Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon
Abstract
1. Introduction
2. Results
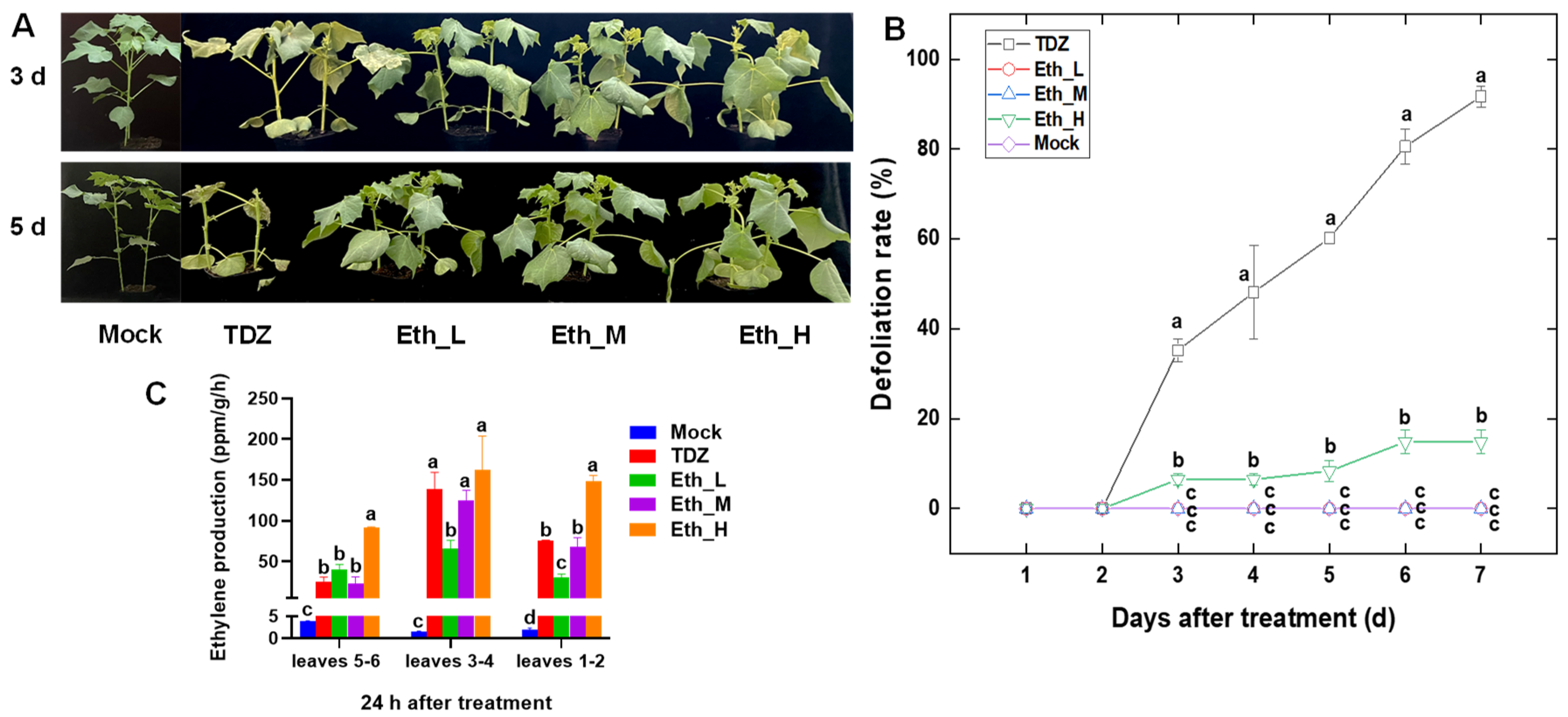
2.1. Effect of TDZ and Ethephon (Eth) on Defoliation and Ethylene Production
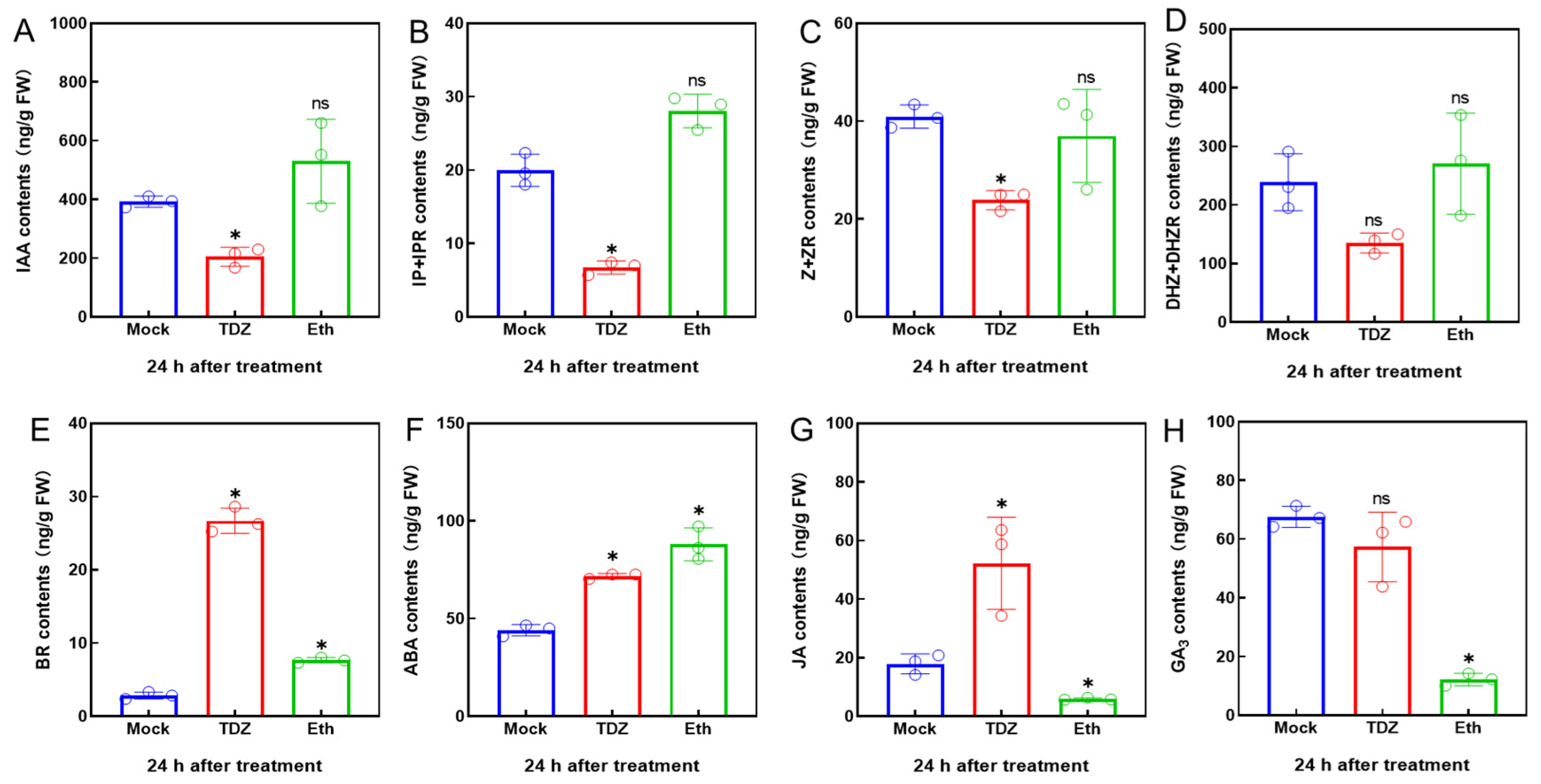
2.2. Effect of TDZ and Eth on Phytohormones Content in Leaves
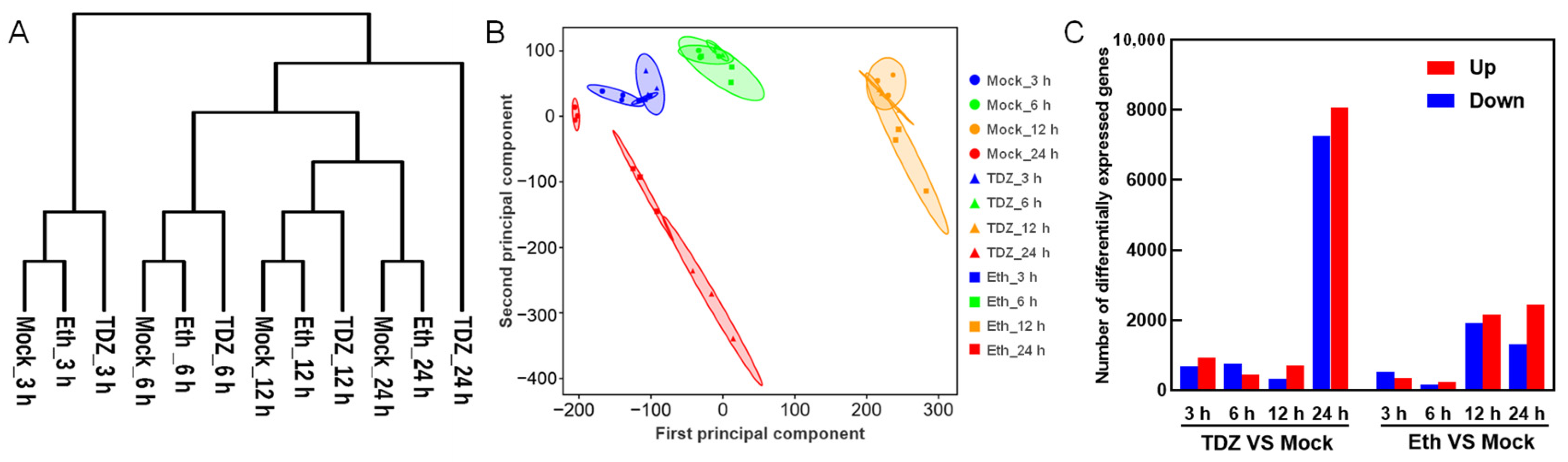
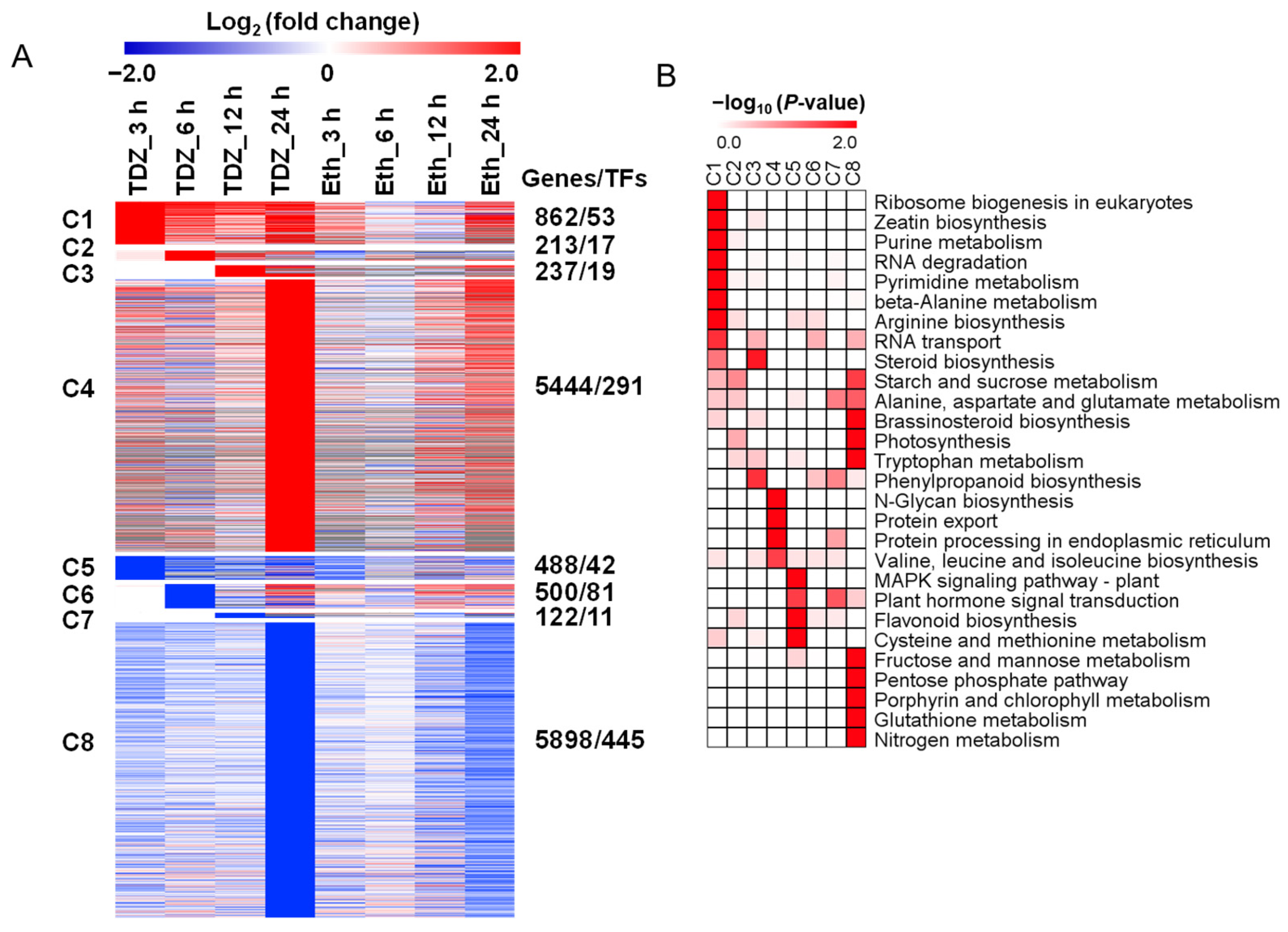
2.3. Dynamic Transcriptome Profiles in Leaves during Chemical Defoliation
2.4. Temporal Expression Pattern of TDZ-SRGs in Leaves
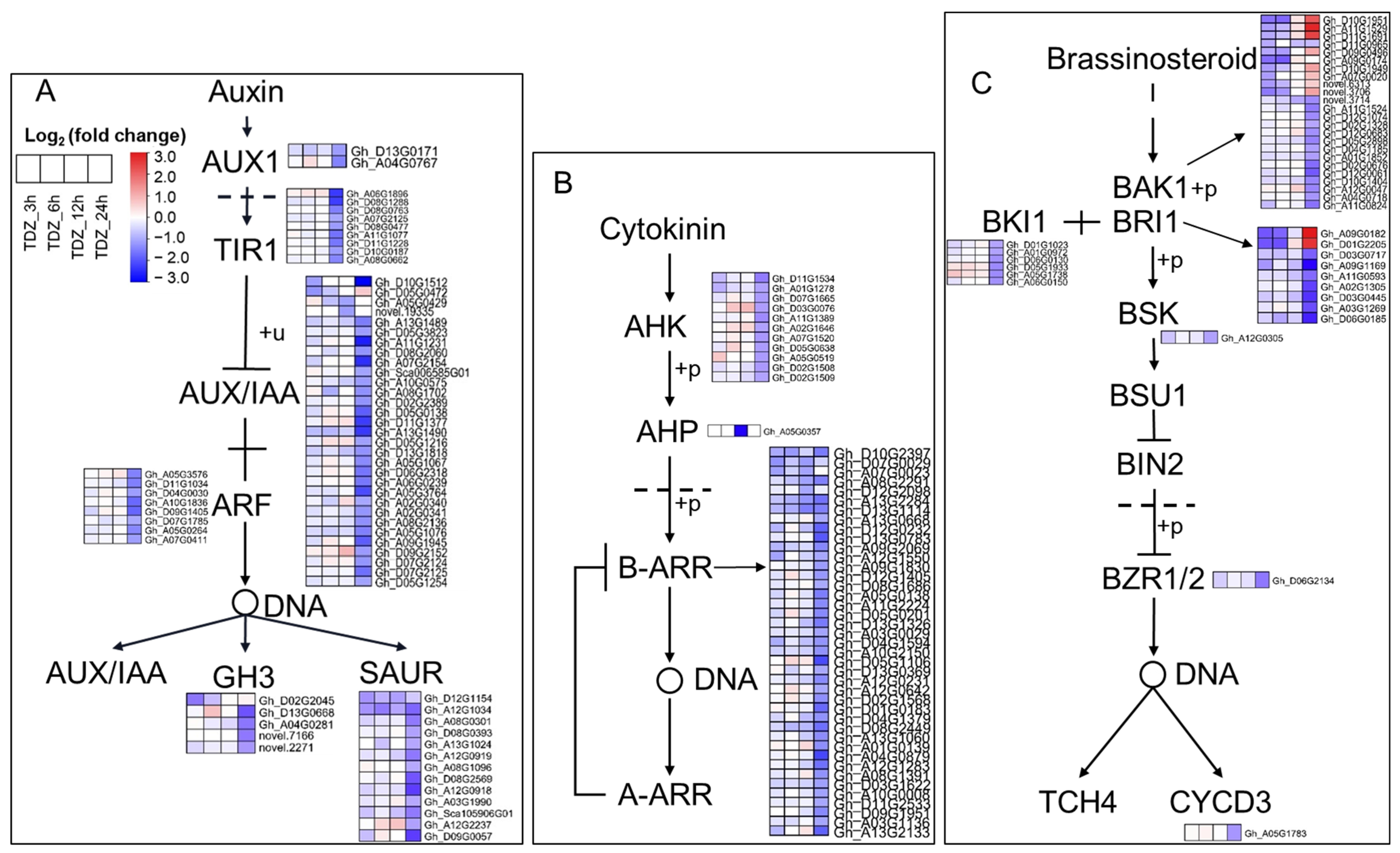
2.5. TDZ-SRGs in Cotton Leaves Involved in Phytohormone Signal Transduction and Hormone Synthesis Pathways
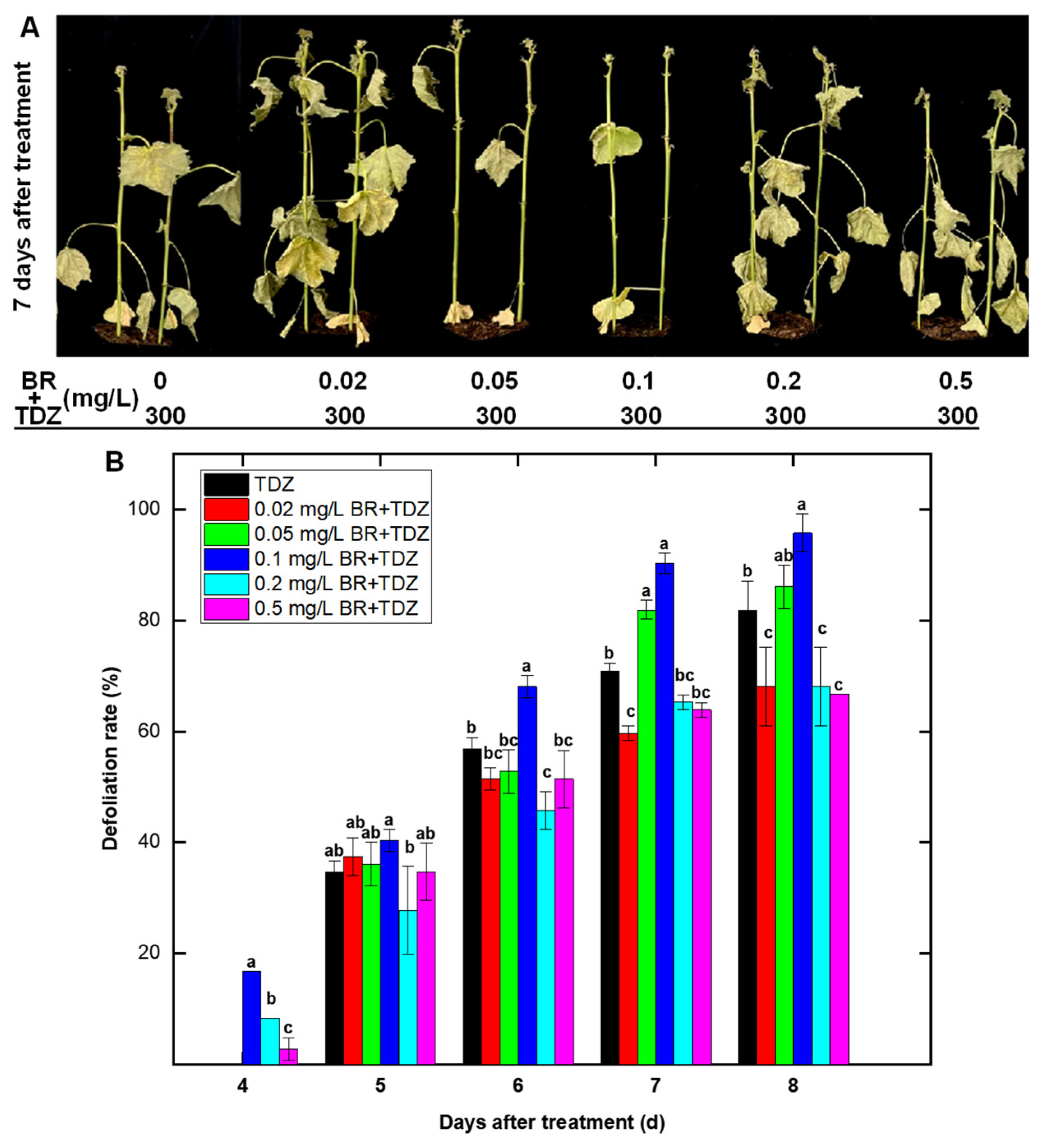
2.6. Effect of Exogenous BR Application on TDZ-Induced Leaf Abscission
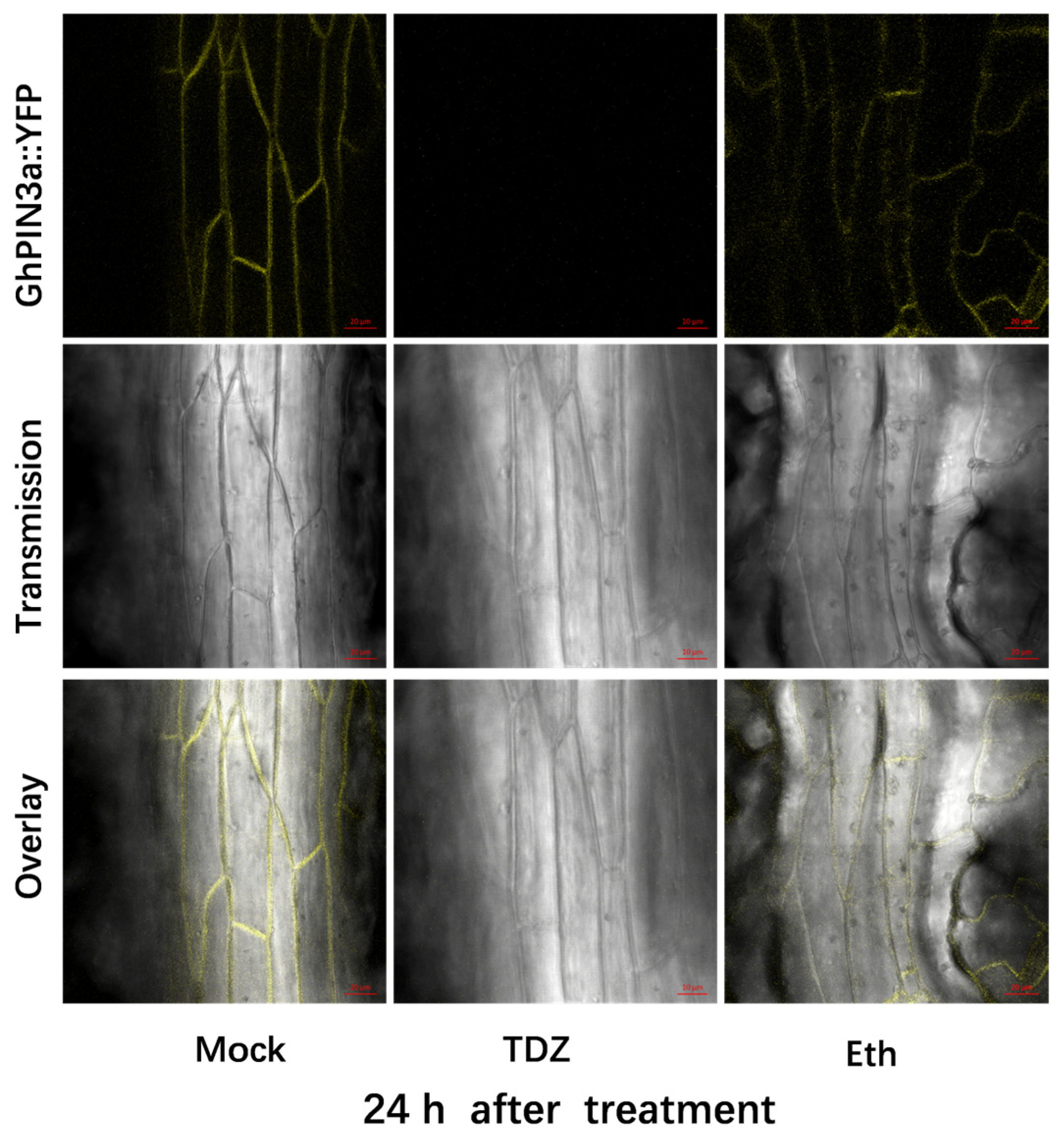
2.7. TDZ-SRGs in Leaves Associated with Auxin Transport
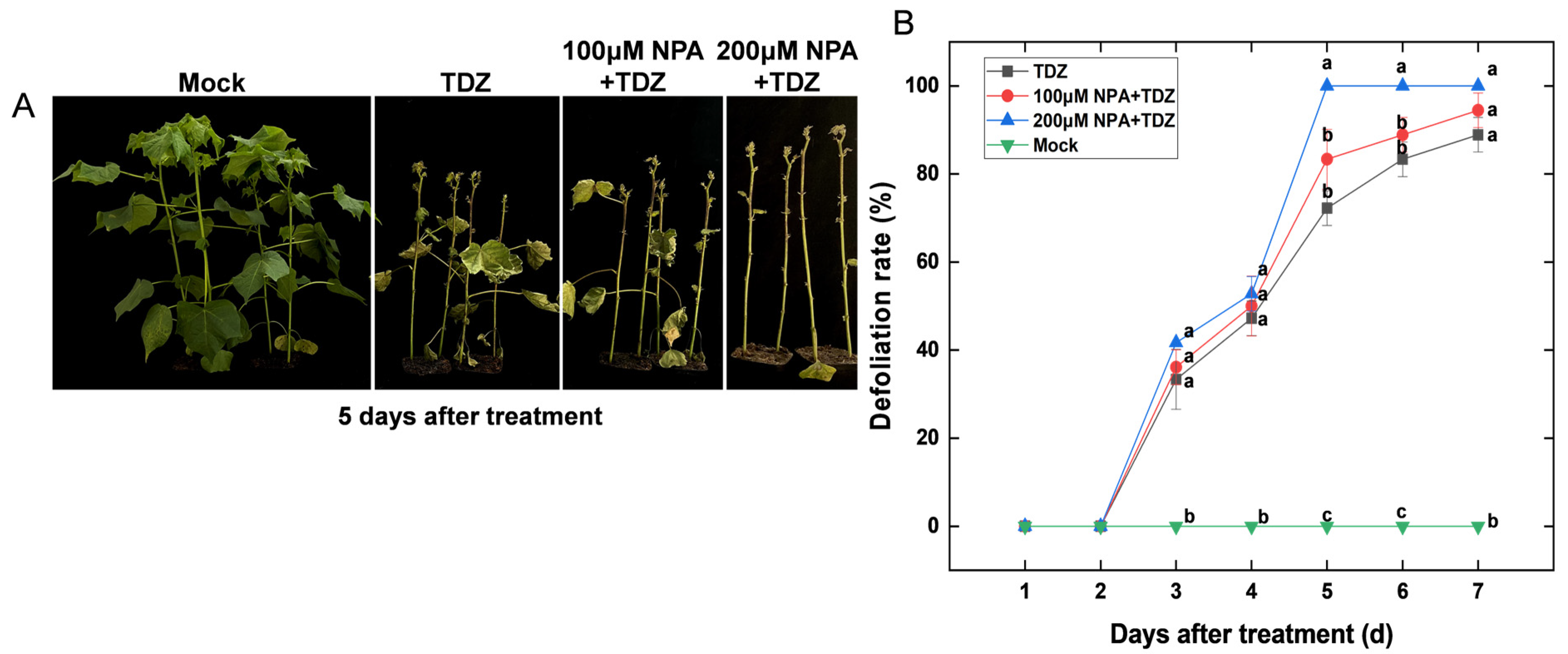
2.8. Effect of Auxin Transport on Chemical Defoliation Induced by TDZ
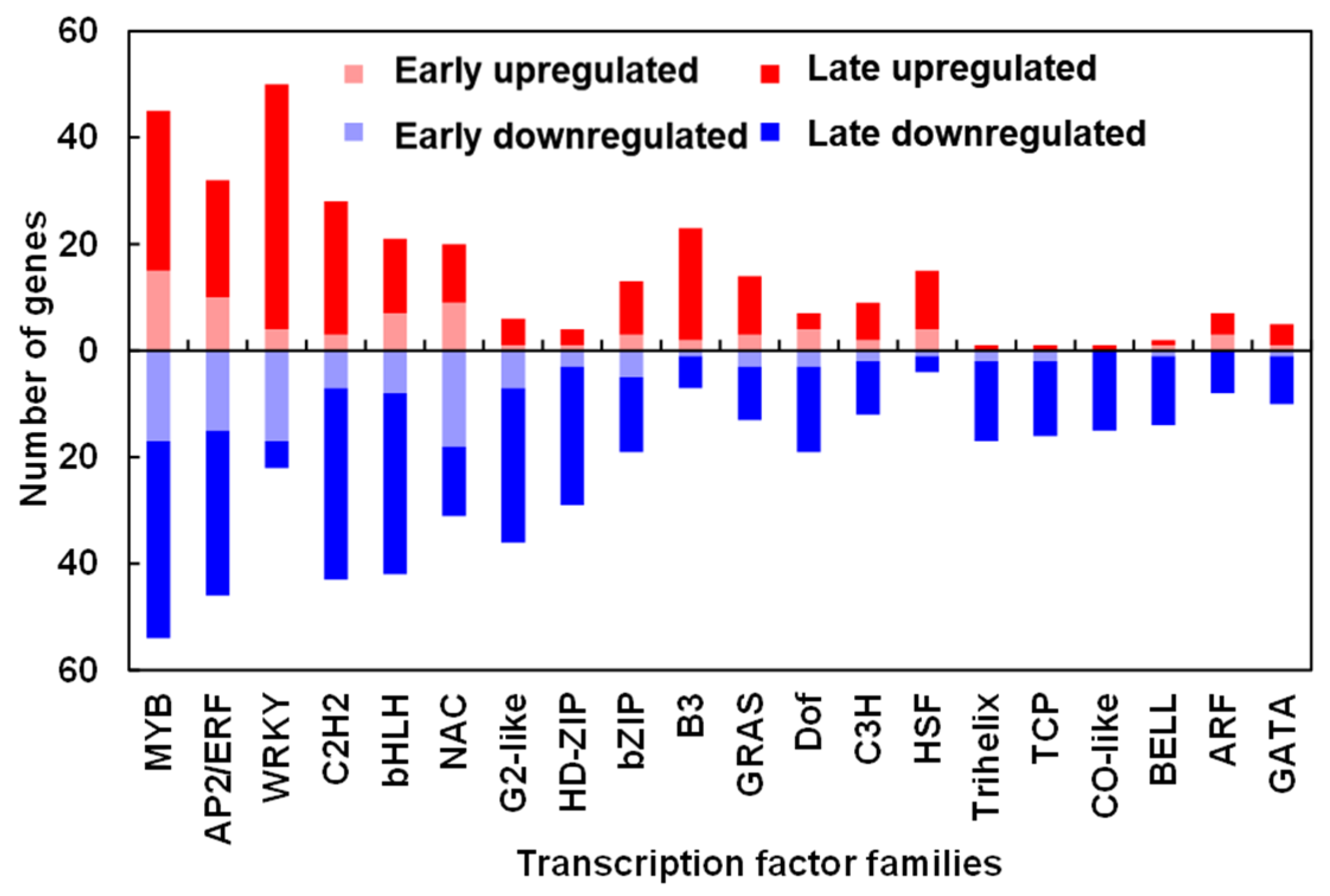
2.9. TDZ-SRGs Encoding for Transcription Factors (TFs) in Cotton Leaves
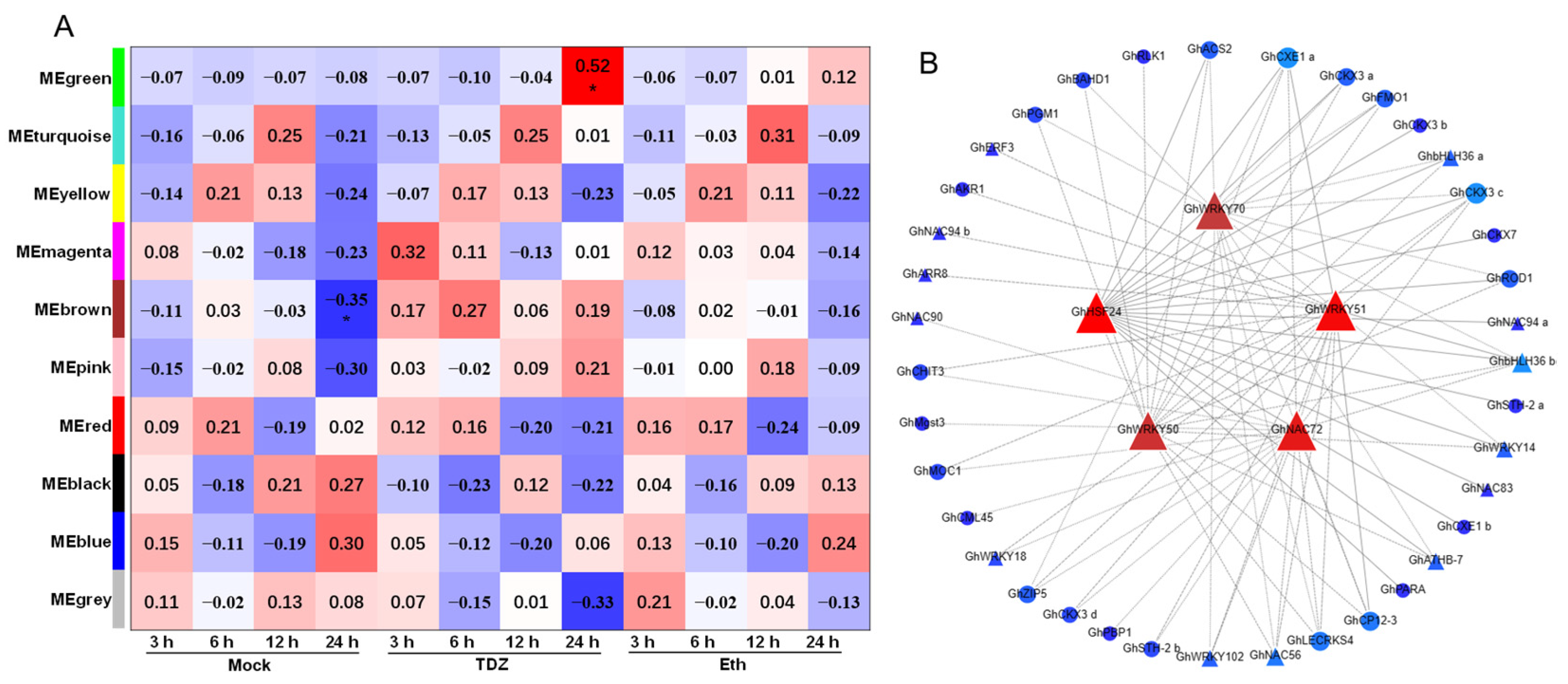
2.10. Co-Expression Network Analysis Associated with TDZ Treatment
3. Discussion
3.1. The Release of Ethylene from Leaves Was Not a Sufficient Condition for Chemical Defoliation
3.2. The Role of Auxin and Cytokinins in TDZ-Induced Cotton Leaf Abscission
3.3. A New Discovery: Brassinosteroid Was Also Involved in TDZ-Induced Chemical Defoliation of Cotton
3.4. Transcription Factors (TFs) in Cotton Leaves Involved in TDZ-Induced Abscission
3.5. Cotton Leaves Undergo Significant Metabolic Changes within 24 h of TDZ Treatment
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Defoliation Treatment
4.3. Measurement of Ethylene Production and Phytohormone Content
4.4. RNA Extraction, cDNA Library Preparation and Sequencing
4.5. Analysis of Differentially Expression Genes
4.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
4.7. Gene Co-Expression Network Analysis
4.8. Microscopic Observations
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Sexton, R.; Roberts, J.A. Cell biology of abscission. Annu. Rev. Plant Physiol. 1982, 33, 133–162. [Google Scholar] [CrossRef]
- Sawicki, M.; Barka, E.A.; Clement, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719. [Google Scholar] [CrossRef]
- Estornell, L.H.; Agusti, J.; Merelo, P.; Talon, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Abeles, F.B.; Rubinstein, B. Regulation of ethylene evolution and leaf abscission by auxin. Plant Physiol. 1964, 39, 963. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Philosoph-Hadas, S.; Sundaresan, S.; Selvaraj, K.; Burd, S.; Ophir, R.; Kochanek, B.; Reid, M.S.; Jiang, C.Z.; Lers, A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol. 2010, 154, 1929–1956. [Google Scholar] [CrossRef]
- Kucko, A.; Wilmowicz, E.; Pokora, W.; Alche, J.D. Disruption of the auxin gradient in the abscission zone area evokes asymmetrical changes leading to flower separation in yellow lupine. Int. J. Mol. Sci. 2020, 21, 3815. [Google Scholar] [CrossRef] [PubMed]
- Louie, D.S.; Addicott, F.T. Applied auxin gradients and abscission in explants. Plant Physiol. 1970, 45, 654–657. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zimmermann, J.; Polle, A.; Fischer, U. Auxin is a long-range signal that acts independently of ethylene signaling on leaf abscission in Populus. Front. Plant Sci. 2015, 6, 634. [Google Scholar] [CrossRef]
- Basu, M.M.; Gonzalez-Carranza, Z.H.; Azam-Ali, S.; Tang, S.; Shahid, A.A.; Roberts, J.A. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant Physiol. 2013, 162, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Jiang, Y.; Han, X.; Liu, X.; Cao, R.; Qi, M.; Xu, T.; Li, T. SlPIN1 regulates auxin efflux to affect flower abscission process. Sci. Rep. 2017, 7, 14919. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wu, J.Y.; Lu, W.J.; Li, J.G. A polygalacturonase gene clustered into clade E involved in lychee fruitlet abscission. Sci. Hortic. 2013, 150, 244–250. [Google Scholar] [CrossRef]
- Meir, S.; Philosoph-Hadas, S.; Riov, J.; Tucker, M.L.; Patterson, S.E.; Roberts, J.A. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J. Exp. Bot. 2019, 70, 1461–1467. [Google Scholar] [CrossRef]
- Mishra, A.; Khare, S.; Trivedi, P.K.; Nath, P. Ethylene induced cotton leaf abscission is associated with higher expression of cellulase (GhCel1) and increased activities of ethylene biosynthesis enzymes in abscission zone. Plant Physiol. Biochem. 2008, 46, 54–63. [Google Scholar] [CrossRef]
- Zahra, T.A. Effect of different ethephon concentrations on olive fruits harvesting at different orchard locations. Palest. Tech. Univ. Res. J. 2014, 2, 9–13. [Google Scholar] [CrossRef]
- Hagemann, M.H.; Winterhagen, P.; Hegele, M.; Wunsche, J.N. Ethephon induced abscission in mango: Physiological fruitlet responses. Front. Plant Sci. 2015, 6, 706. [Google Scholar] [CrossRef]
- Cin, V.D.; Danesin, M.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck). J. Exp. Bot. 2005, 56, 2995–3005. [Google Scholar] [CrossRef]
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission. Plant Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Patterson, S.E. Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 1997, 9, 1169–1179. [Google Scholar] [CrossRef]
- Patterson, S.E.; Bleecker, A.B. Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol. 2004, 134, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.T.; Lada, R.; Martynenko, A.; Dorais, M.; Pepin, S.; Desjardins, Y. Ethylene triggers needle abscission in root-detached balsam fir. Trees 2010, 24, 879–886. [Google Scholar] [CrossRef]
- Najeeb, U.; Sarwar, M.; Atwell, B.J.; Bange, M.P.; Tan, D. Endogenous ethylene concentration is not a major determinant of fruit abscission in heat-stressed cotton (Gossypium hirsutum L.). Front. Plant Sci. 2017, 8, 1615. [Google Scholar] [CrossRef] [PubMed]
- Eder, Z.P.; Singh, S.; Fromme, D.D.; Mott, D.A.; Ibrahim, A.; Morgan, G.D. Cotton harvest aid regimes and their interaction with cotton cultivar characteristics impacting leaf grade. Agron. J. 2017, 109, 2714–2722. [Google Scholar] [CrossRef]
- Suttle, J.C. Involvement of ethylene in the action of the cotton defoliant thidiazuron. Plant Physiol. 1985, 78, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Suttle, J.C. Disruption of the polar auxin transport-system in cotton seedlings following treatment with the defoliant thidiazuron. Plant Physiol. 1988, 86, 241–245. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Sun, H.; Wusiman, N.; Sun, W.N.; Li, B.Q.; Gao, Y.; Kong, J.; Zhang, D.W.; Zhang, X.L.; et al. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J. Exp. Bot. 2019, 70, 1525–1538. [Google Scholar] [CrossRef]
- Li, F.J.; Wu, Q.; Liao, B.P.; Yu, K.K.; Huo, Y.N.; Meng, L.; Wang, S.; Wang, B.M.; Du, M.W.; Tian, X.L.; et al. Thidiazuron promotes leaf abscission by regulating the crosstalk complexities between ethylene, auxin, and cytokinin in cotton. Int. J. Mol. Sci. 2022, 23, 2696. [Google Scholar] [CrossRef]
- Shu, H.M.; Sun, S.W.; Wang, X.J.; Yang, C.Q.; Zhang, G.W.; Meng, Y.L.; Wang, Y.H.; Hu, W.; Liu, R.X. Low temperature inhibits the defoliation efficiency of thidiazuron in cotton by regulating plant hormone synthesis and the signaling pathway. Int. J. Mol. Sci. 2022, 23, 14208. [Google Scholar] [CrossRef]
- Stewart, A.M.; Edmisten, K.L.; Wells, R. Boll openers in cotton: Effectiveness and environmental influences. Field Crops Res. 2000, 67, 83–90. [Google Scholar] [CrossRef]
- Du, M.W.; Ren, X.M.; Tian, X.L.; Duan, L.S.; Zhang, M.C.; Tan, W.M.; Li, Z.H. Evaluation of harvest aid chemicals for the cotton-winter wheat double cropping system. J. Integr. Agric. 2013, 12, 273–282. [Google Scholar] [CrossRef]
- Morgan, P.W.; Jordan, W.R.; Davenport, T.L.; Durham, J.I. Abscission responses to moisture stress, auxin transport inhibitors, and ethephon. Plant Physiol. 1977, 59, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.R.; Liu, C.; Li, X.D.; Xu, H.Q.; Liang, Y.; Ma, N.; Fei, Z.J.; Gao, J.P.; Jiang, C.Z.; Ma, C. Transcriptome profiling of petal abscission zone and functional analysis of an Aux/IAA family gene RhIAA16 involved in petal shedding in rose. Front. Plant Sci. 2016, 7, 01375. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.D.; Qiu, Z.L.; Wen, Z.; Zhang, H.M.; Li, Z.C.; Hong, Y.; Qiao, G.; Wen, X.P. Genome- wide identification of ARF gene family suggests a functional expression pattern during fruitlet abscission in Prunus avium L. Int. J. Mol. Sci. 2021, 22, 11968. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Sundaresan, S.; Riov, J.; Agarwal, I.; Philosoph-Hadas, S. Role of auxin depletion in abscission control. Stewart Posthar. Rev. 2015, 11, 1–5. [Google Scholar]
- Kono, A.; Yin, Y.H. Updates on BES1/BZR1 regulatory networks coordinating plant growth and stress responses. Front. Plant Sci. 2020, 11, 617162. [Google Scholar] [CrossRef]
- He, Y.; Tang, W.; Swain, J.D.; Green, A.L.; Jack, T.P.; Gan, S. Networking senescence-regulating pathways by using Arabidopsis enhancer trap lines. Plant Physiol. 2001, 126, 707–716. [Google Scholar] [CrossRef]
- Han, X.; Kumar, D.; Chen, H.; Wu, S.; Kim, J.Y. Transcription factor-mediated cell-to-cell signalling in plants. J. Exp. Bot. 2014, 65, 1737–1749. [Google Scholar] [CrossRef]
- Liljegren, S.J. Organ abscission: Exit strategies require signals and moving traffic. Curr. Opin. Plant Biol. 2012, 15, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, J.; Yao, L.; Ren, G.; Zhu, X.; Gao, S.; Qiu, K.; Zhou, X.; Kuai, B. The role of ANAC072 in the regulation of chlorophyll degradation during age- and dark-induced leaf senescence. Plant Cell Rep. 2016, 35, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Pang, C.Y.; Hussain, A.; Fan, S.L.; Song, M.Z.; Zamir, R.; Yu, S.X. Molecular cloning and functional analysis of NAC family genes associated with leaf senescence and stresses in Gossypium hirsutum L. Plant Cell Tissue Organ Cult. 2014, 117, 167–186. [Google Scholar] [CrossRef]
- Xiao, S.; Hu, Q.; Zhang, X.; Si, H.; Liu, S.; Chen, L.; Chen, K. Orchestration of plant development and defense by indirect crosstalk of salicylic acid and brassinosteorid signaling via transcription factor GhTINY2. J. Exp. Bot. 2021, 72, 4721–4743. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Wang, X.; Xu, Y.; Gui, H.; Zhang, H.; Dong, Q.; Sikder, R.K.; Yang, G.; Song, M. Chemical defoliant promotes leaf abscission by altering ROS metabolism and photosynthetic efficiency in Gossypium hirsutum. Int. J. Mol. Sci. 2020, 21, 2738. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Y.; Zhang, M.; Hou, L.; Bai, W.Q.; Yan, X.Y.; Hou, N.; Wang, H.X.; Huang, J.; Zhao, J.; Pei, Y. Cytokinin inhibits cotton fiber initiation by disrupting PIN3a-mediated asymmetric accumulation of auxin in the ovule epidermis. J. Exp. Bot. 2019, 70, 3139–3151. [Google Scholar] [CrossRef]
- Xue, J.Q.; Li, Y.H.; Tan, H.; Yang, F.; Ma, N.; Gao, J.P. Expression of ethylene biosynthetic and receptor genes in rose floral tissues during ethylene-enhanced flower opening. J. Exp. Bot. 2008, 59, 2161–2169. [Google Scholar] [CrossRef]
- Siren, J.; Valimaki, N.; Makinen, V. Indexing Graphs for Path Queries with Applications in Genome Research. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014, 11, 375–388. [Google Scholar]
- Zhang, T.Z.; Hu, Y.; Jiang, W.K.; Fang, L.; Guan, X.Y.; Chen, J.D.; Zhang, J.B.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 252–531. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, B.; Li, F.; Yi, F.; Du, M.; Tian, X.; Li, Z. Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon. Int. J. Mol. Sci. 2023, 24, 7590. https://doi.org/10.3390/ijms24087590
Liao B, Li F, Yi F, Du M, Tian X, Li Z. Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon. International Journal of Molecular Sciences. 2023; 24(8):7590. https://doi.org/10.3390/ijms24087590
Chicago/Turabian StyleLiao, Baopeng, Fangjun Li, Fei Yi, Mingwei Du, Xiaoli Tian, and Zhaohu Li. 2023. "Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon" International Journal of Molecular Sciences 24, no. 8: 7590. https://doi.org/10.3390/ijms24087590
APA StyleLiao, B., Li, F., Yi, F., Du, M., Tian, X., & Li, Z. (2023). Comparative Physiological and Transcriptomic Mechanisms of Defoliation in Cotton in Response to Thidiazuron versus Ethephon. International Journal of Molecular Sciences, 24(8), 7590. https://doi.org/10.3390/ijms24087590





