The Role of the Estrogen-Related Receptor Alpha (ERRa) in Hypoxia and Its Implications for Cancer Metabolism
Abstract
1. Introduction
2. HIF-1, -2 and -3 Mediate the Hypoxia Response
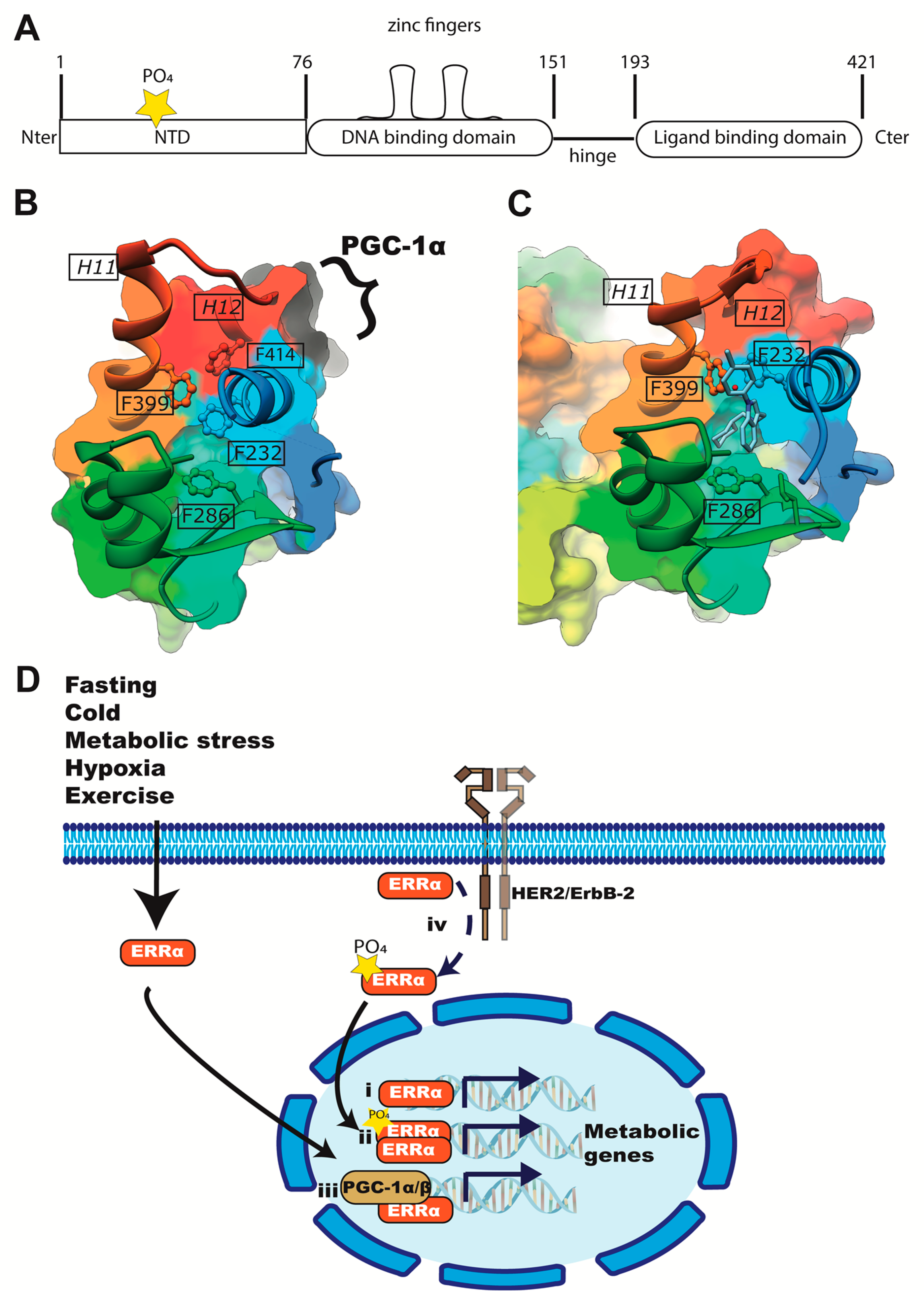
3. Introduction to the Estrogen-Related Receptors (ERR)
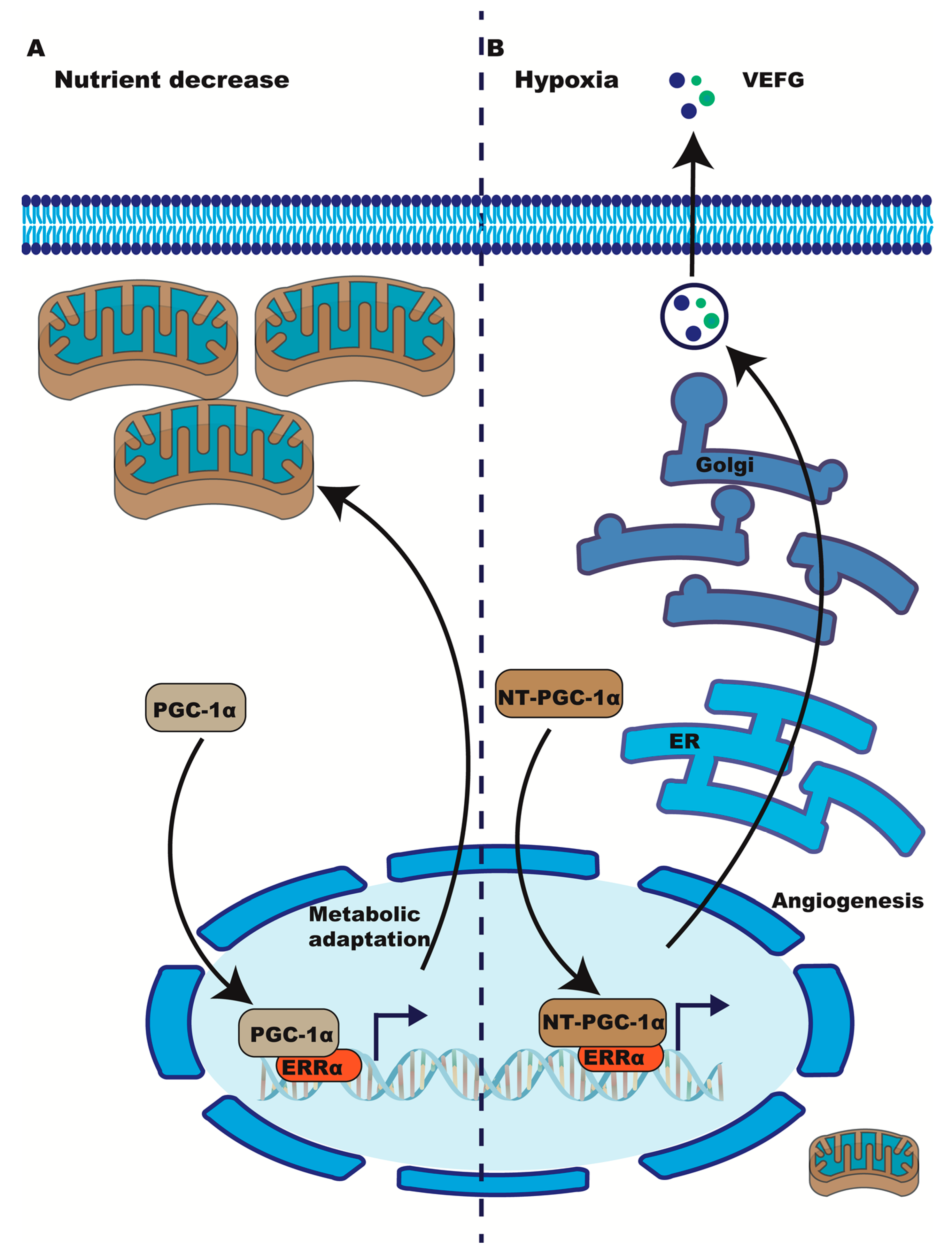
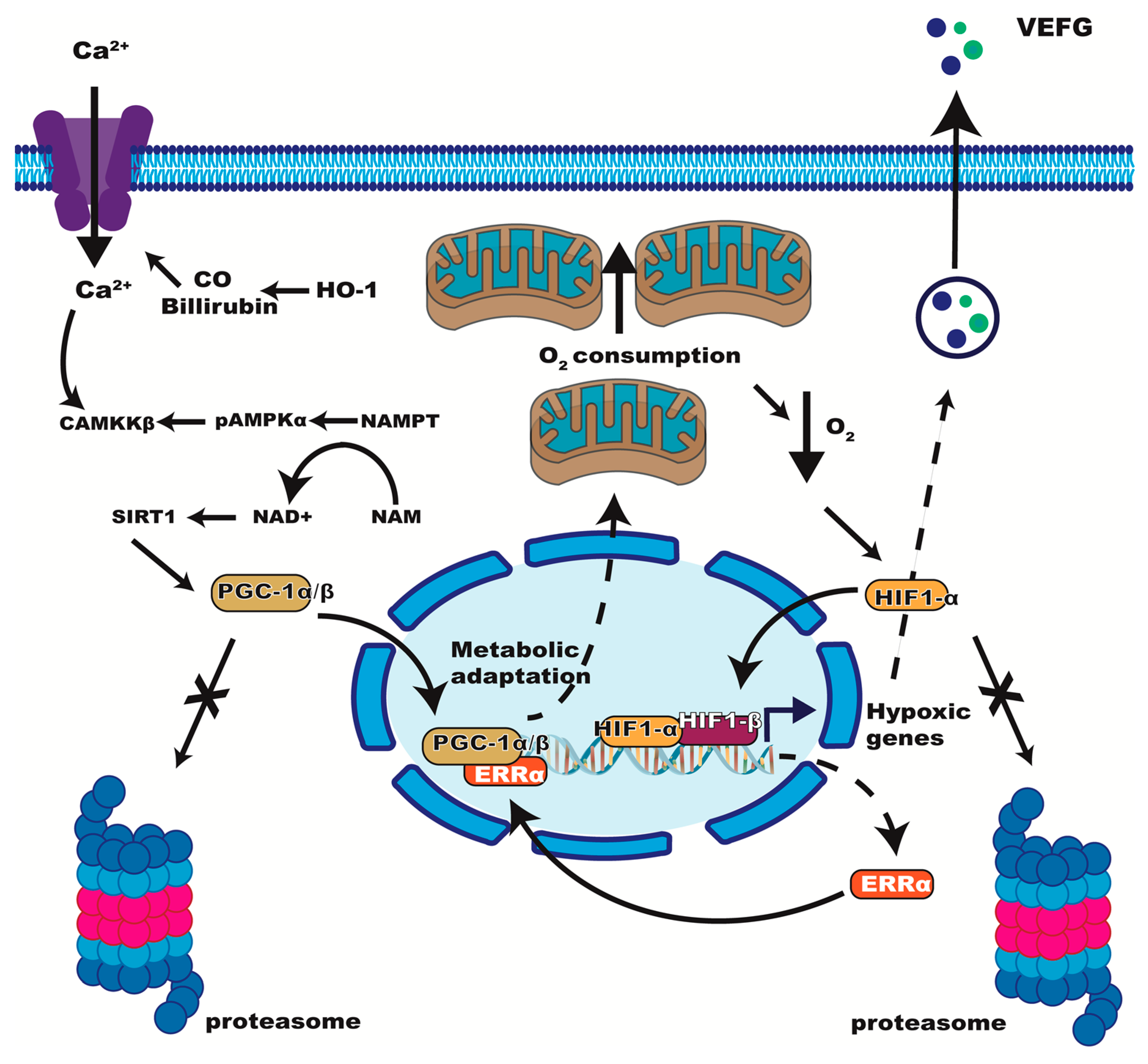
4. Evidence of ERRα’s Participation in the Hypoxia Response
4.1. ERRα Induces VEGF Expression during Muscle Ischemia and Other Models
4.2. ERRα in Brain and Spinal Cord Hypoxia/Ischemia
4.3. ERRα in Hypobaric Hypoxia
4.4. ERRα’s Role in Cancer-Related Hypoxia
4.5. ERRα and Kidney Hypoxia
4.6. Hypoxia in the Invertebrate Fly Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-H.; Zheng, J.Z.; Leung, S.W.; Roe, R.; Semenza, G.L. Transactivation and Inhibitory Domains of Hypoxia-Inducible Factor 1α Modulation of Transcriptional Activity by Oxygen Tension*. J. Biol. Chem. 1997, 272, 19253–19260. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S. Gene Regulation under Low Oxygen: Holding Your Breath for Transcription. Trends Biochem. Sci. 2007, 32, 389–397. [Google Scholar] [CrossRef]
- Seta, K.A.; Spicer, Z.; Yuan, Y.; Lu, G.; Millhorn, D.E. Responding to Hypoxia: Lessons from a Model Cell Line. Sci. Stke. 2002, 2002, re11. [Google Scholar] [CrossRef] [PubMed]
- Fels, D.R.; Koumenis, C. The PERK/eIF2α/ATF4 Module of the UPR in Hypoxia Resistance and Tumor Growth. Cancer Biol. Ther. 2006, 5, 723–728. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A Nuclear Factor Induced by Hypoxia via de Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Tam, I.S.; Giguère, V. There and Back Again: The Journey of the Estrogen-Related Receptors in the Cancer Realm. J. Steroid Biochem. Mol. Biol. 2016, 157, 13–19. [Google Scholar] [CrossRef]
- Matsushima, H.; Mori, T.; Ito, F.; Yamamoto, T.; Akiyama, M.; Kokabu, T.; Yoriki, K.; Umemura, S.; Akashi, K.; Kitawaki, J. Anti-Tumor Effect of Estrogen-Related Receptor Alpha Knockdown on Uterine Endometrial Cancer. Oncotarget 2016, 7, 34131–34148. [Google Scholar] [CrossRef]
- Deblois, G.; Giguère, V. Oestrogen-Related Receptors in Breast Cancer: Control of Cellular Metabolism and Beyond. Nat. Rev. Cancer 2013, 13, 27–36. [Google Scholar] [CrossRef]
- Cai, Q.; Lin, T.; Kamarajugadda, S.; Lu, J. Regulation of Glycolysis and the Warburg Effect by Estrogen-Related Receptors. Oncogene 2013, 32, 2079–2086. [Google Scholar] [CrossRef]
- Advanced Information. The Nobel Prize in Physiology or Medicine 2019. NobelPrize.org Nobel Prize Outreach AB. 2023. Available online: https://www.nobelprize.org/prizes/medicine/2019/advanced-information/ (accessed on 24 March 2023).
- Semenza, G.L. Oxygen Sensing, Homeostasis, and Disease. N. Engl. J. Med. 2011, 365, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. The Genomics and Genetics of Oxygen Homeostasis. Annu. Rev. Genom. Hum. Genet. 2020, 21, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Padwad, Y.S. HIF-1 in Cancer Therapy: Two Decade Long Story of a Transcription Factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Deng, S.; Li, W. The Transcriptional Factors HIF-1 and HIF-2 and Their Novel Inhibitors in Cancer Therapy. Expert. Opin. Drug Dis. 2019, 14, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Leung, I.K.H.; Tian, Y.-M.; Abboud, M.I.; Ge, W.; Domene, C.; Cantrelle, F.-X.; Landrieu, I.; Hardy, A.P.; Pugh, C.W.; et al. Structural Basis for Oxygen Degradation Domain Selectivity of the HIF Prolyl Hydroxylases. Nat. Commun. 2016, 7, 12673. [Google Scholar] [CrossRef]
- Kallio, P.J.; Okamoto, K.; O’Brien, S.; Carrero, P.; Makino, Y.; Tanaka, H.; Poellinger, L. Signal Transduction in Hypoxic Cells: Inducible Nuclear Translocation and Recruitment of theCBP/P300 Coactivator by the Hypoxia-induciblefactor-1α. EMBO J. 1998, 17, 6573–6586. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Michels, C.L.; Leung, M.-K.; Arany, Z.P.; Kung, A.L.; Livingston, D.M. Functional Role of P35srj, a Novel P300/CBP Binding Protein, during Transactivation by HIF-1. Gene Dev. 1999, 13, 64–75. [Google Scholar] [CrossRef]
- Semenza, G.L.; Jiang, B.-H.; Leung, S.W.; Passantino, R.; Concordet, J.-P.; Maire, P.; Giallongo, A. Hypoxia Response Elements in the Aldolase A, Enolase 1, and Lactate Dehydrogenase A Gene Promoters Contain Essential Binding Sites for Hypoxia-Inducible Factor 1*. J. Biol. Chem. 1996, 271, 32529–32537. [Google Scholar] [CrossRef]
- Xia, X.; Lemieux, M.E.; Li, W.; Carroll, J.S.; Brown, M.; Liu, X.S.; Kung, A.L. Integrative Analysis of HIF Binding and Transactivation Reveals Its Role in Maintaining Histone Methylation Homeostasis. Proc. Natl. Acad. Sci. USA 2009, 106, 4260–4265. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.-H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of Vascular Endothelial Growth Factor Gene Transcription by Hypoxia-Inducible Factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.V.; Kotch, L.E.; Agani, F.; Leung, S.W.; Laughner, E.; Wenger, R.H.; Gassmann, M.; Gearhart, J.D.; Lawler, A.M.; Yu, A.Y.; et al. Cellular and Developmental Control of O2 Homeostasis by Hypoxia-Inducible Factor 1α. Gene Dev. 1998, 12, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 Mediates Adaptation to Hypoxia by Actively Downregulating Mitochondrial Oxygen Consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Påhlman, S.; Mohlin, S. Hypoxia and Hypoxia-Inducible Factors in Neuroblastoma. Cell Tissue Res. 2018, 372, 269–275. [Google Scholar] [CrossRef]
- Haase, V.H. Regulation of Erythropoiesis by Hypoxia-Inducible Factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 and HIF-2 Transcription Factors—Similar but Not Identical. Mol. Cells 2010, 29, 435–442. [Google Scholar] [CrossRef]
- Duan, C. Hypoxia-Inducible Factor 3 Biology: Complexities and Emerging Themes. Am. J. Physiol.-Cell Physiol. 2016, 310, C260–C269. [Google Scholar] [CrossRef]
- Bookout, A.L.; Jeong, Y.; Downes, M.; Yu, R.T.; Evans, R.M.; Mangelsdorf, D.J. Anatomical Profiling of Nuclear Receptor Expression Reveals a Hierarchical Transcriptional Network. Cell 2006, 126, 789–799. [Google Scholar] [CrossRef]
- Park, W.; Kim, G.J.; Choi, H.; Vanacker, J.-M.; Sohn, Y.C. Conserved Properties of a Urochordate Estrogen Receptor-Related Receptor (ERR) with Mammalian ERRalpha. Biochim. Biophys. Acta BBA—Gene Regul. Mech. 2009, 1789, 125–134. [Google Scholar] [CrossRef]
- Bardet, P.-L.; Laudet, V.; Vanacker, J.-M. Studying Non-Mammalian Models? Not a Fool’s ERRand! Trends Endocrinol. Metab. 2006, 17, 166–171. [Google Scholar] [CrossRef]
- Maglich, J.M.; Sluder, A.; Guan, X.; Shi, Y.; McKee, D.D.; Carrick, K.; Kamdar, K.; Willson, T.M.; Moore, J.T. Comparison of Complete Nuclear Receptor Sets from the Human, Caenorhabditis Elegans and Drosophila Genomes. Genome Biol. 2001, 2, research0029.1. [Google Scholar] [CrossRef] [PubMed]
- Persson, E.; Sonnhammer, E.L.L. InParanoiDB 9: Ortholog Groups for Protein Domains and Full-Length Proteins. J. Mol. Biol. 2023, 168001. [Google Scholar] [CrossRef] [PubMed]
- Kallen, J.; Schlaeppi, J.-M.; Bitsch, F.; Filipuzzi, I.; Schilb, A.; Riou, V.; Graham, A.; Strauss, A.; Geiser, M.; Fournier, B. Evidence For Ligand-Independent Transcriptional Activation Of The Human Estrogen-Related Receptor A (Errα): Crystal Structure Of Errα Ligand Binding Domain In Complex With Peroxisome Proliferator-Activated Receptor Coactivator-1α. J. Biol. Chem. 2004, 279, 49330–49337. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.; Bader, J.A.; Giguère, V. The Orphan Nuclear Receptor Estrogen-Related Receptor Alpha Is a Transcriptional Regulator of the Human Medium-Chain Acyl Coenzyme A Dehydrogenase Gene. Mol. Cell Biol. 1997, 17, 5400–5409. [Google Scholar] [CrossRef]
- Barry, J.B.; Laganière, J.; Giguère, V. A Single Nucleotide in an Estrogen-Related Receptor α Site Can Dictate Mode of Binding and Peroxisome Proliferator-Activated Receptor γ Coactivator 1α Activation of Target Promoters. Mol. Endocrinol. 2006, 20, 302–310. [Google Scholar] [CrossRef]
- Dufour, C.R.; Wilson, B.J.; Huss, J.M.; Kelly, D.P.; Alaynick, W.A.; Downes, M.; Evans, R.M.; Blanchette, M.; Giguère, V. Genome-Wide Orchestration of Cardiac Functions by the Orphan Nuclear Receptors ERRα and γ. Cell Metab. 2007, 5, 345–356. [Google Scholar] [CrossRef]
- Festuccia, N.; Owens, N.; Navarro, P. Esrrb, an Estrogen-Related Receptor Involved in Early Development, Pluripotency, and Reprogramming. FEBS Lett. 2018, 592, 852–877. [Google Scholar] [CrossRef]
- Giguere, V.; Yang, N.; Segui, P.; Evans, R.M. Identification of a New Class of Steroid Hormone Receptors. Nature 1988, 331, 91–94. [Google Scholar] [CrossRef]
- Ingraham, H.A.; Redinbo, M.R. Orphan Nuclear Receptors Adopted by Crystallography. Curr. Opin. Struc. Biol. 2005, 15, 708–715. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hebert-Losier, A.; Barry, J.; Poirier, D.; Giguere, V.; Mader, S.; Philip, A. Isolation and Functional Characterization of a Novel Endogenous Inverse Agonist of Estrogen Related Receptors (ERRs) from Human Pregnancy Urine. J. Steroid. Biochem. Mol. Biol. 2019, 191, 105352. [Google Scholar] [CrossRef]
- Zhang, Z.; Teng, C.T. Estrogen Receptor-Related Receptor A1 Interacts with Coactivator and Constitutively Activates the Estrogen Response Elements of the Human Lactoferrin Gene*. J. Biol. Chem. 2000, 275, 20837–20846. [Google Scholar] [CrossRef] [PubMed]
- Giguére, V. To ERR in the Estrogen Pathway. Trends Endocrinol. Metab. 2002, 13, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Grasfeder, L.L.; Haeffele, C.L.; Lobenhofer, E.K.; Chu, T.-M.; Wolfinger, R.; Kazmin, D.; Koves, T.R.; Muoio, D.M.; Chang, C.; et al. Receptor-Selective Coactivators as Tools to Define the Biology of Specific Receptor-Coactivator Pairs. Mol. Cell 2006, 24, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Chaveroux, C.; Eichner, L.J.; Dufour, C.R.; Shatnawi, A.; Khoutorsky, A.; Bourque, G.; Sonenberg, N.; Giguère, V. Molecular and Genetic Crosstalks between mTOR and ERRα Are Key Determinants of Rapamycin-Induced Nonalcoholic Fatty Liver. Cell Metab. 2013, 17, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Deblois, G.; Hall, J.A.; Perry, M.-C.; Laganière, J.; Ghahremani, M.; Park, M.; Hallett, M.; Giguère, V. Genome-Wide Identification of Direct Target Genes Implicates Estrogen-Related Receptor Alpha as a Determinant of Breast Cancer Heterogeneity. Cancer Res. 2009, 69, 6149–6157. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.M.; Dufour, C.R.; Ghahremani, M.; Reudelhuber, T.L.; Giguère, V. Physiological Genomics Identifies Estrogen-Related Receptor α as a Regulator of Renal Sodium and Potassium Homeostasis and the Renin-Angiotensin Pathway. Mol. Endocrinol. 2010, 24, 22–32. [Google Scholar] [CrossRef]
- Deblois, G.; Smith, H.W.; Tam, I.S.; Gravel, S.-P.; Caron, M.; Savage, P.; Labbé, D.P.; Bégin, L.R.; Tremblay, M.L.; Park, M.; et al. ERRα Mediates Metabolic Adaptations Driving Lapatinib Resistance in Breast Cancer. Nat. Commun. 2016, 7, 12156. [Google Scholar] [CrossRef]
- Giguère, V. Transcription Initiation by the ERRs: No Ligand but Two Activation Pathways. Cell Res. 2023, 33, 269–270. [Google Scholar] [CrossRef]
- Nakadai, T.; Shimada, M.; Ito, K.; Cevher, M.A.; Chu, C.-S.; Kumegawa, K.; Maruyama, R.; Malik, S.; Roeder, R.G. Two Target Gene Activation Pathways for Orphan ERR Nuclear Receptors. Cell Res. 2023, 33, 165–183. [Google Scholar] [CrossRef]
- Sonoda, J.; Laganière, J.; Mehl, I.R.; Barish, G.D.; Chong, L.-W.; Li, X.; Scheffler, I.E.; Mock, D.C.; Bataille, A.R.; Robert, F.; et al. Nuclear Receptor ERRα and Coactivator PGC-1β Are Effectors of IFN-γ-Induced Host Defense. Gene Dev. 2007, 21, 1909–1920. [Google Scholar] [CrossRef]
- Takacs, M.; Petoukhov, M.V.; Atkinson, R.A.; Roblin, P.; Ogi, F.-X.; Demeler, B.; Potier, N.; Chebaro, Y.; Dejaegere, A.; Svergun, D.I.; et al. The Asymmetric Binding of PGC-1α to the ERRα and ERRγ Nuclear Receptor Homodimers Involves a Similar Recognition Mechanism. PLoS ONE 2013, 8, e67810. [Google Scholar] [CrossRef] [PubMed]
- Sihag, S.; Cresci, S.; Li, A.Y.; Sucharov, C.C.; Lehman, J.J. PGC-1α and ERRα Target Gene Downregulation Is a Signature of the Failing Human Heart. J. Mol. Cell Cardiol. 2009, 46, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Busch, B.B.; Stevens, W.C.; Martin, R.; Ordentlich, P.; Zhou, S.; Sapp, D.W.; Horlick, R.A.; Mohan, R. Identification of a Selective Inverse Agonist for the Orphan Nuclear Receptor Estrogen-Related Receptor Alpha. J. Med. Chem. 2004, 47, 5593–5596. [Google Scholar] [CrossRef] [PubMed]
- Kallen, J.; Lattmann, R.; Beerli, R.; Blechschmidt, A.; Blommers, M.J.J.; Geiser, M.; Ottl, J.; Schlaeppi, J.-M.; Strauss, A.; Fournier, B. Crystal Structure of Human Estrogen-Related Receptor Alpha in Complex with a Synthetic Inverse Agonist Reveals Its Novel Molecular Mechanism. J. Biol. Chem. 2007, 282, 23231–23239. [Google Scholar] [CrossRef]
- Patch, R.J.; Searle, L.L.; Kim, A.J.; De, D.; Zhu, X.; Askari, H.B.; O’Neill, J.C.; Abad, M.C.; Rentzeperis, D.; Liu, J.; et al. Identification of Diaryl Ether-Based Ligands for Estrogen-Related Receptor α as Potential Antidiabetic Agents. J. Med. Chem. 2011, 54, 788–808. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.-Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-Independent Regulation of VEGF and Angiogenesis by the Transcriptional Coactivator PGC-1α. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, J.; Mori, T.; Smith-Powell, L.; Synold, T.W.; Chen, S.; Wen, W. Baicalin Increases VEGF Expression and Angiogenesis by Activating the ERRα/PGC-1α Pathway. Cardiovasc. Res. 2011, 89, 426–435. [Google Scholar] [CrossRef]
- Zhang, L.-D.; Chen, L.; Zhang, M.; Qi, H.-J.; Chen, L.; Chen, H.-F.; Zhong, M.-K.; Shi, X.-J.; Li, Q.-Y. Downregulation of ERRα Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells through Regulating VEGF Production and PI3K/Akt/STAT3 Signaling Pathway. Eur. J. Pharmacol. 2015, 769, 167–176. [Google Scholar] [CrossRef]
- Choi, Y.K.; Park, J.H.; Yun, J.-A.; Cha, J.-H.; Kim, Y.; Won, M.-H.; Kim, K.-W.; Ha, K.-S.; Kwon, Y.-G.; Kim, Y.-M. Heme Oxygenase Metabolites Improve Astrocytic Mitochondrial Function via a Ca2+-Dependent HIF-1α/ERRα Circuit. PLoS ONE 2018, 13, e0202039. [Google Scholar] [CrossRef]
- Stein, R.A.; Gaillard, S.; McDonnell, D.P. Estrogen-Related Receptor Alpha Induces the Expression of Vascular Endothelial Growth Factor in Breast Cancer Cells. J. Steroid Biochem. Mol. Biol. 2009, 114, 106–112. [Google Scholar] [CrossRef]
- Fradet, A.; Sorel, H.; Bouazza, L.; Goehrig, D.; Dépalle, B.; Bellahcène, A.; Castronovo, V.; Follet, H.; Descotes, F.; Aubin, J.E.; et al. Dual Function of ERRα in Breast Cancer and Bone Metastasis Formation: Implication of VEGF and Osteoprotegerin. Cancer Res. 2011, 71, 5728–5738. [Google Scholar] [CrossRef] [PubMed]
- Thom, R.; Rowe, G.C.; Jang, C.; Safdar, A.; Arany, Z. Hypoxic Induction of Vascular Endothelial Growth Factor (VEGF) and Angiogenesis in Muscle by Truncated Peroxisome Proliferator-Activated Receptor γ Coactivator (PGC)-1α. J. Biol. Chem. 2014, 289, 8810–8817. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Graña, O.; et al. MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Sopariwala, D.H.; Likhite, N.; Pei, G.; Haroon, F.; Lin, L.; Yadav, V.; Zhao, Z.; Narkar, V.A. Estrogen-related Receptor α Is Involved in Angiogenesis and Skeletal Muscle Revascularization in Hindlimb Ischemia. FASEB J. 2021, 35, e21480. [Google Scholar] [CrossRef] [PubMed]
- Sopariwala, D.H.; Rios, A.S.; Park, M.K.; Song, M.S.; Kumar, A.; Narkar, V.A. Estrogen-related Receptor Alpha Is an AMPK-regulated Factor that Promotes Ischemic Muscle Revascularization and Recovery in Diet-induced Obese Mice. FASEB Bioadv. 2022, 4, 602–618. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.-H.; Lee, D.-K.; Lee, K.-S.; Won, M.-H.; Jeoung, D.; Lee, H.; Ha, K.-S.; Kwon, Y.-G.; Kim, Y.-M. Carbon Monoxide Potentiation of L-Type Ca2+ Channel Activity Increases HIF-1α-Independent VEGF Expression via an AMPKα/SIRT1-Mediated PGC-1α/ERRα Axis. Antioxid. Redox Sign 2017, 27, 21–36. [Google Scholar] [CrossRef]
- Xiaowei, H.; Ninghui, Z.; Wei, X.; Yiping, T.; Linfeng, X. The Experimental Study of Hypoxia-Inducible Factor-1α and Its Target Genes in Spinal Cord Injury. Spinal Cord 2006, 44, 35–43. [Google Scholar] [CrossRef]
- Chen, M.-H.; Ren, Q.-X.; Yang, W.-F.; Chen, X.-L.; Lu, C.; Sun, J. Influences of HIF-Lα on Bax/Bcl-2 and VEGF Expressions in Rats with Spinal Cord Injury. Int. J. Clin. Exp. Pathol. 2013, 6, 2312–2322.a57. [Google Scholar]
- Mortazavi, M.M.; Verma, K.; Harmon, O.A.; Griessenauer, C.J.; Adeeb, N.; Theodore, N.; Tubbs, R.S. The Microanatomy of Spinal Cord Injury: A Review. Clin. Anat. 2015, 28, 27–36. [Google Scholar] [CrossRef]
- Hu, J.Z.; Long, H.; Wu, T.-D.; Zhou, Y.; Lu, H.-B. The Effect of Estrogen-Related Receptor α on the Regulation of Angiogenesis after Spinal Cord Injury. Neuroscience 2015, 290, 570–580. [Google Scholar] [CrossRef]
- Deng, C.-Y.; Zhu, T.-T.; Lian, S.; Wang, J.-F.; Wu, R.; Zheng, J.-S. Estrogen-Related Receptor α (ERRα) Functions in the Hypoxic Injury of Microglial Cells. J. Vet. Res. 2022, 66, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chitra, L.; Boopathy, R. Adaptability to Hypobaric Hypoxia Is Facilitated through Mitochondrial Bioenergetics: An In Vivo Study. Brit. J. Pharmacol. 2013, 169, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Defining the Role of Hypoxia-Inducible Factor 1 in Cancer Biology and Therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, G.; Jiang, X.; Lu, D.; Mei, H.; Chen, B. Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α (PGC-1α) Regulates the Expression of B-Cell Lymphoma/Leukemia-2 (Bcl-2) and Promotes the Survival of Mesenchymal Stem Cells (MSCs) via PGC-1α/ERRα Interaction in the Absence of Serum, Hypoxia, and High Glucose Conditions. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3451–3460. [Google Scholar] [CrossRef]
- Zhong, H.; Marzo, A.M.D.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Simons, J.W. Overexpression of Hypoxia-Inducible Factor 1alpha in Common Human Cancers and Their Metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar]
- Ariazi, E.A.; Clark, G.M.; Mertz, J.E. Estrogen-Related Receptor Alpha and Estrogen-Related Receptor Gamma Associate with Unfavorable and Favorable Biomarkers, Respectively, in Human Breast Cancer. Cancer Res. 2002, 62, 6510–6518. [Google Scholar]
- Suzuki, T.; Miki, Y.; Moriya, T.; Shimada, N.; Ishida, T.; Hirakawa, H.; Ohuchi, N.; Sasano, H. Estrogen-Related Receptor α in Human Breast Carcinoma as a Potent Prognostic Factor. Cancer Res. 2004, 64, 4670–4676. [Google Scholar] [CrossRef]
- Ariazi, E.A.; Kraus, R.J.; Farrell, M.L.; Jordan, V.C.; Mertz, J.E. Estrogen-Related Receptor A1 Transcriptional Activities Are Regulated in Part via the ErbB2/HER2 Signaling Pathway. Am. Assoc. Cancer Res. 2007, 5, 71–85. [Google Scholar] [CrossRef]
- Barry, J.B.; Giguère, V. Epidermal Growth Factor–Induced Signaling in Breast Cancer Cells Results in Selective Target Gene Activation by Orphan Nuclear Receptor Estrogen-Related Receptor α. Cancer Res. 2005, 65, 6120–6129. [Google Scholar] [CrossRef]
- Jarzabek, K.; Koda, M.; Kozlowski, L.; Sulkowski, S.; Kottler, M.-L.; Wolczynski, S. The Significance of the Expression of ERRα as a Potential Biomarker in Breast Cancer. J. Steroid Biochem. Mol. Biol. 2009, 113, 127–133. [Google Scholar] [CrossRef]
- Cavallini, A.; Notarnicola, M.; Giannini, R.; Montemurro, S.; Lorusso, D.; Visconti, A.; Minervini, F.; Caruso, M.G. Oestrogen Receptor-Related Receptor Alpha (ERRalpha) and Oestrogen Receptors (ERalpha and ERbeta) Exhibit Different Gene Expression in Human Colorectal Tumour Progression. Eur. J. Cancer 2005, 41, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Takahashi, S.; Urano, T.; Kumagai, J.; Ogushi, T.; Horie-Inoue, K.; Ouchi, Y.; Kitamura, T.; Muramatsu, M.; Inoue, S. Increased Expression of Estrogen-Related Receptor Alpha (ERRalpha) Is a Negative Prognostic Predictor in Human Prostate Cancer. Int. J. Cancer 2007, 120, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, A.; von Stedingk, K.; Thorén, M.M.; Mohlin, S.; Påhlman, S. Differential Regulation of HIF-1α and HIF-2α in Neuroblastoma: Estrogen-Related Receptor Alpha (ERRα) Regulates HIF2A Transcription and Correlates to Poor Outcome. Biochem. Biophys. Res. Commun. 2015, 461, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Yu, S.; Xu, Z.; Wu, D.; Ng, C.; Yao, X.; Yew, D.T.; Vanacker, J.; Chan, F.L. ERRα Augments HIF-1 Signalling by Directly Interacting with HIF-1α in Normoxic and Hypoxic Prostate Cancer Cells. J. Pathol. 2014, 233, 61–73. [Google Scholar] [CrossRef]
- Ao, A.; Wang, H.; Kamarajugadda, S.; Lu, J. Involvement of Estrogen-Related Receptors in Transcriptional Response to Hypoxia and Growth of Solid Tumors. Proc. Natl. Acad. Sci. USA 2008, 105, 7821–7826. [Google Scholar] [CrossRef]
- Sahu, A.; Wang, X.; Munson, P.; Klomp, J.P.G.; Wang, X.; Gu, S.S.; Han, Y.; Qian, G.; Nicol, P.; Zeng, Z.; et al. Discovery of Targets for Immune–Metabolic Antitumor Drugs Identifies Estrogen-Related Receptor Alpha. Cancer Discov. 2023, 13, 672–701. [Google Scholar] [CrossRef]
- Zhao, L.; Li, G.; Meng, F.; Sun, Z.; Liu, J. Cortical and Medullary Oxygenation Evaluation of Kidneys with Renal Artery Stenosis by BOLD-MRI. PLoS ONE 2022, 17, e0264630. [Google Scholar] [CrossRef]
- Keppner, A.; Maric, D.; Orlando, I.M.C.; Falquet, L.; Hummler, E.; Hoogewijs, D. Analysis of the Hypoxic Response in a Mouse Cortical Collecting Duct-Derived Cell Line Suggests That Esrra Is Partially Involved in Hif1α-Mediated Hypoxia-Inducible Gene Expression in mCCDcl1 Cells. Int. J. Mol. Sci. 2022, 23, 7262. [Google Scholar] [CrossRef]
- Romero, N.M.; Dekanty, A.; Wappner, P. Cellular and Developmental Adaptations to Hypoxia: A Drosophila Perspective. Methods Enzymol. 2007, 435, 123–144. [Google Scholar] [CrossRef]
- Li, Y.; Padmanabha, D.; Gentile, L.B.; Dumur, C.I.; Beckstead, R.B.; Baker, K.D. HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in Drosophila Melanogaster. PLoS Genet. 2013, 9, e1003230. [Google Scholar] [CrossRef]
- Figueiredo, H.; Figueroa, A.L.C.; Garcia, A.; Fernandez-Ruiz, R.; Broca, C.; Wojtusciszyn, A.; Malpique, R.; Gasa, R.; Gomis, R. Targeting Pancreatic Islet PTP1B Improves Islet Graft Revascularization and Transplant Outcomes. Sci. Transl. Med. 2019, 11, eaar6294. [Google Scholar] [CrossRef] [PubMed]
- Shires, S.E.; Quiles, J.M.; Najor, R.H.; Leon, L.J.; Cortez, M.Q.; Lampert, M.A.; Mark, A.; Gustafsson, Å.B. Nuclear Parkin Activates the ERRα Transcriptional Program and Drives Widespread Changes in Gene Expression Following Hypoxia. Sci. Rep. 2020, 10, 8499. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaltel-Lima, L.; Domínguez, F.; Domínguez-Ramírez, L.; Cortes-Hernandez, P. The Role of the Estrogen-Related Receptor Alpha (ERRa) in Hypoxia and Its Implications for Cancer Metabolism. Int. J. Mol. Sci. 2023, 24, 7983. https://doi.org/10.3390/ijms24097983
Chaltel-Lima L, Domínguez F, Domínguez-Ramírez L, Cortes-Hernandez P. The Role of the Estrogen-Related Receptor Alpha (ERRa) in Hypoxia and Its Implications for Cancer Metabolism. International Journal of Molecular Sciences. 2023; 24(9):7983. https://doi.org/10.3390/ijms24097983
Chicago/Turabian StyleChaltel-Lima, Leslie, Fabiola Domínguez, Lenin Domínguez-Ramírez, and Paulina Cortes-Hernandez. 2023. "The Role of the Estrogen-Related Receptor Alpha (ERRa) in Hypoxia and Its Implications for Cancer Metabolism" International Journal of Molecular Sciences 24, no. 9: 7983. https://doi.org/10.3390/ijms24097983
APA StyleChaltel-Lima, L., Domínguez, F., Domínguez-Ramírez, L., & Cortes-Hernandez, P. (2023). The Role of the Estrogen-Related Receptor Alpha (ERRa) in Hypoxia and Its Implications for Cancer Metabolism. International Journal of Molecular Sciences, 24(9), 7983. https://doi.org/10.3390/ijms24097983







