.; Noel, M.-C.O.; Antonio, N.C.; Teresa, R.A.; Gerardo, L.-T.Z.; Margarita, D.M.; Alejandra, I.M.; Rosalina, C.M.Y.; et al. Polyphenolic compounds in organic and aqueous extracts from Acacia farnesiana pods analyzed by ULPS-ESI-Q-oa/TOF-MS. In Vitro antioxidant activity and anti-Inflammatory response in CD-1 Mice. Molecules 2018, 23, 2386. [Google Scholar] [CrossRef]
- Sánchez, E.; Heredia, N.; Camacho-Corona, M.D.R.; García, S. Isolation, characterization and mode of antimicrobial action against Vibrio cholerae of methyl gallate isolated from Acacia farnesiana. J. Appl. Microbiol. 2013, 115, 1307–1316. [Google Scholar] [CrossRef]
- Hernández-García, E.; García, A.; Garza-González, E.; Alanís, F.G.A.; Rivas-Galindo, V.M.; Rodríguez-Rodríguez, J.; Alcantar-Rosales, V.M.; Delgadillo-Puga, C.; Camacho-Corona, M.D.R. Chemical Composition of Acacia farnesiana (L.) Wild fruits and its activity against Mycobacterium Tuberculosis and dysentery bacteria. J. Ethnoph. 2019, 230, 74–80. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, J.; Ahrén, B.; Wierup, N. Increased β-cell volume in mice fed a high-fat diet: A dynamic study over 12 months. Islets 2010, 2, 353–356. [Google Scholar] [CrossRef]
- Huiyun, L.; Ward, W.F. PGC-1α: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Doan, K.; Ko, C.M.; Kinyua, A.W.; Yang, D.J.; Choi, Y.-H.; Oh, I.Y.; Nguyen, N.M.; Ko, A.; Choi, J.W.; Jeong, Y.; et al. Gallic acid regulates body weight and glucose homeostasis through AMPK Activation. Endocrinology 2015, 1, 157–168. [Google Scholar] [CrossRef]
- Ullah, R.; Rauf, N.; Nabi, G.; Ullah, H.; Shen, Y.; Zhou, Y.D.; Fu, J. Role of nutrition in the pathogenesis and prevention of Non-alcoholic fatty liver disease: Recent Updates. Int. J. Biol. Sci. 2019, 15, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Meng, T.; Gao, P.-J.; Ruan, C.-C. The role of brown adipose tissue dysfunction in the development of cardiovascular disease. Front. Endocrinol. 2021, 12, 652246. [Google Scholar] [CrossRef]
- Tovar-Palacio, C.; Noriega, L.G.; Villalvazo, I.T.; Díaz-Villaseñor, A.; Palacios-González, B. Chapter 5. The Interaction of Nutrition with nuclear receptors in obesity and diabetes. In Nutritional Signaling Pathway Activities in Obesity and Diabetes; The Royal Society of Chemistry: London, UK, 2020; pp. 94–163. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Achaintre, D.; Rothwell, J.A.; Rinaldi, S.; Assi, N.; Ferrari, P.; Leitzmann, M.; Boutron-Ruault, M.-C.; Fagherazzi, G.; Auffret, A.; et al. Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study. Sci. Rep. 2016, 6, 26905. [Google Scholar] [CrossRef]
- Ricciardi, C.A.; Gnudi, L. The endoplasmic reticulum stress and the unfolded protein response in kidney disease: Implications for vascular growth factors. J. Cell Mol. Med. 2020, 24, 12910–12919. [Google Scholar] [CrossRef]
- Figueroa-Juárez, E.; Noriega, L.G.; Pérez-Monter, C.; Alemán, G.; Hernández-Pando, R.; Correa-Rotter, R.; Ramírez, V.; Tovar, A.R.; Torre-Villalvazo, I.; Tovar-Palacio, C. The Role of the Unfolded Protein Response on Renal Lipogenesis in C57BL/6 Mice. Biomolecules 2021, 1, 73. [Google Scholar] [CrossRef]
- Shaker, M.E. The contribution of sterile inflammation to the fatty liver disease and the potential therapies. Biomed. Pharmacother. 2022, 148, 112789. [Google Scholar] [CrossRef]
- Ribeiro, I.A.; Ribeiro, M.H. Naringin and naringenin determination and control in grapefruit juice by a validated HPLC method. Food Control 2008, 19, 432–438. [Google Scholar] [CrossRef]
- Gambardella, J.; Lombardi, A.; Santulli, G. Metabolic Flexibility of Mitochondria Plays a Key Role in Balancing Glucose and Fatty Acid Metabolism in the Diabetic Heart. Diabetes 2020, 69, 2054–2057. [Google Scholar] [CrossRef]
- Zampino, M.; Semba, R.D.; Adelnia, F.; Spencer, R.G.; Fishbein, K.W.; Schrack, J.A.; Simonsick, E.M.; Ferrucci, L. Greater Skeletal Muscle Oxidative Capacity Is Associated with Higher Resting Metabolic Rate: Results from the Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A 2020, 75, 2262–2268. [Google Scholar] [CrossRef]
- Muoio, D.M.; MacLean, P.S.; Lang, D.B.; Li, S.; Houmard, J.A.; Way, J.M.; Winegar, D.A.; Christopher Corton, J.; Lynis Dohm, G.; Kraus, W.E. Fatty Acid Homeostasis and Induction of Lipid Regulatory Genes in Skeletal Muscles of Peroxisome Proliferator-activated Receptor (PPAR) α Knock-out Mice: Evidence for Compensatory Regulation by PPARδ. J. Biol. Chem. 2002, 277, 26089–26097. [Google Scholar] [CrossRef]
- Phua, W.W.T.; Wong, M.X.Y.; Liao, Z.; Tan, N.S. An aPPARent functional consequence in skeletal muscle physiology via peroxisome proliferator-activated receptors. Int. J. Mol. Sci. 2018, 19, 1425. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Pettinelli, P.; Videla, L.A. Up-Regulation of PPAR-γ mRNA expression in the liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metabo. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Sikder, K.; Shukla, S.K.; Patel, N.; Singh, H.; Rafiq, K. High Fat Diet Upregulates Fatty Acid Oxidation and Ketogenesis via Intervention of PPAR-γ. Cell Physiol. Biochem. 2018, 48, 1317–1331. [Google Scholar] [CrossRef]
- Palacios-González, B.; Vargas-Castillo, A.; Velázquez-Villegas, L.A.; Vasquez-Reyes, S.; López, P.; Noriega, L.G.; Aleman, G.; Tovar-Palacio, C.; Torre-Villalvazo, I.; Yang, L.-J.; et al. Genistein increases the thermogenic program of subcutaneous WAT and increases energy expenditure in mice. J. Nutr. Biochem. 2019, 68, 59–68. [Google Scholar] [CrossRef]
- Avior, Y.; Bomze, D.; Ramon, O.; Nahmias, Y. Flavonoids as dietary regulators of nuclear receptor activity. Food Funct. 2013, 4, 831–844. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, M.; Lee, D.; Jang, J.; Shin, S.S.; Yoon, M. Fenofibrate regulates visceral obesity and nonalcoholic steatohepatitis in obese female ovariectomized c57bl/6j mice. Int. J. Mol. Sci. 2021, 22, 3675. [Google Scholar] [CrossRef] [PubMed]
- Menendez, A.; Wanczyk, H.; Walker, J.; Zhou, B.; Santos, M.; Finck, C. Obesity and Adipose Tissue Dysfunction: From Pediatrics to Adults. Genes 2022, 13, 1866. [Google Scholar] [CrossRef] [PubMed]
- Gorenec, L.; Lepej, S.Z.; Grgic, I.; Planinic, A.; Bes, J.I.; Vince, A.; Begovac, J. The comparison of Th1, Th2, Th9, Th17 and Th22 cytokine profiles in acute and chronic HIV-1 infection. Microb. Pathog. 2016, 97, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Abadja, F.; Sarraj, B.; Ansari, M.J. Significance of Th17 immunity in transplantation. Curr. Opin. Organ. Transplant. 2012, 17, 8–14. [Google Scholar] [CrossRef]
- Dardalhon, V.; Korn, T.; Kuchroo, V.K.; Anderson, A.C. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008, 31, 252–256. [Google Scholar] [CrossRef]
- Deteix, C.; Attuil-Audenis, V.; Duthey, A.; Patey, N.; McGregor, B.; Dubois, V.; Caligiuri, G.; Graff-Dubois, S.; Morelon, E.; Thaunat, O. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J. Immunol. 2010, 184, 5344–5351. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Q.; Zheng, D.; Sun, Y.; Wang, C.; Yu, X.; Wang, Y.; Lee, V.W.; Zheng, G.; Tan, T.K.; et al. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013, 84, 745–755. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Wang, X.; Wang, Y.; Niu, A.; Wang, S.; Zou, C.; Harris, R.C. IL-1/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017, 91, 375–386. [Google Scholar] [CrossRef]
- Ricardo, S.D.; van Goor, H.; Eddy, A.A. Macrophage diversity in renal injury and repair. J. Clin. Investig. 2008, 118, 3522–3530. [Google Scholar] [CrossRef]
- Ikezumi, Y.; Suzuki, T.; Karasawa, T.; Hasegawa, H.; Yamada, T.; Imai, N.; Narita, I.; Kawachi, H.; Polkinghorne, K.R.; Nikolic-Paterson, D.J.; et al. Identification of alternatively activated macrophages in new-onset pediatric and adult immunoglobulin A nephropathy: Potential role in mesangial matrix expansion. Histopathol 2011, 8, 198–210. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Durandy, A. Activation-induced cytidine deaminase: A dual role in class-switch recombination and somatic hypermutation. Eur. J. Immunol. 2003, 33, 2069–2073. [Google Scholar] [CrossRef]
- Wynn, T. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Perdigoto, A.L.; Chatenoud, L.; Bluestone, J.A.; Herold, K.C. Inducing and administering Tregs to treat human disease. Front. Immunol. 2015, 6, 654. [Google Scholar] [CrossRef]
- Macfarlane, S.; Steed, H.; Macfarlane, G.T. Intestinal bacteria and inflammatory bowel disease. Crit. Rev. Clin. Lab. Sci. 2009, 46, 25–54. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Fornelos, N.; Franzosa, E.A.; Bishai, J.; Annand, J.W.; Oka, A.; Lloyd-Price, J.; Arthur, T.D.; Garner, A.; Avila-Pacheco, J.; Haiser, H.J.; et al. Growth effects of N-acy lethanolamines on gut bacteria reflect altered bacterial abundances in inflammatory bowel disease. Nat. Microbiol. 2020, 5, 486–497. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Unno, T.; Osakabe, N. Green tea extract and black tea extract differentially influence cecal levels of short-chain fatty acids in rats. Food Sci. Nutr. 2018, 6, 728–735. [Google Scholar] [CrossRef]
- Noratto, G.D.; Garcia-Mazcorro, J.F.; Markel, M.; Martino, H.S.; Minamoto, Y.; Steiner, J.M.; Byrne, D.; Suchodolski, J.S.; Mertens-Talcott, S.U. Carbohydrate-Free Peach (Prunus persica) and Plum (Prunus domestica) Juice Affects Fecal Microbial Ecology in an Obese Animal Model. PLoS ONE 2014, 9, e101723. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Huang, J.; Ding, Y.; Pan, Z.; Zhao, Y.; Zhang, R.; Hu, B.; Zeng, X. In vitro extraction and fermentation of polyphenols from grape seeds (Vitis vinifera) by human intestinal microbiota. Food Funct. 2016, 7, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Venema, K.; Tabernero, M.; Sarriá, B.; Bravo, L.L.; Mateos, R. Bioconversion by gut microbiota of predigested mango (Mangifera indica L.) ‘Ataulfo’ peel polyphenols assessed in a dynamic (TIM-2) in vitro model of the human colon. Food Res. Int. 2021, 139, 109963. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2017, 244, 735–745. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Derrell, C.J.; Gebhart, G.F.; Gonder, J.C.; Keeling, M.E.; Kohn, D.F. The 1996 guide for the care and use of laboratory animals. ILAR 1997, 38, 41–48. [Google Scholar]
- Reeves, P.G.; Forrest, H.N.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Berry, M.N.; Friends, D.S. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef]
- Hetta, M.H.; Owins, A.L.; Haddad, P.S.; Eid, H.M. The fatty acid-rich fraction of Eruca sativa (rocket salad) leaf extract exerts antidiabetic effects in cultured skeletal muscle, adipocytes and liver cells. Pharm. Biol. 2017, 55, 810–818. [Google Scholar] [CrossRef]




| g/kg of Diet | |||||||
|---|---|---|---|---|---|---|---|
| Ingredient (%) | Control | HF | HF + 0.1% Methyl Gallate | HF + 10% VFP | HF + 0.5% VFPE | HF + 1% VFPE | HF + 2% VFPE |
| Casein a | 200 | 200 | 200 | 190 | 200 | 200 | 200 |
| Sucrose b | 100 | 339 | 338 | 271 | 334 | 329 | 319 |
| Maltodextrin d | 132 | 150 | 150 | 150 | 150 | 150 | 150 |
| Cornstarch c | 397 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lard | 0 | 140 | 140 | 128 | 140 | 140 | 140 |
| Soy oil e | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| Cellulose f | 50 | 50 | 50 | 39 | 50 | 50 | 50 |
| Vitamin Mix g | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Mineral Mix h | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| L-Cystine i | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Choline j | 2.5 | 2.5 | 2.5 | 3 | 2.5 | 2.5 | 2.5 |
| Experimental Compounds | 0 | 0 | 1 | 100 | 5 | 10 | 20 |
| g/100 g of diet | |||||||
| Protein | 20.3 | 17.2 | 17.2 | 18.0 | 17.3 | 17.4 | 17.5 |
| Carbohydrates | 63.8 | 42.1 | 42.1 | 40.0 | 41.9 | 41.6 | 41.1 |
| Fat | 16.0 | 40.7 | 40.7 | 42.1 | 40.9 | 41.0 | 41.4 |
| kcal/kg of diet | |||||||
| Energy content | 3946 | 4646 | 4642 | 4228 | 4626 | 4606 | 4566 |
| mg GAE/kg of diet | |||||||
| Total polyphenols content | - | - | - | 399.0 | 199.5 | 399.0 | 798.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgadillo-Puga, C.; Sánchez-Castillo, D.R.; Cariño-Cervantes, Y.Y.; Torre-Villalvazo, I.; Tovar-Palacio, C.; Vásquez-Reyes, S.; Furuzawa-Carballeda, J.; Acevedo-Carabantes, J.A.; Camacho-Corona, M.d.R.; Guzmán-Mar, J.L.; et al. Vachellia farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2023, 24, 7984. https://doi.org/10.3390/ijms24097984
Delgadillo-Puga C, Sánchez-Castillo DR, Cariño-Cervantes YY, Torre-Villalvazo I, Tovar-Palacio C, Vásquez-Reyes S, Furuzawa-Carballeda J, Acevedo-Carabantes JA, Camacho-Corona MdR, Guzmán-Mar JL, et al. Vachellia farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet. International Journal of Molecular Sciences. 2023; 24(9):7984. https://doi.org/10.3390/ijms24097984
Chicago/Turabian StyleDelgadillo-Puga, Claudia, Dulce R. Sánchez-Castillo, Yonatan Y. Cariño-Cervantes, Ivan Torre-Villalvazo, Claudia Tovar-Palacio, Sarai Vásquez-Reyes, Janette Furuzawa-Carballeda, Joshua Ayork Acevedo-Carabantes, María del Rayo Camacho-Corona, Jorge Luis Guzmán-Mar, and et al. 2023. "Vachellia farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet" International Journal of Molecular Sciences 24, no. 9: 7984. https://doi.org/10.3390/ijms24097984
APA StyleDelgadillo-Puga, C., Sánchez-Castillo, D. R., Cariño-Cervantes, Y. Y., Torre-Villalvazo, I., Tovar-Palacio, C., Vásquez-Reyes, S., Furuzawa-Carballeda, J., Acevedo-Carabantes, J. A., Camacho-Corona, M. d. R., Guzmán-Mar, J. L., Cisneros-Zevallos, L., Tovar, A. R., Rebollar-Vega, R., Hernández-Montes, G., Ulloa-Aguirre, A., Palacios-Gonzalez, B., & Noriega, L. G. (2023). Vachellia farnesiana Pods or a Polyphenolic Extract Derived from Them Exert Immunomodulatory, Metabolic, Renoprotective, and Prebiotic Effects in Mice Fed a High-Fat Diet. International Journal of Molecular Sciences, 24(9), 7984. https://doi.org/10.3390/ijms24097984







