Investigation of the Changes in Concentrations of Vitamin D-Binding Protein and Lactoferin in Plasma and Peritoneal Fluid of Patients with Endometriosis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Material
4.3. Laboratory Methods
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef] [PubMed]
- Berker, B.; Seval, M. Problems with the diagnosis of endometriosis. Women’s Health 2015, 11, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Choi, Y.S.; Yim, S.Y.; Yang, H.I.; Jeon, Y.E.; Lee, K.E.; Kim, H.; Seo, S.K.; Lee, B.S. Urinary vitamin D-binding protein is elevated in patients with endometriosis. Hum. Reprod. 2012, 27, 515–522. [Google Scholar] [CrossRef]
- Wrobel, M.; Wielgos, M.; Laudanski, P. Diagnostic delay of endometriosis in adults and adolescence-current stage of knowledge. Adv. Med. Sci. 2022, 67, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Badr, B.; Cassim, S.; Rehman, R. Can low Vitamin D Binding Protein levels be a cause of infertility in females? J. Pak. Med. Assoc. 2019, 69, 1064. [Google Scholar] [PubMed]
- Hwang, J.H.; Wang, T.; Lee, K.S.; Joo, J.K.; Lee, H.G. Vitamin D binding protein plays an important role in the progression of endometriosis. Int. J. Mol. Med. 2013, 32, 1394–1400. [Google Scholar] [CrossRef]
- Laudanski, P.; Gorodkiewicz, E.; Ramotowska, B.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J. Determination of cathepsins B, D and G concentration in eutopic proliferative endometrium of women with endometriosis by the surface plasmon resonance imaging (SPRI) technique. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 80–83. [Google Scholar] [CrossRef]
- Laudanski, P.; Szamatowicz, J.; Oniszczuk, M. Profiling of peritoneal fluid of women with endometriosis by chemokine protein array. Adv. Med. Sci. 2006, 51, 148–152. [Google Scholar]
- Laudanski, P.; Szamatowicz, J.; Ramel, P. Matrix metalloproteinase-13 and membrane type-1 matrix metalloproteinase in peritoneal fluid of women with endometriosis. Gynecol. Endocrinol. 2005, 21, 106–110. [Google Scholar] [CrossRef]
- Szamatowicz, J.; Laudanski, P.; Tomaszewska, I.; Szamatowicz, M. Chemokine growth-regulated-alpha: A possible role in the pathogenesis of endometriosis. Gynecol. Endocrinol. 2002, 16, 137–141. [Google Scholar] [CrossRef]
- Golawski, K.; Soczewica, R.; Kacperczyk-Bartnik, J.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; Banaszewska, B.; et al. The Role of Cadherin 12 (CDH12) in the Peritoneal Fluid among Patients with Endometriosis and Endometriosis-Related Infertility. Int. J. Environ. Res. Public Health 2022, 19, 11586. [Google Scholar] [CrossRef] [PubMed]
- Kacperczyk-Bartnik, J.; Bartnik, P.; Golawski, K.; Sierdzinski, J.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; et al. Plasma and Peritoneal Poly (ADP-Ribose) Polymerase Levels in Patients with Endometriosis. Biomedicines 2022, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, P.; Kacperczyk-Bartnik, J.; Golawski, K.; Sierdzinski, J.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; et al. Plasma and Peritoneal Fluid ZEB Levels in Patients with Endometriosis and Infertility. Biomedicines 2022, 10, 2460. [Google Scholar] [CrossRef] [PubMed]
- Mikus, M.; Goldstajn, M.S.; Brlecic, I.; Dumancic, S.; Lagana, A.S.; Chiantera, V.; Vujic, G.; Coric, M. CTLA4-Linked Autoimmunity in the Pathogenesis of Endometriosis and Related Infertility: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10902. [Google Scholar] [CrossRef]
- Polak, G.; Wertel, I.; Tarkowski, R.; Morawska, D.; Kotarski, J. Decreased lactoferrin levels in peritoneal fluid of women with minimal endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 131, 93–96. [Google Scholar] [CrossRef]
- Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients 2020, 12, 1489. [Google Scholar] [CrossRef]
- Donnez, J.; Cacciottola, L. Endometriosis: An Inflammatory Disease That Requires New Therapeutic Options. Int. J. Mol. Sci. 2022, 23, 1518. [Google Scholar] [CrossRef]
- Riley, C.F.; Moen, M.H.; Videm, V. Inflammatory markers in endometriosis: Reduced peritoneal neutrophil response in minimal endometriosis. Acta Obstet. Gynecol. Scand. 2007, 86, 877–881. [Google Scholar] [CrossRef]
- Skegro, B.; Bjedov, S.; Mikus, M.; Mustac, F.; Lesin, J.; Matijevic, V.; Coric, M.; Elvedi Gasparovic, V.; Medic, F.; Sokol Karadjole, V. Endometriosis, Pain and Mental Health. Psychiatr. Danub. 2021, 33, 632–636. [Google Scholar]
- Mikus, M.; Matak, L.; Vujic, G.; Skegro, B.; Skegro, I.; Augustin, G.; Lagana, A.S.; Coric, M. The short form endometriosis health profile questionnaire (EHP-5): Psychometric validity assessment of a Croatian version. Arch. Gynecol. Obstet. 2023, 307, 87–92. [Google Scholar] [CrossRef]
- Darba, J.; Marsa, A. Economic Implications of Endometriosis: A Review. Pharmacoeconomics 2022, 40, 1143–1158. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B.; Jozwiak-Kisielewska, A.; Lukaszkiewicz, J.; Skarzynska, E. Vitamin D-binding protein as a biomarker to confirm specific clinical diagnoses. Expert Rev. Mol. Diagn. 2020, 20, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, J.; Gmyrek, G.B.; Madej, J.P.; Nowacki, W.; Goluda, M.; Gabrys, M.; Stefaniak, T.; Chelmonska-Soyta, A. Serum and peritoneal evaluation of vitamin D-binding protein in women with endometriosis. Postepy Hig. Med. Dosw. (Online) 2008, 62, 103–109. [Google Scholar]
- Skarzynska, E.; Wrobel, M.; Zborowska, H.; Kolek, M.F.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; et al. The Influence of Lactoferrin in Plasma and Peritoneal Fluid on Iron Metabolism in Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 1619. [Google Scholar] [CrossRef] [PubMed]
- Kew, R.R. The Vitamin D Binding Protein and Inflammatory Injury: A Mediator or Sentinel of Tissue Damage? Front. Endocrinol. (Lausanne) 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Tyryshkin, K.; Monsanto, S.P.; Marks, R.M.; Lingegowda, H.; Vanderbeck, K.; Childs, T.; Young, S.L.; Lessey, B.A.; et al. Neutrophil recruitment and function in endometriosis patients and a syngeneic murine model. FASEB J. 2020, 34, 1558–1575. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.; Shapses, S.; Sun, W.; Scott, R.; Wang, X. Vitamin D binding protein is lower in infertile patients compared to fertile controls: A case control study. Fertil. Res. Pract. 2017, 3, 14. [Google Scholar] [CrossRef]
- Wang, Y.; Sharma, R.K.; Falcone, T.; Goldberg, J.; Agarwal, A. Importance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil. Steril. 1997, 68, 826–830. [Google Scholar] [CrossRef]
- Laudanski, P.; Rogalska, G.; Warzecha, D.; Lipa, M.; Manka, G.; Kiecka, M.; Spaczynski, R.; Piekarski, P.; Banaszewska, B.; Jakimiuk, A.; et al. Autoantibody screening of plasma and peritoneal fluid of patients with endometriosis. Hum. Reprod. 2023, 38, 629–643. [Google Scholar] [CrossRef]
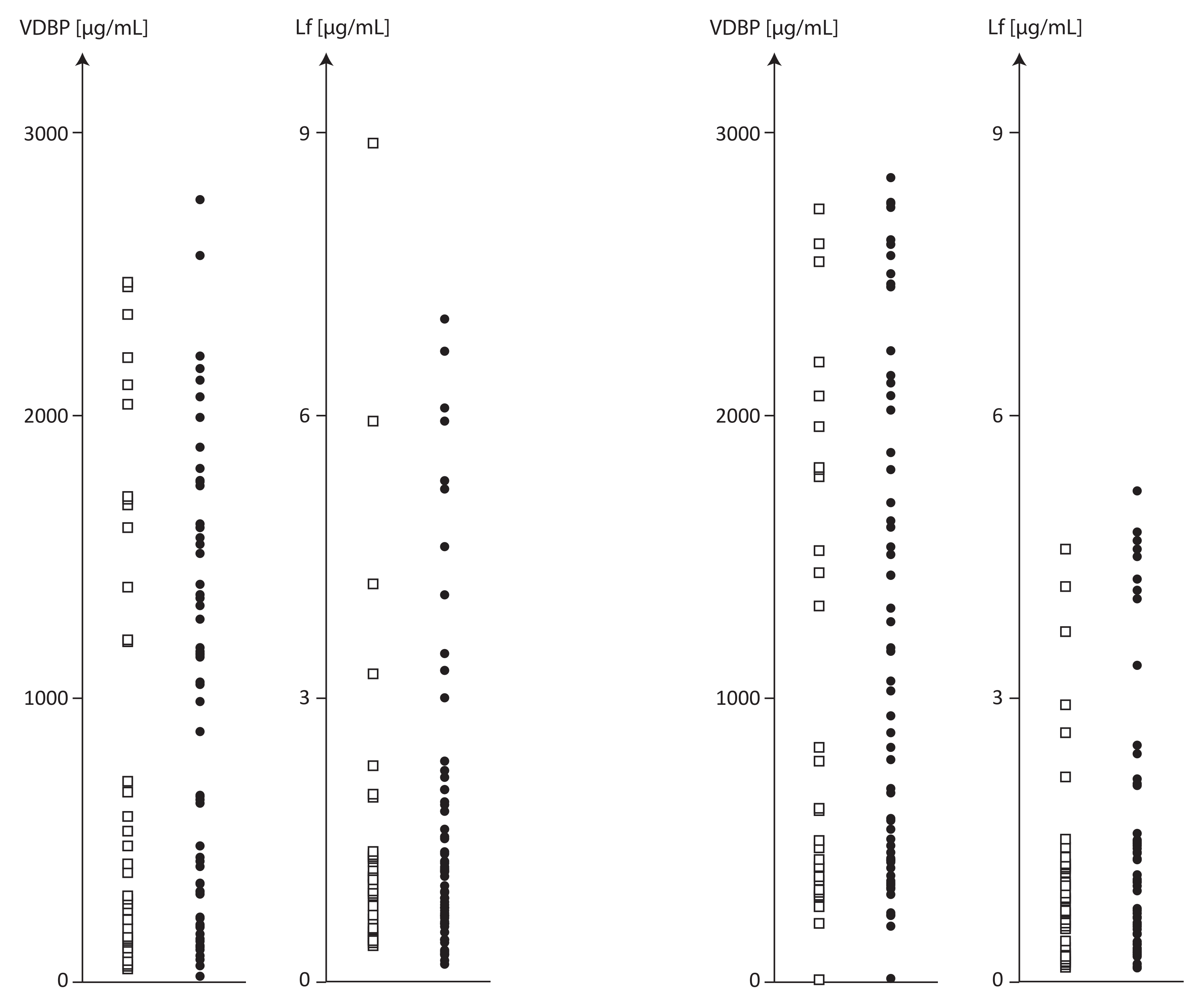
| Parameter Mean ± SD Median (Dispersion)
| Suspected Endometriosis N = 95 | No Endometriosis N = 36 | Endometriosis N = 59 | Differences between Groups p ** | ||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Peritoneal Fluid | Plasma | Peritoneal Fluid | Plasma | Peritoneal Fluid | Plasma | Peritoneal Fluid | |
| VDBP µg/mL | 945.8 ± 779.3 661.8 (20.1–2773.9) 82.4% | 1138.0 ± 856.5 787.1 (14.5–2839.8) 75.3% | 895.6 ± 832.0 510.3 (51.4–2467.5) 92.9% | 1007. ± 833.4 552.8 (212.4–2730.2) 82.7% | 976.4 ± 751.0 993.9 (20.1–2774.0) 77.0% | 1217.7± 867.6 1028.6 (14.5–2839.8) 71.3% | 0.814 | 0.417 |
| LF µg/mL | 1.66 ± 1.72 1.05 (0.16–8.92) 103.8% | 1.31 ± 1.31 0.91 (0.11–5.19) 99.8% | 1.51 ± 1.71 0.97 (0.37–8.92) 113.0% | 1.15 ± 1.12 0.87 (0.12–4.57) 97.1% | 1.74 ± 1.73 1.14 (0.16–6.99) 99.5% | 1.41 ± 1.41 0.98 (0.11–5.19) 100.3% | 0.865 | 0.806 |
| VDBP/LF ratio | 1201.7 ± 1638.4 596.1 (9.1–7682.2) 136.3% | 1997.6 ± 3048.98 647.2 (45.3–15,401.7) 152.6% | 845.8 ± 895.2 488.6 (58.0–3867.1) 105.8% | 2057.5 ± 3639.6 665.9 (183.0–15,401.7) 176.9% | 1418.8 ± 1934.0 630.8 (9.1–7682.2) 136.3% | 1961.1 ± 2658.6 643.6 (45.3–10,991.5) 135.6% | 0.722 | 0.886 |
| Correlations | Study Groups | ||
|---|---|---|---|
| Suspected Endometriosis (n = 95) | No Endometriosis (n = 36) | Endometriosis (n = 59) | |
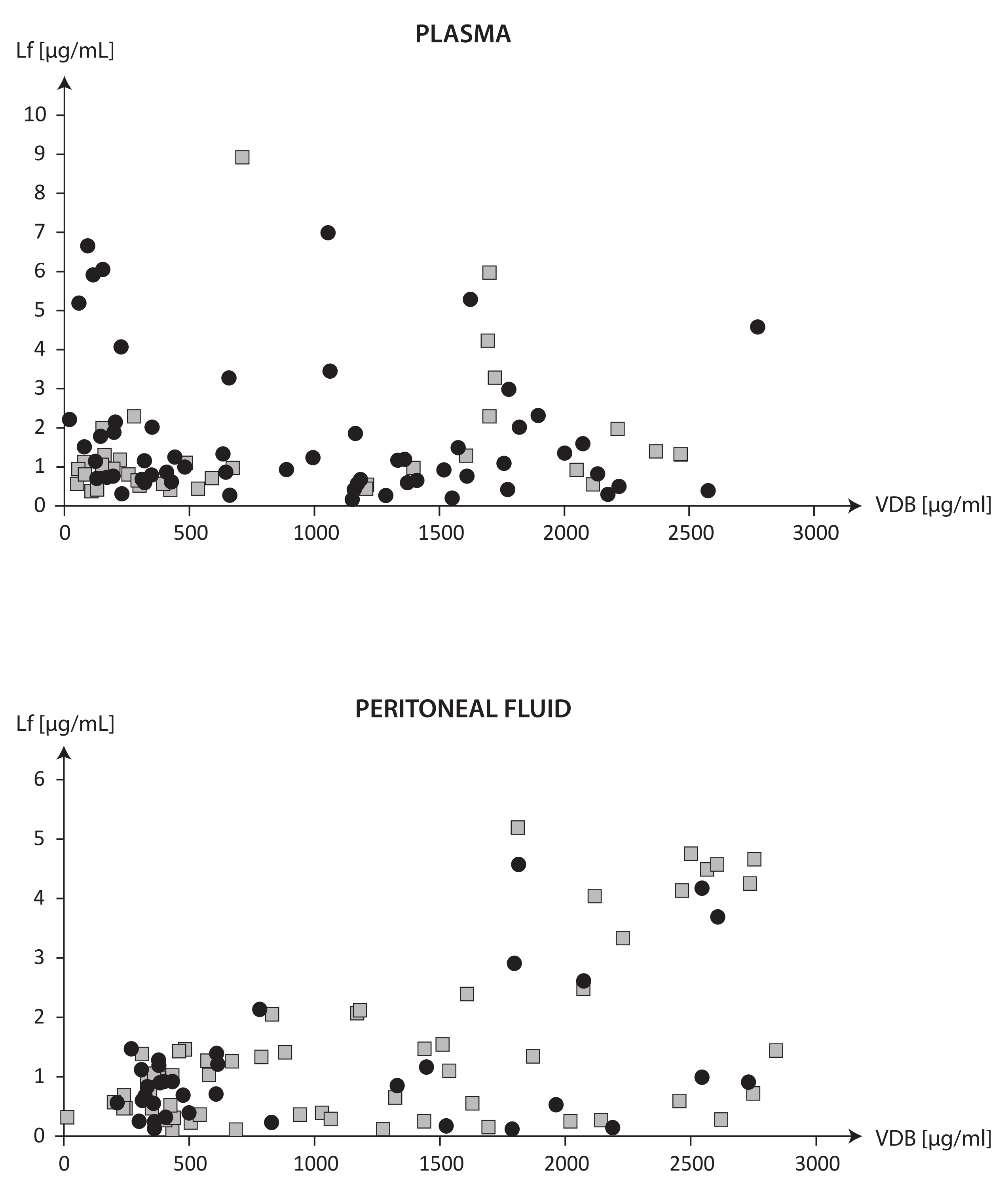
| Plasma VDBP vs. Plasma LF | −0.004, p = 0.968 | 0.374, p = 0.025 | −0.236, p = 0.072 |
| Plasma VDBP vs. PF VDBP | 0.819, p = 0.000 | 0.793, p = 0.000 | 0.821, p = 0.000 |
| Plasma VDBP vs. PF LF | 0.401, p = 0.000 | 0.405, p = 0.014 | 0.413, p = 0.001 |
| PF VDBP vs. PF LF | 0.349, p = 0.001 | 0.256, p = 0.132 | 0.399, p = 0.002 |
| Plasma LF vs. PF LF | 0.184, p = 0.074 | 0.376, p = 0.024 | 0.049, p = 0.714 |
| Parameter Mean ± SD Dispersion | Symptomatic Endometriosis N = 95 | p | No Endometriosis N = 36 | p | Endometriosis N = 59 | p | |||
|---|---|---|---|---|---|---|---|---|---|
| VDBP < 1000 (N = 52) | VDBP > 1000 (N = 43) | VDBP < 1000 (N = 22) | VDBP > 1000 (N = 14) | VDBP < 1000 (N = 30) | VDBP > 1000 (N = 29) | ||||
| Plasma | |||||||||
| VDBP µg/mL | 314.5 ± 229.9 20.1–993.9 | 1709.2 ± 452.6 1055.4–2773.9 | 0.000 | 287.6 ± 203.6 51.4–712.2 | 1851.1 ± 433.4 1207.5–2467.5 | 0.000 | 334.2 ± 249.0 20.1–993.9 | 1640.7 ± 452.9 1055.4–2773.8 | 0.000 |
| LF µg/mL | 1.65 ± 1.81 0.26–8.92 | 1.66 ± 1.63 0.16–6.99 | 0.952 | 1.27 ± 1.77 0.37–8.92 | 1.89 ± 1.59 0.44–5.97 | 0.048 | 1.93 ± 1.81 0.27–6.65 | 1.55 ± 1.66 0.16–6.99 | 0.179 |
| Ratio VDBP/LF | 379.1 ± 421.9 9.1–2469.5 | 2196.4 ± 1985.2 151.1–7682.2 | 0.000 | 369.8 ± 337.5 58.0–1211.7 | 1593.7 ± 993.9 1207.4–2467.5 | <0.001 | 385.9 ± 480.0 9.1–2469.5 | 2487.3 ± 2276.7 151.1–7682.2 | 0.000 |
| Peritoneal fluid | |||||||||
| VDBP µg/mL | 468.7 ± 246.1 14.5–1437.8 | 1947.5 ± 585.5 781.5–2839.8 | 0.000 | 415.6 ± 141.7 245.9–2752.6 | 1937.5 ± 559.6 781.5–2730.2 | 0.000 | 507.6 ± 297.1 14.5–1437.8 | 1952.3 ± 607.2 829.5–2839.8 | 0.000 |
| LF µg/mL | 0.74 ± 0.43 0.11–1.47 | 2.0 ± 1.65 0.12–5.19 | <0.001 | 0.75 ± 0,40 0.13–1.47 | 1.78 ± 1.56 0.12–4.57 | 0.139 | 0.73 ± 0.45 0.11–1.47 | 2.11 ± 1.71 0.12–5.19 | 0.003 |
| Ratio VDBP/LF | 1050.6 ± 1200.0 45.3 –6230.2 | 3142.9 ± 4074.7 348.7–15,401.7 | <0.001 | 862.4 ± 850.0 183.0–3570.8 | 3935.4 ±5318.0 367.1–15,401.7 | 0.013 | 1188.5 ± 1400.5 45.3–6230.2 | 2760.3 ± 3362.8 348.7–10,991.5 | 0.008 |
| Study Groups | Correlations between VDBP and LF Concentrations | |
|---|---|---|
| Plasma VDBP µg/mL | ||
| <1000 µg/mL (n = 52) | >1000 µg/mL (n = 43) | |
| Plasma | ||
| No endometriosis | R = 0.062, p = 0.785 | R = 0.290, p = 0.314 |
| Endometriosis | R = –0.400, p = 0.029 | R = 0.05, p = 0.794 |
| Peritoneal fluid | ||
| No endometriosis | R = 0.159, p = 0.468 | R = 0.289, p = 0.338 |
| Endometriosis | R = 0.231, p = 0.329 | R = 0.416, p = 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowska-Myjak, B.; Skarżyńska, E.; Wróbel, M.; Mańka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczyński, R.; Piekarski, P.; Banaszewska, B.; et al. Investigation of the Changes in Concentrations of Vitamin D-Binding Protein and Lactoferin in Plasma and Peritoneal Fluid of Patients with Endometriosis. Int. J. Mol. Sci. 2023, 24, 7828. https://doi.org/10.3390/ijms24097828
Lisowska-Myjak B, Skarżyńska E, Wróbel M, Mańka G, Kiecka M, Lipa M, Warzecha D, Spaczyński R, Piekarski P, Banaszewska B, et al. Investigation of the Changes in Concentrations of Vitamin D-Binding Protein and Lactoferin in Plasma and Peritoneal Fluid of Patients with Endometriosis. International Journal of Molecular Sciences. 2023; 24(9):7828. https://doi.org/10.3390/ijms24097828
Chicago/Turabian StyleLisowska-Myjak, Barbara, Ewa Skarżyńska, Monika Wróbel, Grzegorz Mańka, Mariusz Kiecka, Michał Lipa, Damian Warzecha, Robert Spaczyński, Piotr Piekarski, Beata Banaszewska, and et al. 2023. "Investigation of the Changes in Concentrations of Vitamin D-Binding Protein and Lactoferin in Plasma and Peritoneal Fluid of Patients with Endometriosis" International Journal of Molecular Sciences 24, no. 9: 7828. https://doi.org/10.3390/ijms24097828
APA StyleLisowska-Myjak, B., Skarżyńska, E., Wróbel, M., Mańka, G., Kiecka, M., Lipa, M., Warzecha, D., Spaczyński, R., Piekarski, P., Banaszewska, B., Jakimiuk, A., Issat, T., Rokita, W., Młodawski, J., Szubert, M., Sieroszewski, P., Raba, G., Szczupak, K., Kluz, T., ... Laudański, P. (2023). Investigation of the Changes in Concentrations of Vitamin D-Binding Protein and Lactoferin in Plasma and Peritoneal Fluid of Patients with Endometriosis. International Journal of Molecular Sciences, 24(9), 7828. https://doi.org/10.3390/ijms24097828





