Abstract
Extracellular vesicles (EVs) mediate cell interactions in biological processes, such as receptor activation or molecule transfer. Estimates of variation by age and sex have been limited by small sample size, and no report has assessed the contribution of genetic factors to levels of EVs. Here, we evaluated blood levels of 25 EV and 3 platelet traits in 974 individuals (933 genotyped) and reported the first genome-wide association study (GWAS) on levels of these traits. EV levels all decreased with age, whereas the trend for their surface markers was more heterogeneous. Platelets and CD31dim platelet EVs significantly increased in females compared to males, although CD31 expression on both platelets and platelet EVs decreased in females. Levels of the other EV subsets were similar between sexes. GWAS revealed three statistically significant genetic signals associated with EV levels in the F10 and GBP1 genes and in the intergenic region between LRIG1 and KBTBD8. These add to a signal in the 3′UTR of RHOF associated with CD31 expression on platelets that was previously found to be associated with other platelet traits. These findings suggest that EV formation is not a simple, constant adjunct of metabolism but is under both age-related and genetic control that can be independent of the regulation of the levels of the cells from which the EVs derive.
1. Introduction
Extracellular vesicles are small lipid-encapsulated bits of cells secreted into the extracellular space during biological processes such as cellular activation, maturation, proliferation, aging and apoptosis [1]. Recently, they have been shown to participate in intercellular signaling and communication and in modulating both homeostatic and pathological processes [2,3]. Indeed, by transferring nucleic acids and a range of proteins to recipient cells, EVs are implicated in tumor cell invasion and metastasis, metabolic and cardiovascular disease, angiogenesis, immune response, and antigen presentation. The identification and characterization of EVs from multiple body fluids—urine [4], breast milk [5], cerebrospinal fluid [6], blood [7], saliva [8], and bile [9]—thus become important to clarify their function in health and disease.
EVs are heterogeneous in size, cellular origin, and composition and content of proteins, nucleic acids, lipids, surface receptors and glycans. In the blood, EVs can be classified into three major groups: exosomes, microvesicles, and apoptotic bodies. Exosomes ranging in size from 30 nm to 100 nm in diameter are considered intraluminal vesicles generated by the inward budding of the multivesicular body (MVB) membrane and are secreted following the fusion of the MVB with the cell membrane [10]. Microvesicles from 50 nm to 1000 nm in size are formed directly by the outward budding and fission of the plasma membrane. In contrast, apoptotic bodies from 500 nm to 5 μm in diameter are vesicles shed by cells undergoing programmed death [11].
EVs are characterized by markers derived from the cell of origin, but they also contain miRNA, lipid and protein profiles that differ from those of their cell of origin [12]. Interestingly, their content can reflect both physiologic and pathogenic conditions, and indeed, many studies have identified variations in EV counts and content in different human diseases. For instance, changes in EV concentrations have been observed in cardiovascular disease [13]. Elevated levels of platelet-derived microvesicles have also been observed in diabetes mellitus [14]. Other evidence shows that EVs appear at significantly higher levels in systemic lupus erythematosus and rheumatoid arthritis cases than in healthy controls [15]. Moreover, EVs contain different RNA species that have been examined as potential biomarkers for various diseases, including different types of cancer [16,17]. Such biomarkers could be especially useful for pathologies for which invasive diagnostic approaches are currently used.
Flow cytometry, enabling multi-parameter single-particle analysis, is a promising technique to measure EVs. A recent article by Marchisio and colleagues [18] described the EV detection method used in the present study based on lipophilic cationic dye positivity and phalloidin negativity. To demonstrate the correct morphology and size of EVs separated by flow cytometry, they used transmission electron microscopy and control beads with a size similar to EVs. Other articles related to SARS-CoV-2 status have been published using the same EV detection approach [19,20], and several other studies have identified EVs by flow cytometry instead of using classical EV detection methods [21]. Previous studies have measured EVs in samples of a very small size [22], and no study, to our knowledge, has investigated the possible genetic regulation of their levels or has systematically assessed their trends during lifespan or by sex in a large general population cohort.
Here, we measured 28 circulating traits including 25 EV and 3 platelet traits, in up to 974 individuals from a general population and performed a genome-wide association study (GWAS). We also assessed age- and sex-related differences in these traits.
2. Results
2.1. Age Effects on EV and Platelet Traits
We assessed 28 peripheral blood traits on up to 974 Sardinian volunteers. Twelve traits were absolute counts (AC, including 11 EV subsets and platelets, expressed as events/μL) and 16 were median fluorescence intensities of surface antigens (MFI, 14 assessed on EVs and 2 on platelets), as further described in Section 4 and Supplementary Table S1 and Figure 1.
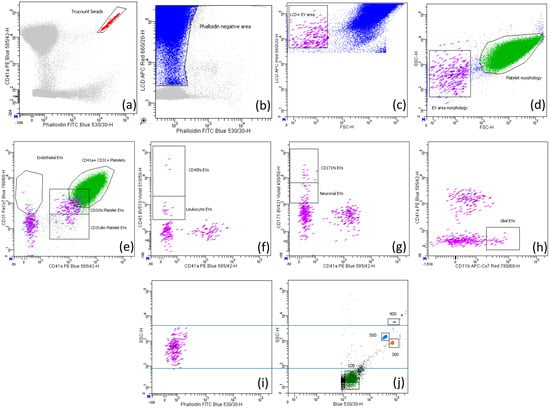
Figure 1.
Gating strategy to identify EVs and platelets. (a) Counting beads (red) were identified based on their high fluorescence for FITC and PE fluorochromes; (b) the phalloidin negative area (blue) contained both extracellular vesicles and platelets. The EV area (violet) was identified by intersecting the lipophilic cationic dye (LCD) positive EV area in (c) with the EV area morphologically assessed in (d). Platelets (green) were identified by intersecting platelets morphologically detected in (d) with those identified by CD31 and CD41a positivity in (e). (e) EVs double positive for CD31 and CD41a were considered of platelet origin and further divided into CD31dim and CD31hi; EVs positive for CD31 but negative for CD41a were considered of endothelial origin. EVs positive for CD45, CD171 and CD11b were considered leukocyte (f), neuronal (g), and glial (h) origins, respectively. In (f,g), we also gated CD45hi and CD171hi events, respectively, that could be either EVs expressing high levels of these specific markers or crystals. The comparison between the size of EVs (i) and of the Megamix beads (j) is shown.
All of the absolute counts measured showed a downward trend across the lifespan (Supplementary Table S2), which was highly significant for platelet and CD31dim platelet EVs (Figure 2a,b), significant for neuronal and leukocyte EVs (Figure 2c,d), and nominal for endothelial and glial EVs (Figure 2e,f). This is in line with previous observations of EV counts [23,24,25]. We also evaluated the phenotypic correlations between the most represented trait, platelet count, and the levels of the EV subpopulations. We observed a significant positive correlation of platelet levels with platelet EVs; EVs from other cell types showed no significant correlation with platelet counts (Supplementary Table S3).
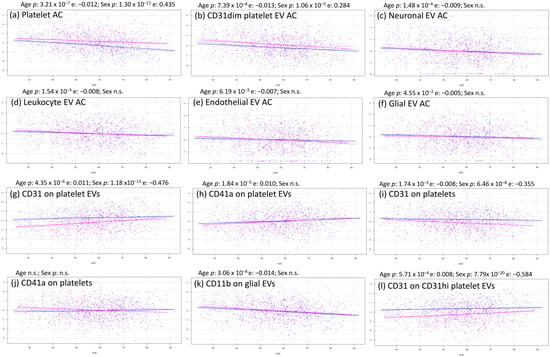
Figure 2.
Most relevant age–sex tendencies of EVs and platelets. Each scatter plot represented males in blue and females in pink, age in the x axis, and normalized trait in the y axis.
The reduction of EV levels was not necessarily accompanied by a reduction in the expression of their characteristic surface antigens; in fact, both CD31 and CD41a expression on platelet EVs increased with aging (Figure 2g,h). Importantly, CD31 expression on platelets decreased, while CD41a expression on platelets was quite stable (Figure 2i,j), indicating that EVs have their own idiosyncratic regulation independent of the cell from which they derive. In contrast, glial EVs, which did not significantly decrease with aging (Figure 2f), were characterized by a strong reduction in CD11b expression (Figure 2k).
2.2. Sex Effects on EV and Platelet Traits
Sex significantly affected platelet traits: both platelets and CD31dim platelet EVs increased in females compared to males (Figure 2a,b), although levels of the other EV subsets were similar between sexes. Regarding the expression level of the surface antigens, CD31 was reduced in females compared to males in both platelets (Figure 2i) and platelet EVs (Figure 2g,l) and, to a lesser extent, in endothelial EVs (Supplementary Table S2). The other proteins measured on platelet and EV surfaces showed no significant differences between males and females.
2.3. Genome Wide Association Study of EV and Platelet Traits
We performed a GWAS that, correcting for the number of the assessed traits, had a p-value for statistical significance of 2.47 × 10−10 (see Section 4, Table 1). We identified one significant signal led by SNP rs11553699 in the 3′UTR of the RHOF and TMEM120B genes on chromosome 12, associated with the expression of CD31 on platelets (p-value = 1.97 × 10−15, beta = 0.46) (Figure 3a). This variant was also an s-QTL (splicing quantitative trait locus) for TMEM120B [26]; however, the role of this gene in platelet regulation or function is not known. RHOF belongs to the GTPase family, which is critical for platelet function. It is expressed in platelets and seems to interact with cytoskeleton regulators, but no role in platelets has been proposed [27].

Table 1.
GWAS results for EVs and platelets. p-values lower than 5.00 × 10−8 were shown. Beta + referred to the alternative (Alt) allele. Minor allele frequency (MAF); Standard error of beta (SE beta); Reference allele (Ref).
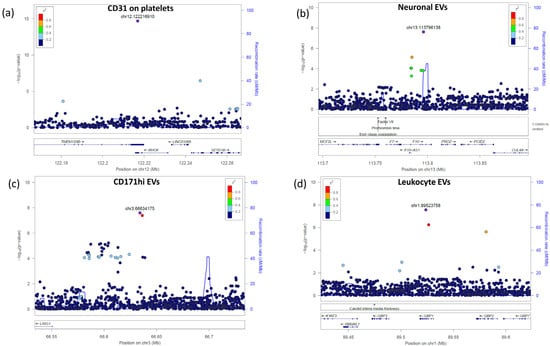
Figure 3.
Regional association plots of the most significant identified signals. The significance of the association (−log10[p value]; left y axis) for each trait was plotted relative to the genomic positions on the hg19/GRCh37 genomic build (x axis). SNPs were colored to reflect their linkage disequilibrium with the top SNP (indicated with a purple dot and with its genomic position at the built GRCh37/hg19).
Three novel signals did not reach the p-value of 2.47 × 10−10 but met the classical genome-wide significance threshold of 5.00 × 10−8 and were associated with the level of EV subsets. The first signal, led by rs3213004 in the intronic region of the F10 gene on chromosome 13, was associated with neuronal EVs (p = 2.47 × 10−8, beta = 0.51) (Figure 3b). F10 is the vitamin K-dependent coagulation factor X of the blood coagulation cascade, which, when activated, converts prothrombin to thrombin.
The second signal, led by rs116060256 in the intergenic region between the LRIG1 and KBTBD8 genes on chromosome 3, was associated with CD171hi EVs (p = 2.51 × 10−8, beta = −0.50) (Figure 3c). These two genes are involved in neural precursor cell proliferation (LRIG1) [28] and neural crest specification (KBTBD8) [29].
The third signal was led by a missense variant, rs148526074 (c.C793A:p.Q265K), in exon 6 of the GBP1 gene (chromosome 1) and was associated with leukocyte EV counts (p = 2.80 × 10−8, beta = 1.02) (Figure 3d). This gene belongs to the group of guanylate-binding proteins and is involved in inflammasome activation following microbial insults [30,31].
3. Discussion
Previous studies used a very small sample size [22] that precluded the possibility of performing GWAS and often focused on a specific EV subtype or on EVs in toto [23]. The present study assessed the genetic regulation of EVs for the first time.
Initially, EVs were isolated by ultracentrifugation and density gradient flotation or other time-consuming approaches [32,33,34]. More recently, they began to be detected by alternative approaches, such as flow cytometry, staining biological liquids (blood, milk, tears, saliva) with two reagents: a lipophilic cationic dye that diffuses into EVs by their membrane potential, and phalloidin, which interacts with cytoskeleton actin only when EV membranes are damaged, and thus does not bind intact EVs (see Section 4). This procedure enriches EVs for further subsequent subset detection using surface markers that characterize the cells/fragments from which EVs derive [18,19,20]. This approach identifies EVs more rapidly and easily than previously used, facilitating the processing of the large number of samples necessary to perform GWAS. However, because profiling and enumeration of EVs usually requires two or more complementary techniques to aid in their definitive identification, controls to verify their size are always recommended. Furthermore, EV detection by flow cytometry also needs to be improved because the EV area is often enriched with non-EV fragments that can hinder correct EV counting.
Here, we assessed about 1000 individuals, still relatively small for GWAS, but sufficient for an exploratory analysis that already yielded suggestive results. Thus, a larger number of samples should provide additional and more robust genetic signals.
Among the 4 signals we described, the most significant was in the 3′UTR region of RHOF, associated with the expression of CD31 in platelets. This variant was already known to be strongly associated with platelet volume, count and distribution width [35]; thus, the identified association can be considered a proof of concept of the method using flow cytometry to measure small molecules, such as EVs and platelets.
The other three novel genetic signals included an association of neuronal EVs with the F10 gene, consistent with the suggested involvement of coagulation factors, such as F10, in neurodegeneration [36,37,38]. A second signal linked CD171hi EVs, which are mainly derived from the nervous system, with LRIG1 and KBTBD8, both genes involved in neuronal development and differentiation [39]. The last signal associated leukocyte EVs, known to induce immune and inflammatory responses [40], with GBP1, a gene involved in the inflammatory response. All of these genes are correspondingly implicated in the function of the EV subsets with which they are associated.
Given the global increase in lifespan, there is growing interest in identifying circulating factors that correlate with biological or chronological age. Because EVs appear to undergo alterations in number and internal content during senescence [41], we tested the correlation of EV subsets with age. We observed a general reduction in the levels of EV subsets, suggesting that the EV-mediated interaction of cells may decline with age. This might cause an increased risk for several diseases more common in the elderly. Indeed, neural stem cell-derived EVs have been suggested to have a neuroprotective function by reducing reactive oxygen species and inflammatory cytokines [42] and may thus have therapeutic potential in neurodegenerative disorders. Furthermore, native and engineered EVs have been suggested for the treatment of different brain pathologies [43].
On the other hand, it has been observed that neuronal EVs derived from Parkinson’s patients and transferred to mice may exacerbate the disease in a Parkinson mouse model [44]. Furthermore, in the area of blood banking, extracellular vesicles derived from red blood cells and accumulated during blood storage might have proinflammatory and procoagulant effects and be implicated in adverse transfusion events and posttransfusion outcomes [21,45]. Therefore, while the reduction of circulating EVs may result in detrimental reduction of communication between cells, an increase in EV levels could still be harmful depending on their cargo. Any therapeutic interventions based on EVs thus remain conjectural, requiring further study.
In contrast to the general downtrend of EV levels with age, the expression tendencies of their surface proteins during aging were less homogeneous, with some of them increasing and others decreasing. For example, the levels of glial EVs, characterized by CD11b positivity, did not significantly decrease during aging, but the expression of CD11b on their surface was strongly reduced. This may indicate that aging primarily causes reduced levels of circulating EVs, but when such a reduction does not occur, energy is saved by a decreased production of surface proteins.
Furthermore, sex-specific dissection of EVs (and, more generally, of any quantitative trait involved in disease susceptibility or providing a therapeutic target) is relevant to better understand diseases with a disproportionate incidence in males and females. It can also help to assess correct dosages of drugs. Considering the growing interest in the use of EVs as therapeutic targets [46], we analyzed sex-specific differences in EV levels but observed a significant impact of sex only on those derived from platelets.
Overall, we infer that EVs are not simply mirrors of the cells/fragments from which they are derived. This is clearly demonstrated by the increase of CD31 on platelet EVs during the lifespan, while the same marker decreased in platelets. We also infer that markers are specifically regulated on different EV subtypes, e.g., over the lifespan, CD31 changes in platelet EVs but not in endothelial EVs.
Thus, EVs can be treated like any other component measured in blood, such as neurofilaments or fatty acids, whose increasingly relevant roles in disease susceptibility may provide therapeutic targets or prognostic/diagnostic markers [47,48,49]. EV assessment in large cohorts could further analyze their involvement in complex diseases through the colocalization of GWAS signals for EV levels and diseases and Mendelian randomization [50].
4. Materials and Methods
4.1. Samples
This study was approved by the Sardinian Regional Ethics Committee (protocol no. 2171/CE) and all participants provided written informed consent. The 974 participants (aged 23–92 years, 61% females) belonged to the general population SardiNIA cohort originating from four towns located on the central east coast of Sardinia, Italy [51]. Of 974 volunteers, 933 have been genetically characterized for about 18.4 million variants [52].
4.2. Sample Preparation and Sample Staining
Fresh peripheral blood from volunteers was collected into sodium citrate tubes. The tubes were inverted five times and processed within 2 h of blood drawing to minimize time-dependent artifacts. To obtain absolute cell counts, TruCount absolute counting tubes were used (BD Biosciences, San Jose, CA, USA, Cat#340334). For staining whole peripheral blood, 95 μL of filtered phosphate buffered saline (PBS, Sigma, St. Louis, MO, USA, Cat#D8537; 0.22 μm filter unit; Millipore, Carrigtwohill, Ireland, Cat#SLAGP033RB) was dispensed in BD Trucount tubes without disturbing the bead pellet. Five microliters of blood was added using reverse pipetting. Characterization of extracellular vesicles was carried out using two custom kits named “Dye Integer EV Detection Kit” (BD Biosciences, Cat#626267) and “CD41a/CD31/CD45 EV Detection Kit” (BD Biosciences, Cat#626266) that are not present in the standard catalogue but are available on demand. The first kit is based on lipophilic cationic dye that diffuses into EVs by their membrane potential; the kit also contains phalloidin FITC, which binds the cytoskeleton protein actin only in particles having damaged membranes. We then prepared a reagent mix, as described in Supplementary Table S4. To eliminate crystal molecules, the mix was spun down at 16,000× g for 20 min immediately before use. We then added 100 μL of the mix to the TruCount tubes containing 100 μL of diluted sample (95 μL of PBS and 5 μL of blood). After incubation for 45 min in the dark at room temperature, 1.5 mL of filtered PBS was added to the samples and finally acquired by a FACSCanto II (BD Biosciences) flow cytometer and analyzed by FACSDiva software, version 8.0.1 (BD Biosciences). To eliminate the background, we used a threshold on the fluorescence channel of the lipophilic probe. However, because events below 100 nm (such as exosomes) could not be distinguished from the background, with the threshold used, we eliminated both the background and the exosomes; thus, exosomes were not considered in this work.
4.3. Gating Strategy Analysis
Phalloidin negative and lipophilic cationic dye positive events were considered enriched in EVs, as previously described [18]. Leukocyte-derived EVs were identified as CD45 positive and further subdivided into CD45dim EVs and CD45high EVs. CD31 and CD41a were evaluated on CD45-negative EVs to define EVs derived from endothelial cells (CD31+ CD41a−) and platelets (CD31+ CD41a+). Within CD45 negative EVs, we also used the CD11b marker to identify glial-derived EVs and the neuronal surface marker CD171 to characterize EVs of neuronal origin. Outside the EV area, platelets were identified based on their positivity for both CD31 and CD41a markers. Red blood cells were identified by their morphology and lipophilic cationic dye positivity and were not acquired to avoid storing heavy files.
To evaluate the correct size (50 nm 1000 nm) of the events acquired in the EV area, we performed set-up experiments using a mix of beads with the following diameters: 100, 300, 500, and 900 nm, called Megamix-Plus FSC (Biocytex, Marseille, France, #7802), which have been specifically generated for cytometer settings in microparticle analysis (Figure 1i–j). A further internal control was the parallel acquisition of platelets, which measure 1500–3000 nm in diameter; indeed, as we observed in Figure 1d, the EVs were always smaller than platelets.
4.4. Statistical Analysis
4.4.1. Genotyping and Imputation
Genetic analyses were performed on 933 samples genotyped with OmniExpress Illumina array, as previously described for the entire SardiNIA cohort [52]. Imputation was performed on a genome-wide scale using a Sardinian sequence-based reference panel of 3514 individuals and Minimac software, version 4.0 [53] on pre-phased genotypes [52]. After imputation, only markers with imputation quality (RSQR) >0.3 for estimated MAF ≥ 1% or >0.6 for MAF < 1% were retained for association analyses [54], yielding 18,425,755 variants (17,209,121 SNPs and 1,216,634 indels) useful for analyses.
4.4.2. Association Analysis
Genome-wide association analysis for each quantitative trait was carried out using the q.emmax (quantitative EMMAX–Efficient Mixed Model Association eXpedited) function included in EPACTS-3.2.6 (https://genome.sph.umich.edu/wiki/EPACTS, accessed on 10 July 2014). The method implemented in this software accounts for a wide range of sample structures, such as cryptic relatedness and population stratification, by applying a linear mixed model adjusted for a genomic-based kinship matrix obtained from quality-checked genotyped autosomal SNPs with MAF > 1% [52]. All assessed traits were normalized with inverse-normal transformation and adjusted for sex and age as covariates. To adjust for multiple testing, Bonferroni correction was applied to the empirical significance threshold (p = 6.91 × 10−9) by considering the total number of absolute cell counts and MFIs assessed here (n = 28), yielding a final threshold of p < 2.47 × 10−10. Variants in the sex chromosomes were not analyzed.
4.4.3. Age, Sex, and Correlation Analysis
To assess the impact of age and sex on the 28 traits measured, we applied a linear regression model and used R software, version 4.2.2. Traits were normalized before the analyses using inverse normal transformation. The significant threshold of 1.79 × 10−3 was obtained by dividing the nominal p-value of 0.05 for the number of traits assessed (n = 28). Phenotypic correlations between platelet and EV levels were calculated using Spearman’s coefficient in R software, version 4.2.2. The significant threshold of 4.55 × 10−3 was obtained by dividing the nominal p-value of 0.05 for the number of traits expressed assessed (n = 11 EV absolute counts vs. platelet count).
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24087183/s1.
Author Contributions
Conceptualization, V.O. and E.F.; methodology, F.V., V.O. and V.S.; software, V.O.; formal analysis, M.M.; investigation, V.O.; writing—original draft preparation, V.O. and F.V.; writing—review and editing, E.F., D.S., M.D. and F.C.; visualization, V.O.; supervision, V.O.; project administration, E.F. and V.O.; funding acquisition, F.C. All authors have read and agreed to the published version of the manuscript.
Funding
The preparation of this manuscript was supported by the Intramural Research Program of the National Institute on Ageing, National Institutes of Health (NIH) (contract 75N95021C00012-SardiNIA5), by the Horizon 2020 Research and Innovation Program of the European Union (grant 633964), by Consiglio Nazionale delle Ricerche DSB.AD001.149/Identificazione dei marcatori cellulari coinvolti nella predisposizione a patologie complesse (MarCellCo), and by Virus Memory (FOE 2020).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee ASL 1 Sassari (protocol no. 2171/CE 19 May 2015).
Informed Consent Statement
All participants signed informed consent approved by the Sardinian Regional Ethics Committee (protocol no. 2171/CE).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the volunteers who generously participated in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Harrington, M.G.; Fonteh, A.N.; Oborina, E.; Liao, P.; Cowan, R.P.; McComb, G.; Chavez, J.N.; Rush, J.; Biringer, R.G.; Hühmer, A.F. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cereb. Fluid Res. 2009, 6, 10. [Google Scholar] [CrossRef]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Sturk, A.; van Tienen, L.M.; Schaap, M.C.; Nieuwland, R. Cell-derived vesicles exposing coagulant tissue factor in saliva. Blood 2011, 117, 3172–3180. [Google Scholar] [CrossRef]
- Severino, V.; Dumonceau, J.M.; Delhaye, M.; Moll, S.; Annessi-Ramseyer, I.; Robin, X.; Frossard, J.L.; Farina, A. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology 2017, 153, 495–504.e8. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zaldivia, M.T.K.; McFadyen, J.D.; Lim, B.; Wang, X.; Peter, K. Platelet-Derived Microvesicles in Cardiovascular Diseases. Front. Cardiovasc. Med. 2017, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Nikolaidou, B.; Gavriilaki, E.; Lazaridis, A.; Yiannaki, E.; Anyfanti, P.; Zografou, I.; Markala, D.; Douma, S. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diab. Vasc. Dis. Res. 2019, 16, 458–465. [Google Scholar] [CrossRef]
- Sellam, J.; Proulle, V.; Jüngel, A.; Ittah, M.; Miceli Richard, C.; Gottenberg, J.E.; Toti, F.; Benessiano, J.; Gay, S.; Freyssinet, J.M.; et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 2009, 11, R156. [Google Scholar] [CrossRef]
- Liang, B.; Peng, P.; Chen, S.; Li, L.; Zhang, M.; Cao, D.; Yang, J.; Li, H.; Gui, T.; Li, X.; et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteom. 2013, 80, 171–182. [Google Scholar] [CrossRef]
- Park, J.O.; Choi, D.Y.; Choi, D.S.; Kim, H.J.; Kang, J.W.; Jung, J.H.; Lee, J.H.; Kim, J.; Freeman, M.R.; Lee, K.Y.; et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics 2013, 13, 2125–2134. [Google Scholar] [CrossRef]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Del Zotto, G.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2020, 22, 48. [Google Scholar] [CrossRef]
- Cappellano, G.; Raineri, D.; Rolla, R.; Giordano, M.; Puricelli, C.; Vilardo, B.; Manfredi, M.; Cantaluppi, V.; Sainaghi, P.P.; Castello, L.; et al. Circulating Platelet-Derived Extracellular Vesicles Are a Hallmark of SARS-CoV-2 Infection. Cells 2021, 10, 85. [Google Scholar] [CrossRef]
- Raineri, D.; Venegoni, C.; Calella, M.G.; Vaschetto, R.; Scotti, L.; Canciani, E.; Manfredi, M.; Gavelli, F.; Castello, L.; Chiocchetti, A.; et al. Worse Disease Prognosis Is Associated to an Increase of Platelet-Derived Extracellular Vesicles in Hospitalized SARS-CoV-2 Patients. Dis. Markers 2022, 2022, 8074655. [Google Scholar] [CrossRef]
- Hermida-Nogueira, L.; García, Á. Extracellular vesicles in the transfusion medicine field: The potential of proteomics. Proteomics 2021, 21, e2000089. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Byappanahalli, A.M.; Vannoy, M.; Omoniyi, V.; Evans, M.K. Influences of age, race, and sex on extracellular vesicle characteristics. Theranostics 2022, 12, 4459–4476. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [PubMed]
- Enjeti, A.K.; Ariyarajah, A.; D’Crus, A.; Seldon, M.; Lincz, L.F. Circulating microvesicle number, function and small RNA content vary with age, gender, smoking status, lipid and hormone profiles. Thromb. Res. 2017, 156, 65–72. [Google Scholar] [CrossRef]
- Zhang, X.; Hubal, M.J.; Kraus, V.B. Immune cell extracellular vesicles and their mitochondrial content decline with ageing. Immun. Ageing 2020, 17, 1. [Google Scholar] [CrossRef]
- Pala, M.; Zappala, Z.; Marongiu, M.; Li, X.; Davis, J.R.; Cusano, R.; Crobu, F.; Kukurba, K.R.; Gloudemans, M.J.; Reinier, F.; et al. Population- and individual-specific regulatory variation in Sardinia. Nat. Genet. 2017, 49, 700–707. [Google Scholar] [CrossRef]
- Goggs, R.; Williams, C.M.; Mellor, H.; Poole, A.W. Platelet Rho GTPases—A focus on novel players, roles and relationships. Biochem. J. 2015, 466, 431–442. [Google Scholar] [CrossRef]
- Jeong, D.; Lozano Casasbuenas, D.; Gengatharan, A.; Edwards, K.; Saghatelyan, A.; Kaplan, D.R.; Miller, F.D.; Yuzwa, S.A. LRIG1-Mediated Inhibition of EGF Receptor Signaling Regulates Neural Precursor Cell Proliferation in the Neocortex. Cell Rep. 2020, 33, 108257. [Google Scholar] [CrossRef]
- Werner, A.; Iwasaki, S.; McGourty, C.A.; Medina-Ruiz, S.; Teerikorpi, N.; Fedrigo, I.; Ingolia, N.T.; Rape, M. Cell-fate determination by ubiquitin-dependent regulation of translation. Nature 2015, 525, 523–527. [Google Scholar] [CrossRef]
- Gomes, M.T.R.; Cerqueira, D.M.; Guimarães, E.S.; Campos, P.C.; Oliveira, S.C. Guanylate-binding proteins at the crossroad of noncanonical inflammasome activation during bacterial infections. J. Leukoc. Biol. 2019, 106, 553–562. [Google Scholar] [CrossRef]
- Honkala, A.T.; Tailor, D.; Malhotra, S.V. Guanylate-Binding Protein 1: An Emerging Target in Inflammation and Cancer. Front. Immunol. 2020, 10, 3139. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pertierra, E.; Oliveira-Rodríguez, M.; Matos, M.; Gutiérrez, G.; Moyano, A.; Salvador, M.; Rivas, M.; Blanco-López, M.C. Extracellular Vesicles: Current Analytical Techniques for Detection and Quantification. Biomolecules 2020, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D.; Bao, E.L.; Akbari, P.; Lareau, C.A.; Mousas, A.; Jiang, T.; Chen, M.-H.; Raffield, L.M.; Tardaguila, M.; Huffman, J.E.; et al. The Polygenic and Monogenic Basis of Blood Traits and Diseases. Cell 2020, 182, 1214–1231.e11. [Google Scholar] [CrossRef]
- Ziliotto, N.; Bernardi, F.; Jakimovski, D.; Zivadinov, R. Coagulation Pathways in Neurological Diseases: Multiple Sclerosis. Front. Neurol. 2019, 10, 409. [Google Scholar] [CrossRef]
- De Luca, C.; Virtuoso, A.; Maggio, N.; Papa, M. Neuro-Coagulopathy: Blood Coagulation Factors in Central Nervous System Diseases. Int. J. Mol. Sci. 2017, 18, 2128. [Google Scholar] [CrossRef]
- Lorenzano, S.; Inglese, M.; Koudriavtseva, T. Editorial: Role of Coagulation Pathways in Neurological Diseases. Front. Neurol. 2019, 10, 791. [Google Scholar] [CrossRef]
- Gomes, D.E.; Witwer, K.W. L1CAM-associated extracellular vesicles: A systematic review of nomenclature, sources, separation, and characterization. J. Extracell. Biol. 2022, 1, e35. [Google Scholar] [CrossRef]
- Xu, L.; Liang, Y.; Xu, X.; Xia, J.; Wen, C.; Zhang, P.; Duan, L. Blood cell-derived extracellular vesicles: Diagnostic biomarkers and smart delivery systems. Bioengineered 2021, 12, 7929–7940. [Google Scholar] [CrossRef]
- Hooten, N.N. Extracellular vesicles and extracellular RNA in aging and age-related disease. Transl. Med. Aging 2020, 4, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Choi, Y.; Lee, H.J.; Hwang, D.W.; Lee, D.S. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 2022, 20, 198. [Google Scholar] [CrossRef] [PubMed]
- Lino, M.M.; Simões, S.; Tomatis, F.; Albino, I.; Barrera, A.; Vivien, D.; Sobrino, T.; Ferreira, L. Engineered extracellular vesicles as brain therapeutics. J. Control. Release 2021, 338, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Z.; Li, N.; Tian, C.; Yang, H.; Huo, Y.; Li, Y.; Zhang, J.; Yu, Z. Parkinson’s Disease Derived Exosomes Aggravate Neuropathology in SNCA*A53T Mice. Ann. Neurol. 2022, 92, 230–245. [Google Scholar] [CrossRef]
- Bebesi, T.; Kitka, D.; Gaál, A.; Szigyártó, I.C.; Deák, R.; Beke-Somfai, T.; Koprivanacz, K.; Juhász, T.; Bóta, A.; Varga, Z.; et al. Storage conditions determine the characteristics of red blood cell derived extracellular vesicles. Sci. Rep. 2022, 12, 977. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Too complex to fail? Targeting fatty acid metabolism for cancer therapy. Prog. Lipid Res. 2022, 85, 101143. [Google Scholar] [CrossRef]
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020, 143, 1975–1998. [Google Scholar] [CrossRef]
- Zamzam, A.; Syed, M.H.; Rotstein, O.D.; Eikelboom, J.; Klein, D.J.; Singh, K.K.; Abdin, R.; Qadura, M. Validating fatty acid binding protein 3 as a diagnostic and prognostic biomarker for peripheral arterial disease: A three-year prospective follow-up study. EClinicalMedicine 2022, 55, 101766. [Google Scholar] [CrossRef]
- Yang, C.; Fagan, A.M.; Perrin, R.J.; Rhinn, H.; Harari, O.; Cruchaga, C. Mendelian randomization and genetic colocalization infer the effects of the multi-tissue proteome on 211 complex disease-related phenotypes. Genome Med. 2022, 14, 140. [Google Scholar] [CrossRef]
- Pilia, G.; Chen, W.M.; Scuteri, A.; Orrú, M.; Albai, G.; Dei, M.; Lai, S.; Usala, G.; Lai, M.; Loi, P.; et al. Heritability of cardiovascular and personality traits in 6148 Sardinians. PLoS Genet. 2006, 2, e132. [Google Scholar]
- Sidore, C.; Busonero, F.; Maschio, A.; Porcu, E.; Naitza, S.; Zoledziewska, M.; Mulas, A.; Pistis, G.; Steri, M.; Danjou, F.; et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat. Genet. 2015, 47, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Pistis, G.; Porcu, E.; Vrieze, S.I.; Sidore, C.; Steri, M.; Danjou, F.; Busonero, F.; Mulas, A.; Zoledziewska, M.; Maschio, A.; et al. Rare variant genotype imputation with thousands of study-specific whole-genome sequences: Implications for cost-effective study designs. Eur. J. Hum. Genet. 2015, 23, 975–983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).