GSPE Pre-Treatment Exerts Long-Lasting Preventive Effects against Aging-Induced Changes in the Colonic Enterohormone Profile of Female Rats
Abstract
1. Introduction
2. Results
2.1. Differential Effects of Aging and GSPE Pre-Treatment on the Upper GIT Secretory Profile
2.2. The Effects of Aging and GSPE on the GLP-1 System Differ between the Ileum and the Colon
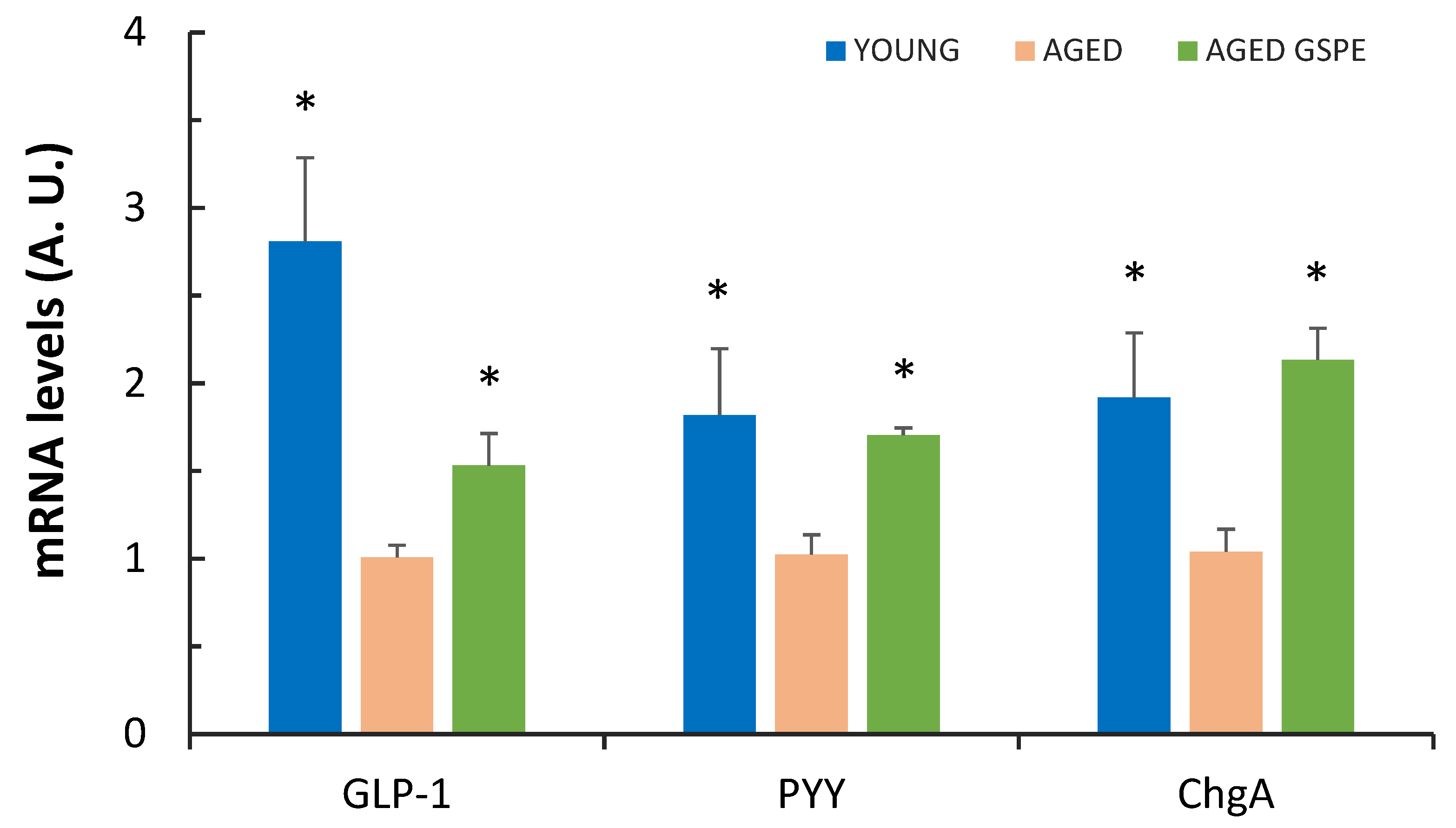
2.3. GSPE Pre-Treatment Prevents an Aging-Induced Decrease in Colonic Enterohormone Expression
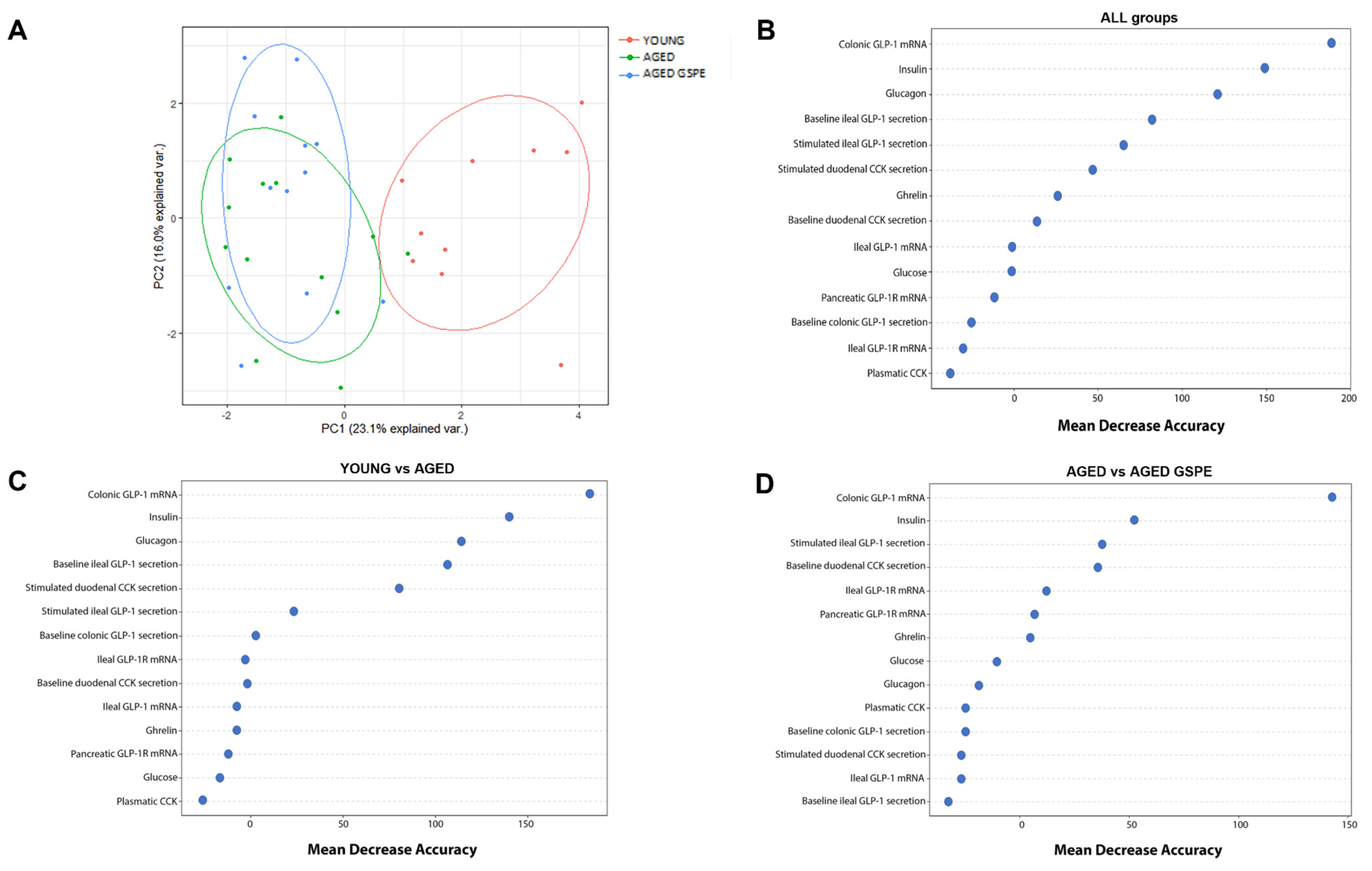
2.4. The Colonic Expression of GLP-1 Is the Most Relevant Factor for Discriminating between Groups
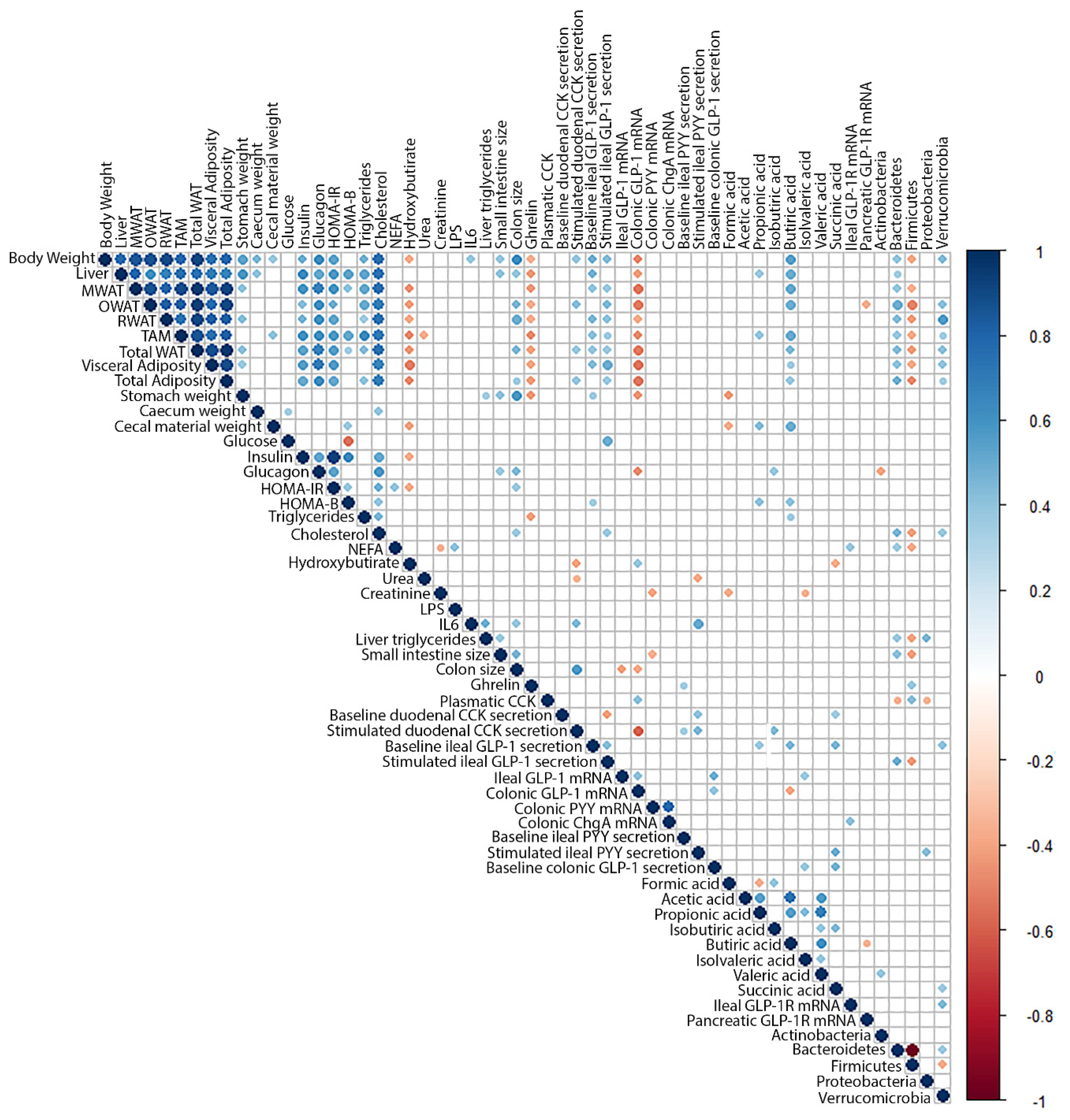
3. Discussion
4. Materials and Methods
4.1. Proanthocyanidin Extract
4.2. Animal Study
4.3. Blood and Tissue Collection
4.4. Ex Vivo Study
Enterohormone Quantification
4.5. Gene Expression Analysis
4.6. Quantification of Short-Chain Fatty Acids
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Sternini, C.; De Giorgio, R.; Greenwood-Van Meerveld, B. Enteroendocrine cells: A review of their role in brain-gut communication. Neurogastroenterol. Motil. 2016, 28, 620–630. [Google Scholar] [CrossRef]
- Bouskra, D.; Brézillon, C.; Bérard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Calçada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A.; et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136–137, 138–147. [Google Scholar] [CrossRef]
- Scheen, A.J.; Van Gaal, L.F. Combating the dual burden: Therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet. Diabetes Endocrinol. 2014, 2, 911–922. [Google Scholar] [CrossRef]
- Balaskó, M.; Rostás, I.; Füredi, N.; Mikó, A.; Tenk, J.; Cséploo, P.; Koncsecskó-Gáspár, M.; Soós, S.; Székely, M.; Pétervári, E. Age and nutritional state influence the effects of cholecystokinin on energy balance. Exp. Gerontol. 2013, 48, 1180–1188. [Google Scholar] [CrossRef]
- Sandströ, O.; El-Salhy, M. Ageing and endocrine cells of human duodenum. Mech. Ageing Dev. 1999, 108, 39–48. [Google Scholar] [CrossRef]
- Sandström, O.; El-Salhy, M. Duodenal endocrine cells in mice with particular regard to age-induced changes. Histol. Histopathol. 2000, 15, 347–353. [Google Scholar]
- MacIntosh, C.G.; Andrews, J.M.; Jones, K.L.; Wishart, J.M.; Morris, H.A.; Jansen, J.B.M.J.; Morley, J.E.; Horowitz, M.; Chapman, I.M. Effects of age on concentrations of plasma cholecystokinin, glucagon- like peptide 1, and peptide YY and their relation appetite and pyloric motility. Am. J. Clin. Nutr. 1999, 69, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Britton, E.; McLaughlin, J.T. Ageing and the gut. Proc. Nutr. Soc. 2013, 72, 173–177. [Google Scholar] [CrossRef]
- Moss, C.; Dhillo, W.S.; Frost, G.; Hickson, M. Gastrointestinal hormones: The regulation of appetite and the anorexia of ageing. J. Hum. Nutr. Diet. 2012, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Lizárraga, E.; Álvarez-Cilleros, D.; Pérez-Pardo, P.; Sanmartín-Salinas, P.; Val Toledo-Lobo, M.; Alvarez, C.; Escrivá, F.; Fernández-Lobato, M.; Guijarro, L.G.; et al. Aging in Male Wistar Rats Associates with Changes in Intestinal Microbiota, Gut Structure, and Cholecystokinin-Mediated Gut-Brain Axis Function. Journals Gerontol.-Ser. A Biol. Sci. Med. Sci. 2021, 76, 1915–1921. [Google Scholar] [CrossRef]
- Suzuki, T.; Aoki, K.; Shimokobe, K.; Omiya, S.; Funayama, C.; Takahashi, T.; Kato, M. Age-related morphological and functional changes in the small intestine of senescence-accelerated mouse. Exp. Gerontol. 2022, 163, 111795. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Quesada, H.; del Bas, J.M.; Pajuelo, D.; Díaz, S.; Fernandez-Larrea, J.; Pinent, M.; Arola, L.; Salvadó, M.J.; Bladé, C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. (Lond) 2009, 33, 1007–1012. [Google Scholar] [CrossRef]
- Castell-Auví, A.; Cedó, L.; Pallarès, V.; Blay, M.; Ardévol, A.; Pinent, M. The effects of a cafeteria diet on insulin production and clearance in rats. Br. J. Nutr. 2012, 108, 1155–1162. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.L.; Blay, M.; Ardevol, A.; González-Abuín, N.; Pinent, M.; Casanova-Martí, A.; Arola, L.L.; et al. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Montagut, G.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.J.; Arola, L.L.; Pinent, M.; Ardévol, A.; Blade, C.; et al. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J. Nutr. Biochem. 2010, 21, 961–967. [Google Scholar] [CrossRef]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Ardévol, A.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-promoting properties of proanthocyanidins for intestinal dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Depoortere, I.; Blay, M.T.M.T.; Terra, X.; Pinent, M.; Ardévol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016, 60, 2554–2564. [Google Scholar] [CrossRef] [PubMed]
- Grau-bové, C.; Ginés, I.; Beltrán-Debón, R.; Terra, X.; Blay, M.; Pinent, M.; Ardévol, A. Glucagon Shows Higher Sensitivity than Insulin to Grapeseed. Nutrients 2021, 13, 1084. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-cardoso, K.; Serrano, J.; Casanova-marti, À.; Lobato, M.; Terra, X.; Blay, M.T.; Ard, A. Proanthocyanidins Limit Adipose Accrual Induced by a Cafeteria Diet, Several Weeks after the End of the Treatment. Genes (Basel) 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Grau-Bové, C.; Sierra-Cruz, M.; Miguéns, A.; Rodríguez-gallego, E.; Miguéns-Gómez, A.; Rodríguez-gallego, E.; Beltrán-Debón, R.; Blay, M.; Terra, X.; Pinent, M.; et al. A ten-day grape seed procyanidin treatment prevents certain ageing processes in female rats over the long term. Nutrients 2020, 12, 3647. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Cruz, M.; Miguéns-Gómez, A.; Grau-Bové, C.; Rodríguez-Gallego, E.; Blay, M.; Pinent, M.; Ardévol, A.; Terra, X.; Beltrán-Debón, R. Grape-seed proanthocyanidin extract reverts obesity-related metabolic derangements in aged female rats. Nutrients 2021, 13, 2059. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; Serrano, J.; Casanova-Martí, À.; Blay, M.; Pinent, M.; Ardévol, A.; Terra, X. Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients 2018, 10, 315. [Google Scholar] [CrossRef]
- Thorburn, A.W.; Baldwin, M.E.; Rosella, G.; Zajac, J.D.; Fabris, S.; Song, S.; Proietto, J. Features of syndrome X develop in transgenic rats expressing a non-insulin responsive phosphoenolpyruvate carboxykinase gene. Diabetologia 1999, 42, 419–426. [Google Scholar] [CrossRef]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef]
- Kraegen, E.W.; Cooney, G.J. Free fatty acids and skeletal muscle insulin resistance. Curr. Opin. Lipidol. 2008, 19, 235–241. [Google Scholar] [CrossRef]
- Ding, Y.-Y.; Fang, Y.; Pan, Y.; Lan, J.; Xu, T.; Zhang, W.; Mao, H.; Gu, Z.; Chen, X.; Shen, Q. Orally administered octacosanol improves liver insulin resistance in high-fat diet-fed mice through the reconstruction of the gut microbiota structure and inhibition of the TLR4/NF-κB inflammatory pathway. Food Funct. 2023, 14, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Jeong Park, Y.; Kim, Y.-R.; Na Kim, Y.; Ka, S.; Young Lee, H.; Kyung Seong, J.; Seok, Y.-J.; Bum Kim, J. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015, 29, 2397–2411. [Google Scholar] [CrossRef]
- Pearce, S.C.; Weber, G.J.; Van Sambeek, D.M.; Soares, J.W.; Racicot, K.; Breault, D.T. Intestinal enteroids recapitulate the effects of short-chain fatty acids on the intestinal epithelium. PLoS ONE 2020, 15, e0230231. [Google Scholar] [CrossRef]
- Miguéns-Gómez, A.; Sierra-Cruz, M.; Pérez-Vendrell, A.M.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Terra, X.; Ardévol, A.; Pinent, M. Differential effects of a cafeteria diet and GSPE preventive treatments on the enterohormone secretions of aged vs. young female rats. Food Funct. 2022, 10491–10500. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; D’addario, C.; Falconi, A.; Bellia, F.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M.; Beltrán-Debón, R. Long-lasting effects of gspe on ileal GLP-1R gene expression are associated with a hypomethylation of the GLP-1R promoter in female wistar rats. Biomolecules 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Iglesias-Carres, L.; Pons, Z.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Age related differences in the plasma kinetics of flavanols in rats. J. Nutr. Biochem. 2016, 29, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Marti, A.; Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef]
- van Zanten, G.C.; Knudsen, A.; Röytiö, H.; Forssten, S.; Lawther, M.; Blennow, A.; Lahtinen, S.J.; Jakobsen, M.; Svensson, B.; Jespersen, L.; et al. The Effect of Selected Synbiotics on MicrobialComposition and Short-Chain Fatty Acid Production in aModel System of the Human Colon. PLoS ONE 2012, 7, e4721. [Google Scholar] [CrossRef]
- Casanova-martí, À.; González-abuín, N.; Serrano, J.; Blay, M.T.; Terra, X.; Frost, G.; Ardévol, A. Long Term Exposure to a Grape Seed Proanthocyanidin Extract Enhances L-Cell Differentiation in Intestinal Organoids. Mol. Nutr. Food Res. 2020, 64, 2000303. [Google Scholar] [CrossRef] [PubMed]
- Lastya, A.; Saraswati, M.R.; Suastika, K. The low level of glucagon-like peptide-1 (glp-1) is a risk factor of type 2 diabetes mellitus. BMC Res. Notes 2014, 7, 849. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; Terra, X.; Blay, M.; Pérez-Vendrell, A.M.; Pinent, M.; Ardévol, A. Grape Seed Proanthocyanidins Target the Enteroendocrine System in Cafeteria-Diet-Fed Rats. Mol. Nutr. Food Res. 2019, 63, 1800912. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; et al. Defining conditions for optimal inhibition of food intake in rats by a grape-seed derived proanthocyanidin extract. Nutrients 2016, 8, 652–666. [Google Scholar] [CrossRef] [PubMed]
| YOUNG | AGED | AGED GSPE | |
|---|---|---|---|
| CCK plasmatic levels (ng mL−1) | 0.72 ± 0.05 | 0.64 ± 0.03 | 0.61 ± 0.03 |
| Baseline CCK (ng mL−1) | 0.23 ± 0.03 | 0.30 ± 0.04 | 0.18 ± 0.02 * |
| Stimulated CCK (ng mL−1) | 0.50 ± 0.04 * | 0.72 ± 0.09 | 0.64 ± 0.05 |
| YOUNG | AGED | AGED GSPE | |
|---|---|---|---|
| Baseline aGLP-1 (pM) | 59.4 ± 10.0 * | 156.5 ± 38.4 | 135.5 ± 19.7 |
| Stimulated aGLP-1 (pM) | 196.7 ± 44.8 # | 368.2 ± 82.4 | 607.4 ± 90.2 # |
| Baseline PYY (pg mL−1) | 21.3 ± 5.7 | 25.6 ± 4.9 | 32.3 ± 1.2 |
| Stimulated PYY (pg mL−1) | n.a. | 107.5 ± 15.7 | 101.3 ± 7.4 |
| GLP-1 mRNA (A. U.) | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.8 ± 0.1 # |
| YOUNG | AGED | AGED GSPE | |
|---|---|---|---|
| Formic | 10.1 ± 1.6 | 7.7 ± 1.2 | 4.7 ± 1.3 # |
| Acetic | 25.6 ± 4.9 | 26.6 ± 2.2 | 28.0 ± 3.6 |
| Propionic | 5.2 ± 1.4 | 7.6 ± 0.7 | 6.5 ± 1.4 |
| Isobutyric | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.2 |
| Butyric | 4.5 ± 0.5 * | 9.1 ± 1.2 | 6.7 ± 1.7 |
| Isovaleric | 0.3 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.1 |
| Valeric | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Lactic | 0.0 | 0.0 | 0.0 |
| Succinic | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguéns-Gómez, A.; Sierra-Cruz, M.; Blay, M.T.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Terra, X.; Pinent, M.; Ardévol, A. GSPE Pre-Treatment Exerts Long-Lasting Preventive Effects against Aging-Induced Changes in the Colonic Enterohormone Profile of Female Rats. Int. J. Mol. Sci. 2023, 24, 7807. https://doi.org/10.3390/ijms24097807
Miguéns-Gómez A, Sierra-Cruz M, Blay MT, Rodríguez-Gallego E, Beltrán-Debón R, Terra X, Pinent M, Ardévol A. GSPE Pre-Treatment Exerts Long-Lasting Preventive Effects against Aging-Induced Changes in the Colonic Enterohormone Profile of Female Rats. International Journal of Molecular Sciences. 2023; 24(9):7807. https://doi.org/10.3390/ijms24097807
Chicago/Turabian StyleMiguéns-Gómez, Alba, Marta Sierra-Cruz, M. Teresa Blay, Esther Rodríguez-Gallego, Raúl Beltrán-Debón, Ximena Terra, Montserrat Pinent, and Anna Ardévol. 2023. "GSPE Pre-Treatment Exerts Long-Lasting Preventive Effects against Aging-Induced Changes in the Colonic Enterohormone Profile of Female Rats" International Journal of Molecular Sciences 24, no. 9: 7807. https://doi.org/10.3390/ijms24097807
APA StyleMiguéns-Gómez, A., Sierra-Cruz, M., Blay, M. T., Rodríguez-Gallego, E., Beltrán-Debón, R., Terra, X., Pinent, M., & Ardévol, A. (2023). GSPE Pre-Treatment Exerts Long-Lasting Preventive Effects against Aging-Induced Changes in the Colonic Enterohormone Profile of Female Rats. International Journal of Molecular Sciences, 24(9), 7807. https://doi.org/10.3390/ijms24097807










