Metabolic Effects of New Glucose Transporter (GLUT-1) and Lactate Dehydrogenase-A (LDH-A) Inhibitors against Chemoresistant Malignant Mesothelioma
Abstract
1. Introduction
2. Results
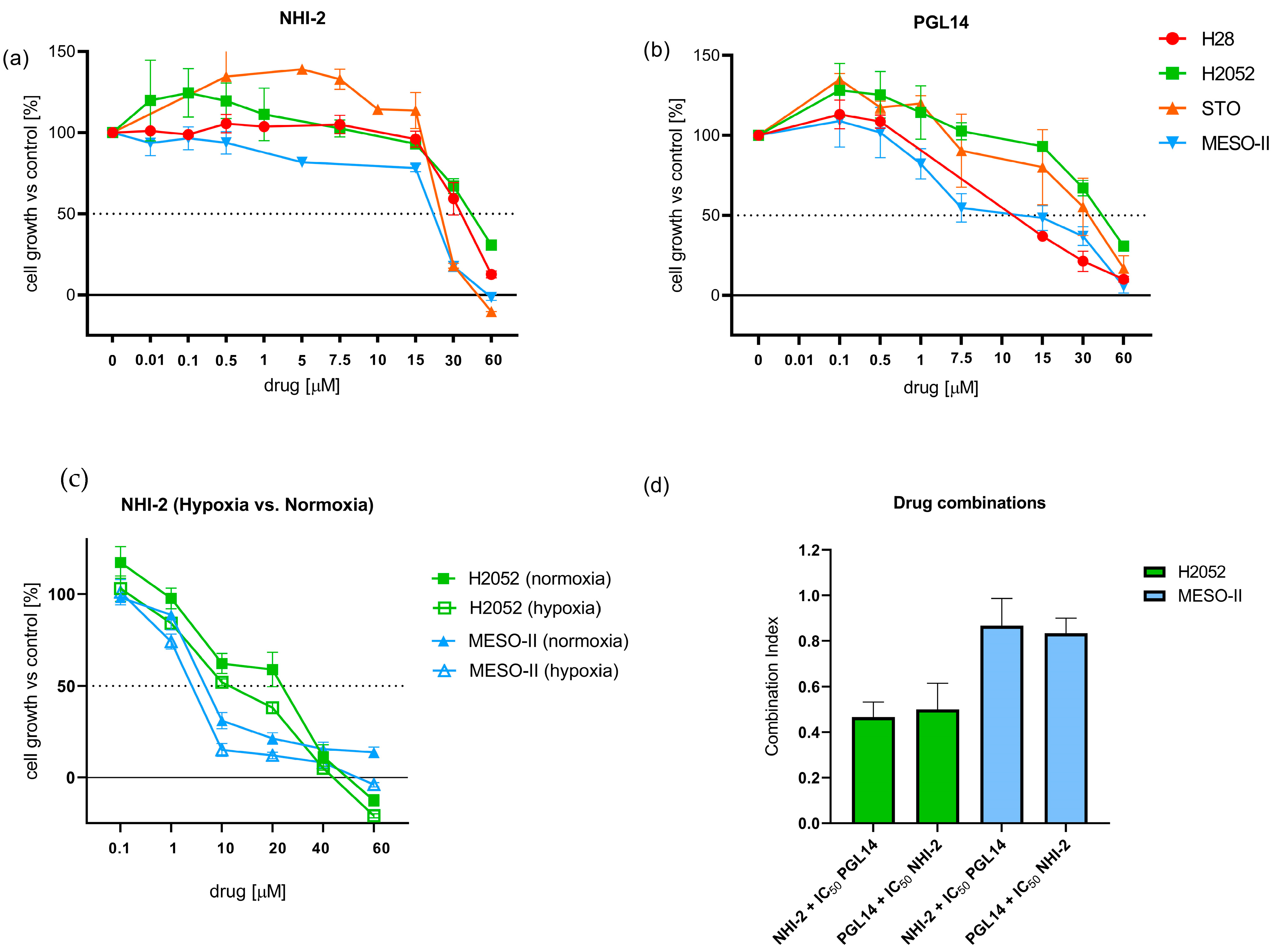
2.1. Effect of GLUT-1 and LDH-A Inhibitors on the Growth of Mesothelioma and HMEC-1 Cells
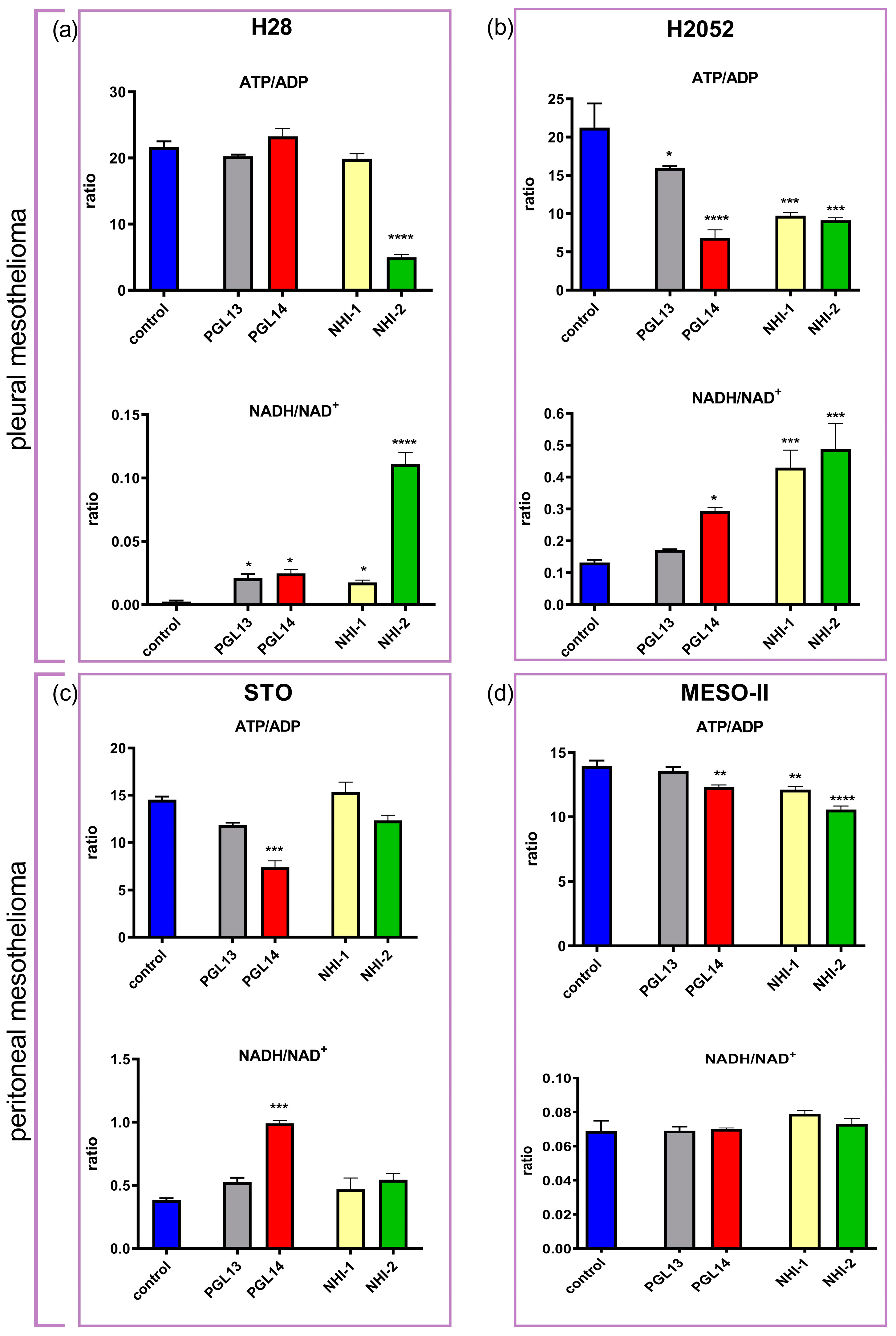
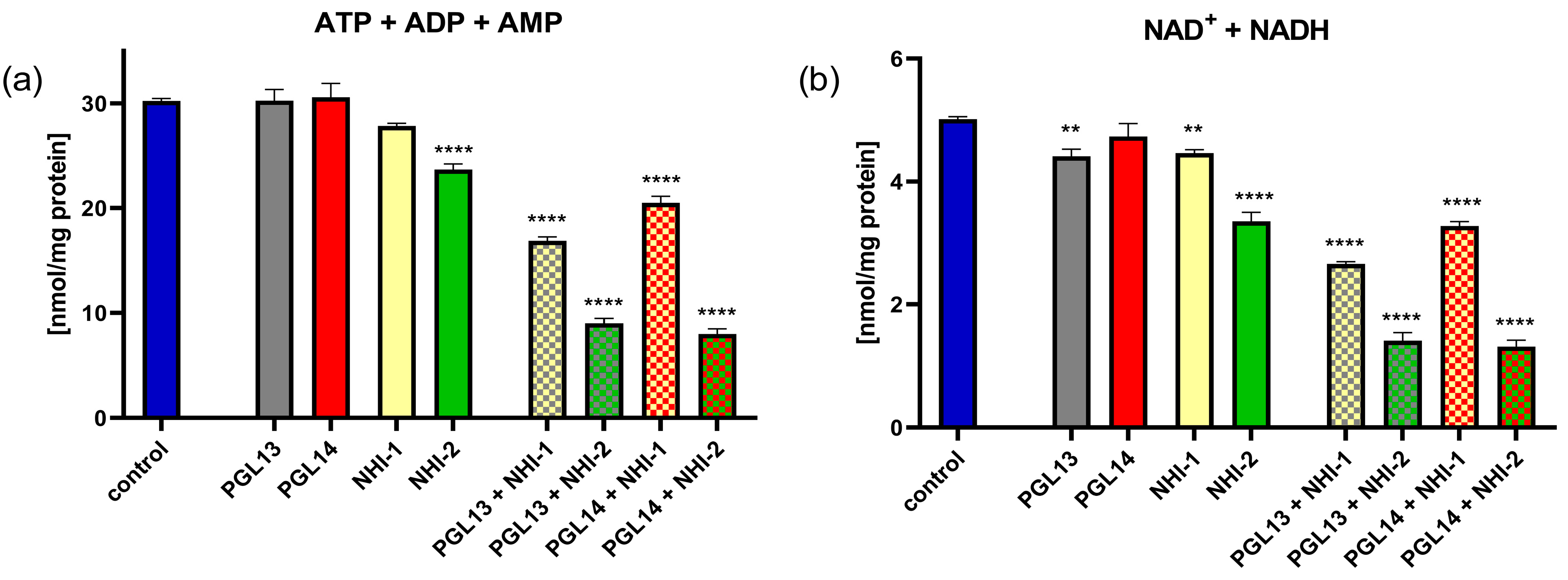
2.2. Effect of GLUT-1 and LDH-A Inhibitors, as Single Agents or in Combination, on Intracellular Nucleotide Concentrations in Mesothelioma Cell Lines
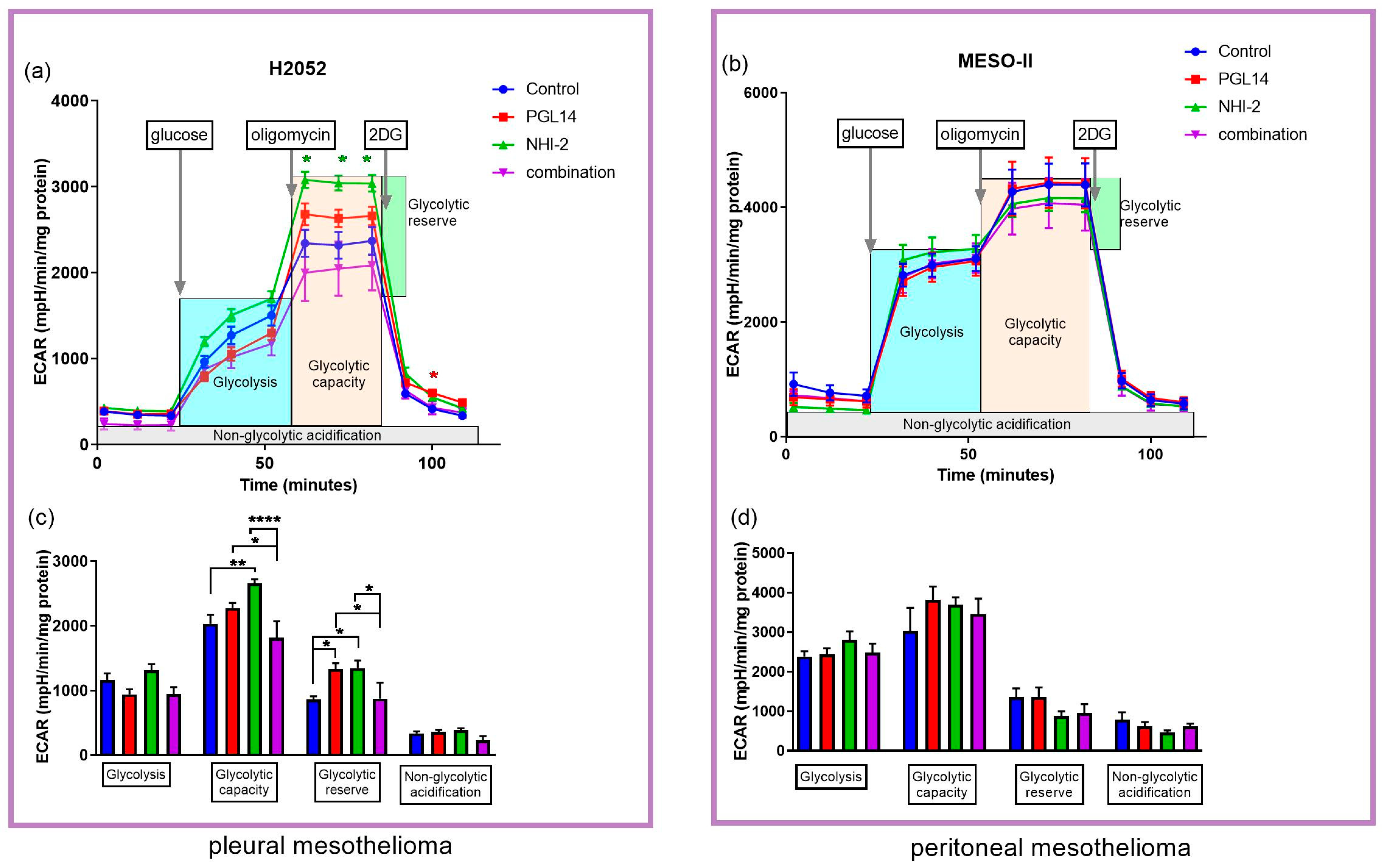
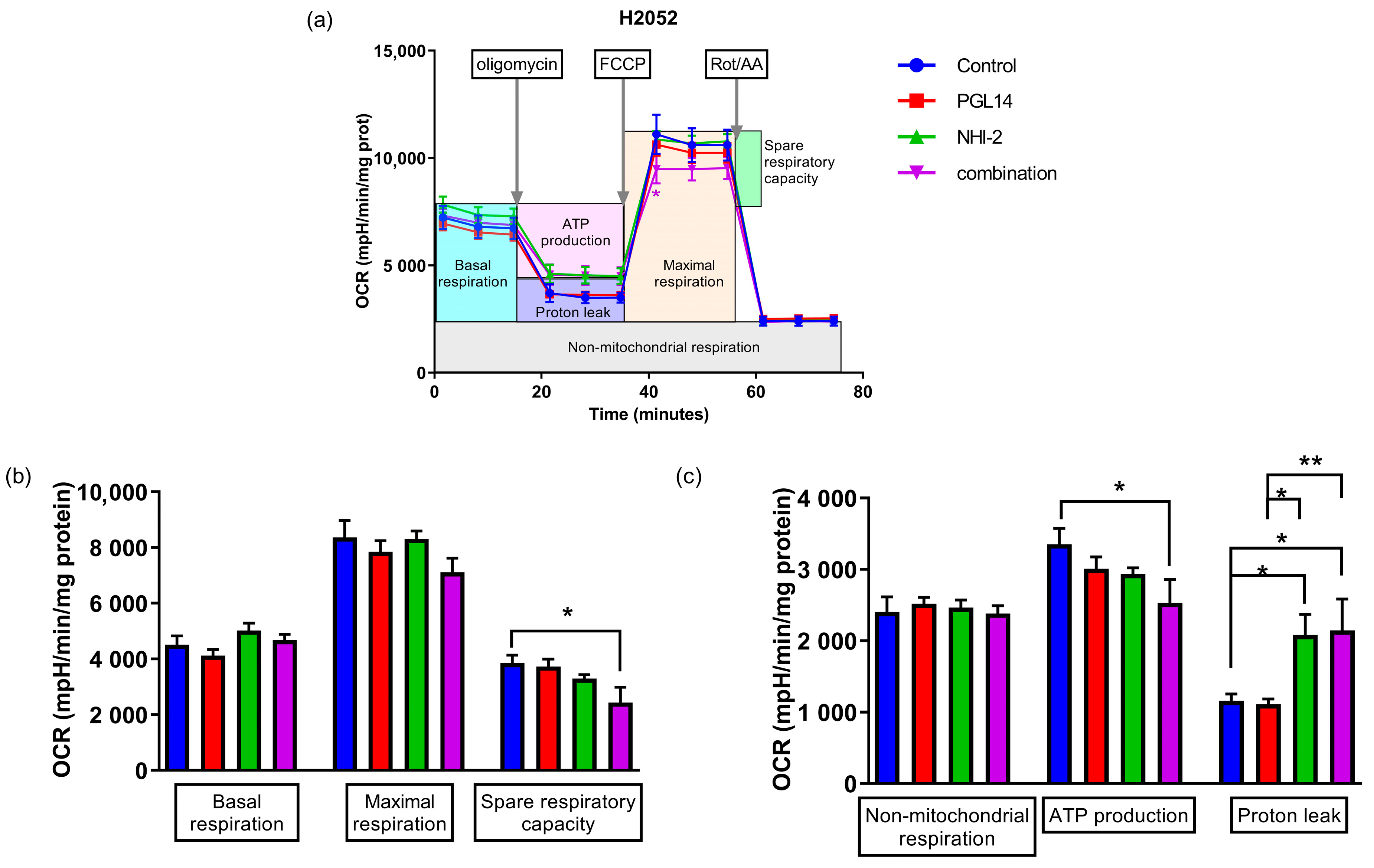
2.3. The Effect of GLUT-1 and LDH-A Inhibitors on Glycolytic Parameters and Mitochondrial Respiration of Mesothelioma Cell Lines
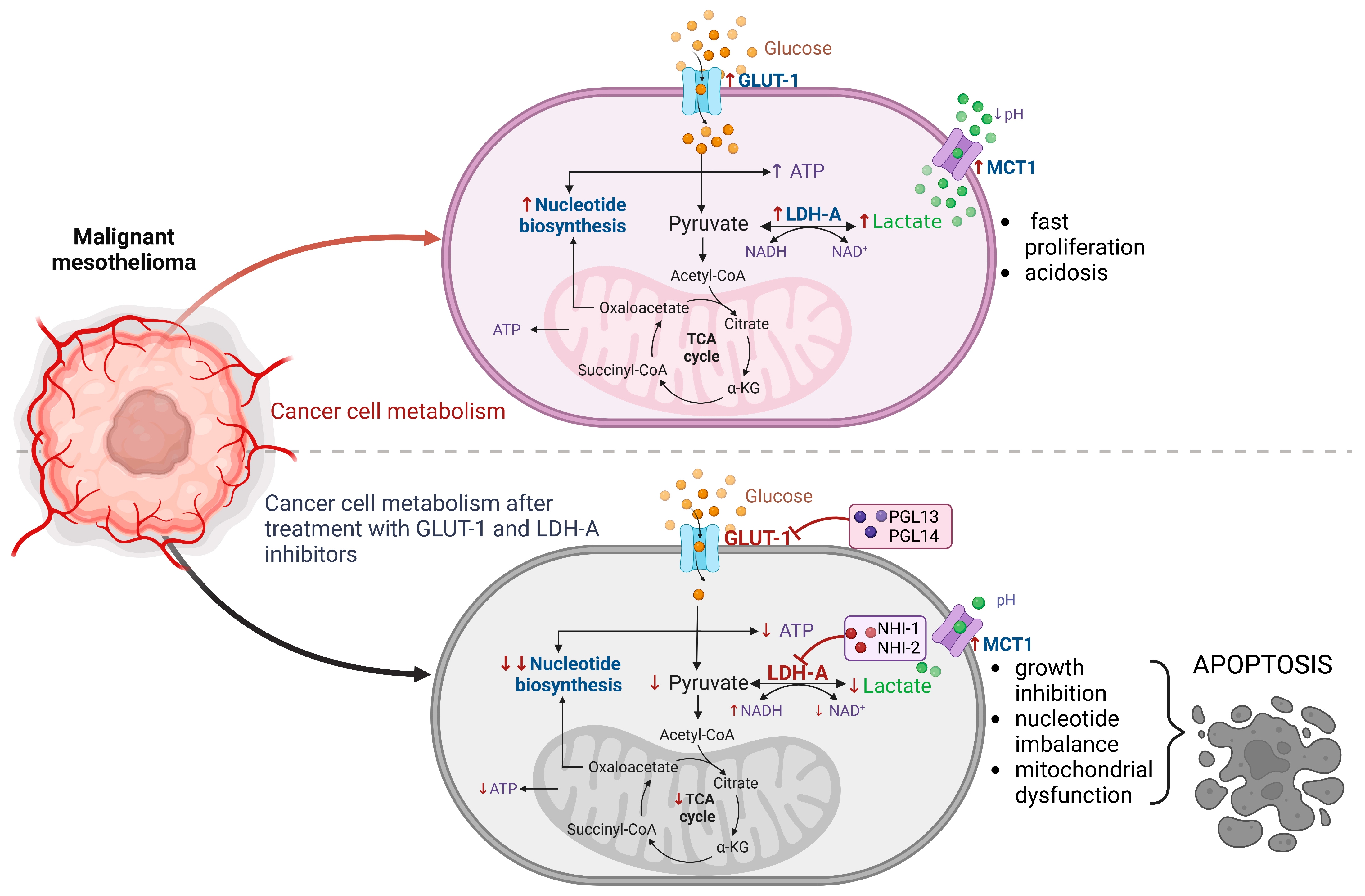
3. Discussion
4. Materials and Methods
4.1. Materials
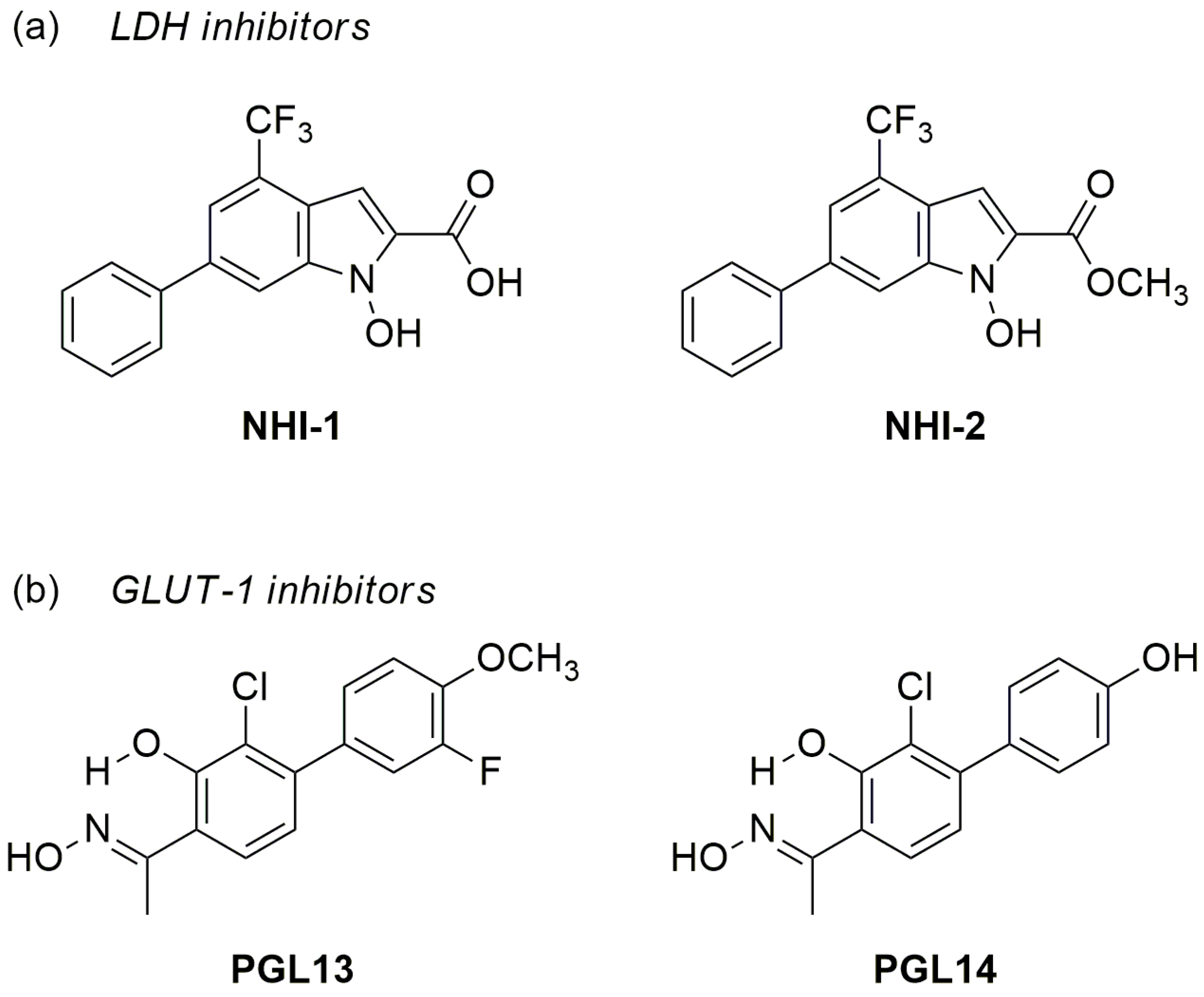
4.1.1. LDH-A and GLUT-1 Inhibitors
4.1.2. Cell Culture
4.2. Methods
4.2.1. Analysis of Inhibition of Cell Growth
4.2.2. Evaluation of Intracellular Nucleotide Concentrations by Reversed-Phase High-Performance Liquid Chromatography
4.2.3. Analysis of Glycolysis and Cell Mito Stress Tests Using the Seahorse Analyzer
4.2.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, D.; Banerjee, D. Lactate Dehydrogenases as Metabolic Links between Tumor and Stroma in the Tumor Microenvi-ronment. Cancers 2019, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Meskers, C.J.; Franczak, M.; Smolenski, R.T.; Giovannetti, E.; Peters, G.J. Are we still on the right path(way)?: The altered expression of the pentose phosphate pathway in solid tumors and the potential of its inhibition in combination therapy. Expert Opin. Drug Metab. Toxicol. 2022, 18, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol. Biol. Lett. 2020, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Radenkovic, S.; Konjevic, G. The Actual Role of LDH as Tumor Marker, Biochemical and Clinical Aspects. In Advances in Cancer Biomarkers; Scatena, R., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2015; Volume 867, pp. 115–124. [Google Scholar]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R. Lactate dehydrogenase 5: An old friend and a new hope in the war on cancer. Cancer Lett. 2015, 358, 1–7. [Google Scholar] [CrossRef]
- Comandatore, A.; Franczak, M.; Smolenski, R.T.; Morelli, L.; Peters, G.J.; Giovannetti, E. Lactate Dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin. Cancer Biol. 2022, 86, 93–100. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Petri, G.L.; El Hassouni, B.; Sciarrillo, R.; Funel, N.; Mantini, G.; van der Laan, E.A.Z.; Cascioferro, S.; Avan, A.; Zucali, P.A.; Zaffaroni, N.; et al. Impact of hypoxia on chemoresistance of mesothelioma mediated by the proton-coupled folate transporter, and preclinical activity of new anti-LDH-A compounds. Br. J. Cancer 2020, 123, 644–656. [Google Scholar] [CrossRef]
- El Hassouni, B.E.; Franczak, M.; Capula, M.; Vonk, C.M.; Gomez, V.M.; Smolenski, R.T.; Granchi, C.; Peters, G.J.; Minutolo, F.; Giovannetti, E. Lactate dehydrogenase A inhibition by small molecular entities: Steps in the right direction. Oncoscience 2020, 7, 76–80. [Google Scholar] [CrossRef]
- Kanno, T.; Sudo, K.; Maekawa, M.; Nishimura, Y.; Ukita, M.; Fukutake, K. Lactate dehydrogenase M-subunit deficiency: A new type of hereditary exertional myopathy. Clin. Chim. Acta 1988, 173, 89–98. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, M.; Gupta, R.; Kumar, V.; Kumar, V.; Rani, R. Intervention on lactate in cancer: A promising approach for the development of cancer therapeutics. Adv. Cancer Biol.-Metastasis 2022, 5, 100058. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.; Sharma, D.; Kumar, V.; Rani, R. Tumor Metabolism: Focused on Tumor Glycolysis, Progress, and Prospects in Cancer Therapy. In Burger’s Medicinal Chemistry and Drug Discovery; Wiley: New York, NY, USA, 2021; pp. 1–33. [Google Scholar]
- Li, J.; Eu, J.Q.; Kong, L.R.; Wang, L.; Lim, Y.C.; Goh, B.C.; Wong, A.L.A. Targeting Metabolism in Cancer Cells and the Tumour Microenvironment for Cancer Therapy. Molecules 2020, 25, 4831. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, Y.; Fan, Z. The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front. Oncol. 2021, 11, 698023. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.-C.; Ao, X.-J.; Yu, S.-H. Diagnostic value of GLUT-1 in distinguishing malignant mesothelioma from reactive mesothelial cells: A meta-analysis. Biomarkers 2020, 25, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, Z.; Li, B. The clinicopathologic impacts and prognostic significance of GLUT1 expression in patients with lung cancer: A meta-analysis. Gene 2018, 689, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, S.; Hong, W.; Gu, Y.; Huang, B.; Lin, Y.; Zhou, Y.; Jin, H.; Deng, Y.; Tu, L.; et al. Prognostic role of glycolysis for cancer outcome: Evidence from 86 studies. J. Cancer Res. Clin. Oncol. 2019, 145, 967–999. [Google Scholar] [CrossRef]
- Tsao, A.S.; Lindwasser, O.W.; Adjei, A.A.; Adusumilli, P.S.; Beyers, M.L.; Blumenthal, G.M.; Bueno, R.; Burt, B.M.; Carbone, M.; Dahlberg, S.E.; et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J. Thorac. Oncol. 2018, 13, 1655–1667. [Google Scholar] [CrossRef]
- Giovannetti, E.; Leon, L.G.; Gómez, V.E.; Zucali, P.A.; Minutolo, F.; Peters, G.J. A specific inhibitor of lactate dehydrogenase overcame the resistance toward gemcitabine in hypoxic mesothelioma cells, and modulated the expression of the human equilibrative transporter-1. Nucleosides Nucleotides Nucleic Acids 2016, 35, 643–651. [Google Scholar] [CrossRef]
- Granchi, C.; Roy, S.; Giacomelli, C.; Macchia, M.; Tuccinardi, T.; Martinelli, A.; Lanza, M.; Betti, L.; Giannaccini, G.; Lucacchini, A.; et al. Discovery of N-Hydroxyindole-Based Inhibitors of Human Lactate Dehydrogenase Isoform A (LDH-A) as Starvation Agents against Cancer Cells. J. Med. Chem. 2011, 54, 1599–1612. [Google Scholar] [CrossRef]
- Tuccinardi, T.; Granchi, C.; Iegre, J.; Paterni, I.; Bertini, S.; Macchia, M.; Martinelli, A.; Qian, Y.; Chen, X.; Minutolo, F. Oxime-based inhibitors of glucose transporter 1 displaying antiproliferative effects in cancer cells. Bioorganic Med. Chem. Lett. 2013, 23, 6923–6927. [Google Scholar] [CrossRef]
- Young, A.; Oldford, C.; Mailloux, R.J. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2019, 28, 101339. [Google Scholar] [CrossRef]
- Varma, G.; Seth, P.; de Souza, P.C.; Callahan, C.; Pinto, J.; Vaidya, M.; Sonzogni, O.; Sukhatme, V.; Wulf, G.M.; Grant, A.K. Visualizing the effects of lactate dehydrogenase (LDH) inhibition and LDH-A genetic ablation in breast and lung cancer with hyperpolarized pyruvate NMR. NMR Biomed. 2021, 34, e4560. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.; Molt, M.; Uribe, E.; Salas, M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. Int. J. Mol. Sci. 2019, 20, 3374. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 3828. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Taniguchi, K.; Tokumaru, Y.; Inomata, Y.; Arima, J.; Lee, S.-W.; Takabe, K.; Yoshida, K.; Uchiyama, K. Glucose transporter-1 inhibition overcomes imatinib resistance in gastrointestinal stromal tumor cells. Oncol. Rep. 2021, 47, 1–13. [Google Scholar] [CrossRef]
- Lennon, F.E.; Cianci, G.C.; Kanteti, R.; Riehm, J.J.; Arif, Q.; Poroyko, V.A.; Lupovitch, E.; Vigneswaran, W.; Husain, A.; Chen, P.; et al. Unique fractal evaluation and therapeutic implications of mitochondrial morphology in malignant mesothelioma. Sci. Rep. 2016, 6, 24578. [Google Scholar] [CrossRef]
- Du, M.; Yu, T.; Zhan, Q.; Li, H.; Zou, Y.; Geng, M.; Meng, T.; Xie, Z. Development of a novel lactate dehydrogenase A inhibitor with potent antitumor activity and immune activation. Cancer Sci. 2022, 113, 2974–2985. [Google Scholar] [CrossRef]
- Jiang, J.; Roman, J.; Xu, H.N.; Li, L.Z. An Observation on Enhanced Extracellular Acidification and Lactate Production Induced by Inhibition of Lactate Dehydrogenase, A. In Oxygen Transport to Tissue XLII; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1269, pp. 163–167. [Google Scholar] [CrossRef]
- Yang, Y.; Su, D.; Zhao, L.; Zhang, D.; Xu, J.; Wan, J.; Fan, S.; Chen, M. Different effects of LDH-A inhibition by oxamate in non-small cell lung cancer cells. Oncotarget 2014, 5, 11886–11896. [Google Scholar] [CrossRef]
- Hahne, M.; Schumann, P.; Mursell, M.; Strehl, C.; Hoff, P.; Buttgereit, F.; Gaber, T. Unraveling the role of hypoxia-inducible factor (HIF)-1α and HIF-2α in the adaption process of human microvascular endothelial cells (HMEC-1) to hypoxia: Redundant HIF-dependent regulation of macrophage migration inhibitory factor. Microvasc. Res. 2018, 116, 34–44. [Google Scholar] [CrossRef]
- Wu, H.; He, L.; Shi, J.; Hou, X.; Zhang, H.; Zhang, X.; An, Q.; Fan, F. Resveratrol inhibits VEGF-induced angiogenesis in human endothelial cells associated with suppression of aerobic glycolysis via modulation of PKM2 nuclear translocation. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1265–1273. [Google Scholar] [CrossRef]
- Minutolo, F.; Bertini, S.; Granchi, C.; Marchitiello, T.; Prota, G.; Rapposelli, S.; Tuccinardi, T.; Martinelli, A.; Gunther, J.R.; Carlson, K.E.; et al. Structural Evolutions of Salicylaldoximes as Selective Agonists for Estrogen Receptor β. J. Med. Chem. 2009, 52, 858–867. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Kawecka, A.; Braczko, A.; Franczak, M.; Slominska, E.M.; Giovannoni, R.; Smolenski, R.T. CoCl2-Mimicked Endothelial Cell Hypoxia Induces Nucleotide Depletion and Functional Impairment That Is Reversed by Nucleotide Precursors. Biomedicines 2022, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Keepers, Y.P.; Pizao, P.E.; Peters, G.J.; van Ark-Otte, J.; Winograd, B.; Pinedo, H.M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur. J. Cancer Clin. Oncol. 1991, 27, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, E.; Zucali, P.A.; Assaraf, Y.G.; Leon, L.G.; Smid, K.; Alecci, C.; Giancola, F.; Destro, A.; Gianoncelli, L.; Lorenzi, E.; et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: Molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br. J. Cancer 2011, 105, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, R.; Lachno, D.; Ledingham, S.; Yacoub, M. Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in hearts for transplantation. J. Chromatogr. B Biomed. Sci. Appl. 1990, 527, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Franczak, M.; Kutryb-Zajac, B.; El Hassouni, B.; Giovannetti, E.; Granchi, C.; Minutolo, F.; Smolenski, R.T.; Peters, G.J. The effect of lactate dehydrogenase-A inhibition on intracellular nucleotides and mitochondrial respiration in pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids 2022, 41, 1375–1385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franczak, M.A.; Krol, O.; Harasim, G.; Jedrzejewska, A.; Zaffaroni, N.; Granchi, C.; Minutolo, F.; Avan, A.; Giovannetti, E.; Smolenski, R.T.; et al. Metabolic Effects of New Glucose Transporter (GLUT-1) and Lactate Dehydrogenase-A (LDH-A) Inhibitors against Chemoresistant Malignant Mesothelioma. Int. J. Mol. Sci. 2023, 24, 7771. https://doi.org/10.3390/ijms24097771
Franczak MA, Krol O, Harasim G, Jedrzejewska A, Zaffaroni N, Granchi C, Minutolo F, Avan A, Giovannetti E, Smolenski RT, et al. Metabolic Effects of New Glucose Transporter (GLUT-1) and Lactate Dehydrogenase-A (LDH-A) Inhibitors against Chemoresistant Malignant Mesothelioma. International Journal of Molecular Sciences. 2023; 24(9):7771. https://doi.org/10.3390/ijms24097771
Chicago/Turabian StyleFranczak, Marika A., Oliwia Krol, Gabriela Harasim, Agata Jedrzejewska, Nadia Zaffaroni, Carlotta Granchi, Filippo Minutolo, Amir Avan, Elisa Giovannetti, Ryszard T. Smolenski, and et al. 2023. "Metabolic Effects of New Glucose Transporter (GLUT-1) and Lactate Dehydrogenase-A (LDH-A) Inhibitors against Chemoresistant Malignant Mesothelioma" International Journal of Molecular Sciences 24, no. 9: 7771. https://doi.org/10.3390/ijms24097771
APA StyleFranczak, M. A., Krol, O., Harasim, G., Jedrzejewska, A., Zaffaroni, N., Granchi, C., Minutolo, F., Avan, A., Giovannetti, E., Smolenski, R. T., & Peters, G. J. (2023). Metabolic Effects of New Glucose Transporter (GLUT-1) and Lactate Dehydrogenase-A (LDH-A) Inhibitors against Chemoresistant Malignant Mesothelioma. International Journal of Molecular Sciences, 24(9), 7771. https://doi.org/10.3390/ijms24097771







