Abstract
Our previous study identified that the RepA protein encoded by the oat dwarf virus (ODV) was responsible for inducing a strong hypersensitive response (HR) during the virus infection in non-host tobacco plants. However, little was known about the molecular mechanism of the RepA-elicited HR. Here, a RING-finger protein, which is described as NbRFP1 and is mainly located in the cytoplasm and nucleus in Nicotiana benthamiana cells, was confirmed to interact with RepA. In addition, the accumulation level of NbRFP1 in N. benthamiana leaves was enhanced by either ODV infection or by only RepA expression. The knockdown of NbRFP1 by a TRV-mediated virus-induced gene silencing markedly delayed the ODV or RepA-elicited HR. By contrast, the overexpression of NbRFP1 in N. benthamiana conferred enhanced resistance to ODV infection and promoted RepA-induced HR. Further mutation analysis showed that a RING-finger domain located in NbRFP1 plays important roles in modulating RepA-induced HR, as well as in mediating the interaction between NbRFP1 and RepA.
1. Introduction
Plants often experience various biotic and abiotic stresses in natural ecosystems. In order to survive, plants have gradually formed multilayer defense mechanisms to adapt to various external stimuli [1]. PAMP-activated immunity (PTI), which is triggered by pathogen-associated molecular patterns (PAMP), and effector-activated immunity (ETI) are mainly represented in the plant defense portal [2]. The hypersensitive response (HR) is a typical manifestation of the ETI disease resistance, which is common in plant resistance responses to various pathogens, such as fungi, bacteria, viruses, and nematodes [3,4]. HR is usually associated with rapid cell death in the vicinity of pathogen primary infection, resulting in restricting pathogen spread [5]. The typical characteristics of HR include a burst of reactive oxygen species (ROS), the reprogramming of defense-related genes, the deposition of callose, and the activation of hormone signaling and the MAPK cascade [6,7,8,9,10].
Recent studies have shown that the ubiquitination-mediated 26S proteasome degradation system (UPS) plays an important role in the plant PTI-ETI defense system [11,12,13]. Protein ubiquitination is a common form of post-translational modification, which contains a series of cascade reactions catalyzed by the ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3) [14]. Currently, the identified E3 ubiquitin ligases mainly include two types: single-subunit E3 and multi-subunit E3 [15]. The single-subunit E3 is further divided into three types: HECT type, U-box type, and RING type. Meanwhile, the multi-subunit E3 mainly includes the SCF type, APC type, and VBC type [16].
The RING-type E3 ubiquitin ligase has a typical RING finger domain, which is necessary to maintain E3 ubiquitin ligase activity [17,18]. The RING domain contains 40–60 amino acids that bind the E2 in order to induce a closed E2-Ub conformation and to bind together the substrate [19]. Although many RING-type E3 proteins are found in plants, only some of them are reported to be involved in response to biotic stresses [20,21]. Emerging evidence has revealed that the pepper E3 ubiquitin ligase CaRING1 is induced by Xanthomonas campestris pv vesicatoria (XCV); furthermore, it serves in positive roles for HR production and disease resistance against XCV infection [22]. Similarly, rice OsBBI1, identified as RING E3 ligase, is induced by Magnaporthe oryzae (M. oryzae) and confers a broad resistance against M. oryzae [23]. In contrast, the type-III-secreted effector RipAC, which is obtained from Ralstonia solanacearum, was reported to interact with E3 ubiquitin ligase PUB4 in order to regulate kinase BIK1 homeostasis for the suppression of plant PTI immunity [24]. Furthermore, the M. oryzae avirulence effector AvrPiz-t was confirmed to target the RING E3 ubiquitin ligase APIP6 to promote the degradation of both proteins and to suppress PAMP-triggered immune responses in rice [25].
The oat dwarf virus (ODV) belongs to the genus Mastrevirus in the Geminiviridae family, which possess a circular single-stranded DNA genome with a full length of 2740 base pairs (bp) [26,27]. ODV encodes four proteins: a movement protein (MP or V1) and a coat protein (CP or V2) on the viral-sense strand, and two replication-associated proteins (RepA and Rep) on the complementary-sense strand [27]. Our previous reports revealed that the infection of ODV in non-host tobacco plants elicited a HR-like response. Subsequently, the ODV RepA protein was demonstrated to be responsible for HR-type cell death [9]. Further gene expression profiling that was produced by RNA-Seq confirmed 7878 significantly differentially expressed genes (DEGs) responses to the transient expression of ODV RepA, suggesting a complex and dynamic regulatory network involved in modulating RepA-induced HR [28].
Although ODV RepA had been manifested to trigger HR-like plant immunity, up to date, little was known about the host factor(s) that are involved in modulating RepA-induced HR. In this study, we identified that the RING-finger protein NbRFP1 is responsible for ODV infection and that it interacts with ODV RepA. An analysis of the knockdown and overexpression demonstrated that NbRFP1 positively regulates ODV RepA-induced HR. Additionally, the RING domain located in NbRFP1 serves important roles in regulating RepA-induced HR and the interaction with RepA. Our findings will provide new insights for a better understanding of the molecular mechanism of ODV RepA-induced HR.
2. Results
2.1. The Isolation and Sequence Analysis of NbRFP1
RING-typed E3 ligases are widely reported to be involved in plant defense responses. Previously, a tobacco RING-finger protein that was designated as NtRFP1 was demonstrated to be a functional E3 ubiquitin ligase and was found to mediate the degradation of geminivirus-encoded βC1 [17]. Here, a predicted RING-finger protein 1 (NbS00031234g0005.1) was cloned from the N. benthamiana cDNA library, where it is named NbRFP1. The NbRFP1 coding region is 1464 bp in length and encodes a 487-amino-acid (aa) protein with an estimated molecular mass of 53.84 kDa (Figure 1a). A conserved domain search through the online InterPro (https://www.ebi.ac.uk/interpro/, accessed on 18 February 2022) revealed that the NbRFP1 protein contains a von Willebrand factor type A (vWFA) domain (which is located between 109–312 aa) and a RING-finger domain (spanning the region between 444–477 aa) (Figure 1b). A Blastx search, which was conducted using the complete coding sequence of NbRFP1 in NCBI, and further sequence alignments revealed that NbRFP1 shared the highest amino acid sequence similarity (98.1%) with Nicotiana tabacum NtRFP1 (GenBank accession: AGL95792) and the N. tabacum E3 ubiquitin-protein ligase RGLG2-like (NtRGLG2L) (GenBank accession: NP_001312603). In addition, phylogenetic analysis showed that NbRFP1 is more closely related to NtRFP1, NtRGLG2L, N. tomentosiformis E3 ubiquitin-protein ligase RGLG2 (NtRGLG2) (GenBank accession: XP_009599492), and N. attenuata E3 ubiquitin-protein ligase RGLG2-like (NaRGLG2L) (GenBank accession: XP_019223978) from Nicotiana plants (Figure 1c). These results suggest that NbRFP1 is a RING-finger protein that is closer to the NtRFP1 and NtRGLG2L from N. tabacum.
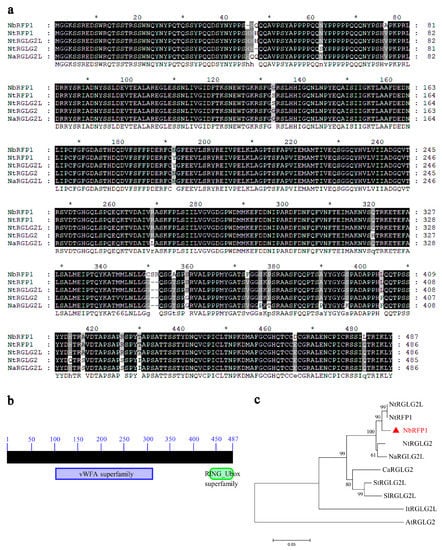
Figure 1.
Bioinformatics analysis of NbRFP1. (a) The amino acid sequence alignment was carried out using MEGA 11 software and visualized using GeneDoc v2.7 software. (b) The conserved domain of NbRFP1 was predicted through the online InterPro. (c) The phylogenetic analysis based on the amino acid sequences derived from NbRFP1 and its homologs was performed using MEGA 11 software.
2.2. Expression Pattern and Subcellular Localization of NbRFP1
We evaluated the expression levels of NbRFP1 in the leaves of N. benthamiana in response to ODV infection or the transient expression of ODV RepA. As shown in Figure 2a,b, when compared with the empty vector control, the expression of NbRFP1 was significantly up-regulated by more than 5.0-fold and 4.0-fold following ODV infection and the transient expression of ODV RepA, respectively. These results suggest that both ODV and ODV RepA can positively regulate the transcript levels of NbRFP1 in N. benthamiana.
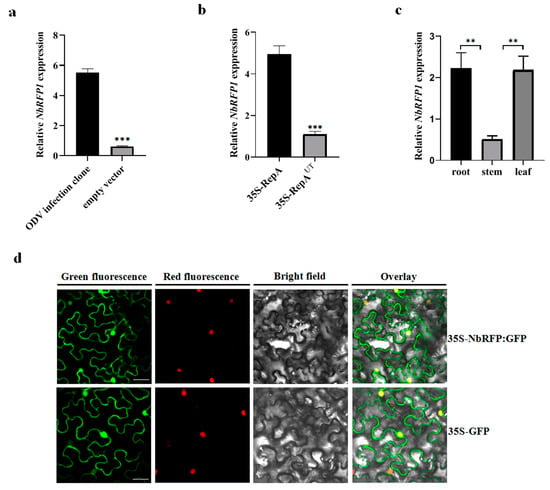
Figure 2.
The expression pattern and subcellular localization of NbRFP1. (a,b) Reverse transcription-quantitative PCR (RT-qPCR) analysis of the expression levels of NbRFP1 in the leaves of N. benthamiana following the ODV infection or transient expression of ODV RepA. 35S-RepAUT served as an untranslatable mutant control. The data are given as the means ± the standard deviation (SD) of the three biological replicates. Asterisk indicated significant difference between treatments (** p ≤ 0.01; *** p ≤ 0.001). (c) The expression pattern of NbRFP1 in the different tissues of N. benthamiana during the 5–6 leaf stage. Histone H3 was used as an internal reference. The data are given as the means ± the SD of the three biological replicates. (d) The sublocalization of the NbRFP1 in RFP-H2B transgenic N. benthamiana leaf epidermis. The GFP fluorescence was observed via confocal microscopy at 48 h post-infiltration (hpi). Histone 2B-RFP was used as a marker for the nucleus. Bar scale = 20 μm.
To further investigate the biological functions of NbRFP1, we determined its tissue expression in N. benthamiana. As shown in Figure 2c, NbRFP1 was ubiquitous in the examined roots, stems and leaves, displaying a similar expression pattern to that of the NtRFP1 in N. tabacum [17]. Further analysis of RT-qPCR revealed the highest levels of NbRFP1 transcripts were detected in roots and leaves at the 5–6 leave stage.
Subsequently, we examined the subcellular localization of NbRFP1 in leaf epidermis through the transient expression of the NbRFP1-GFP fusion protein in transgenic N. benthamiana overexpressing an RFP protein fused to a histone 2B (RFP-H2B) nuclear marker. The NbRFP1-GFP fluorescence was uniformly distributed in both the cytoplasm and the nucleus in the epidermal cells of N. benthamiana (Figure 2d), which was similar to that of the N. benthamiana that was infiltrated with 35S-GFP alone (Figure 2d). In order to further evaluate whether NbRFP1 is located in cytoplasm, PIP2A-mCherry, a marker for the plasma membrane, was co-agroinoculated with 35S-NbRFP1:GFP. The merged image revealed NbRFP1 was not closely associated with the plasma membrane (Figure S1). Taken together, these observations suggest that NbRFP1 is primarily localized in the cytoplasm and nucleus.
2.3. Silencing of NbRFP1 Delays ODV RepA-Induced HR
Our previous study has shown that ODV RepA is responsible for eliciting HR-type cell death during ODV infection [9]. To investigate the role of NbRFP1 in the induction of HR caused by ODV infection or ODV RepA, we used the tobacco rattle virus (TRV)-based virus-induced gene silencing system to silence the endogenous NbRFP1 in N. benthamiana seedlings (Figure S2). At 10 days post inoculation (dpi) with TRV-derived constructs (Figure 3a), systemic leaves were sampled and subjected to RT-qPCR analysis. Compared with the TRV1 and TRV2:GFP-infiltrated control plants, the transcript level of NbRFP1 in plants co-agroinfiltrated with TRV1 and TRV2:NbRFP1 decreased by approximately 80% (Figure 3b). Afterward, the control and silenced plants were infiltrated with agrobacterium cultures containing 35S-RepA or the infectious clone of ODV, respectively. They were then monitored for symptom development over time. In NbRFP1-silenced N. benthamiana plants, 50% of the leaves inoculated with ODV infectious clone presented HR-like symptoms at 7.5 dpi, while all inoculated leaves showed cell death at 9.5 dpi. In contrast, in the TRV1 and TRV2:GFP-infiltrated control plants, approximately 50% of the leaves that were inoculated with ODV infectious clones showed cell death at 6.5 dpi and all inoculated leaves presented HR-like symptoms at 8.5 dpi (Figure 3c). Similarly, HR-like cell death caused by 35S-RepA in NbRFP1-silenced plants was severely delayed compared to the control plants (Figure 3d). These results suggest that the silencing of NbRFP1 negatively regulates ODV RepA-elicited HR.
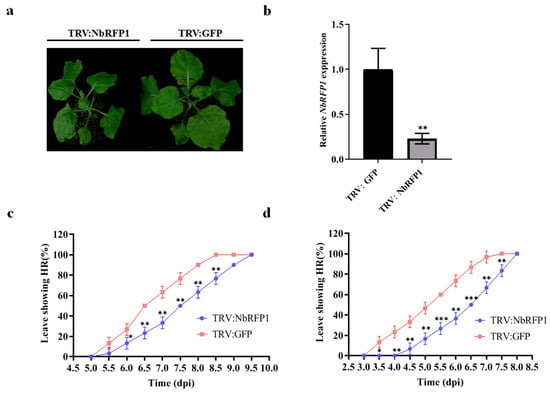
Figure 3.
The effects of silencing NbRFP1 on ODV or RepA-induced HR. (a,b) Phenotype in NbRFP1-silenced N. benthamiana plants (a) and the silencing efficiency (b) of NbRFP1 through the TRV-mediated VIGS system. The photograph was taken and the silencing efficiency was examined at 10 dpi, and N. benthamiana glyceraldehyde-3-phosphate dehydrogenase (NbGADPH) was used as an internal reference gene for RT-qPCR analysis. The data represent the means ± the SD of the three biological replicates. (c,d) The percentage of exhibiting HR symptoms in the control (TRV:GFP) and NbRFP1-silenced N. benthamiana plants (TRV:NbRFP1) that were agroinfiltrated with ODV infectious clones (c) or with 35S-RepA (d) over time. The data represent the means ± the SD of the three biological replicates. Statistical analysis was performed using two-way ANOVA followed by Student’s t test, and significant difference between treatments was indicated by asterisk (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
2.4. The Overexpression of NbRFP1 Enhances ODV RepA-Induced HR
Since the down-regulated expression of NbRFP1 in N. benthamiana plants by VIGS-mediated gene silencing could postpone HR induced by ODV or ODV RepA, we next determined whether the overexpression of NbRFP1 produced opposite effects on the production of RepA-elicited HR. As shown in Figure 4a,b, cell death elicited by ODV or RepA was produced faster while overexpressing NbRFP1. Further time-course observation identified 50% of the N. benthamiana leaves co-agroinfiltrated with 35S-NbRFP1 and ODV infectious clones demonstrated HR-like necrosis at approximately 6 dpi, while all infiltrated leaves showed cell death closely at 8 dpi (Figure 4c). Meanwhile, the control plants co-agroinfiltrated with empty vectors and ODV infectious clones showed HR-like symptoms with approximately a one-day delay, indicating that the transient overexpression of NbRFP1 could promote the production of HR that are caused by ODV infectious clones (Figure 4c). Similarly, the results showed approximately 50% of N. benthamiana leaves co-agroinfiltrated with 35S-NbRFP1 and 35S-RepA demonstrated cell death at approximately 4.5 dpi, and all inoculated leaves showed HR symptoms at 6.0 dpi (Figure 4d). However, 50% of the N. benthamiana leaves were co-agroinfiltrated with empty vectors and 35S: RepA showed HR phenotypes until 5.0 dpi; all plants showed HR-like cell death at 7.0 dpi (Figure 4d). These results indicate that the transient overexpression of NbRFP1 can significantly promote ODV RepA-induced HR.
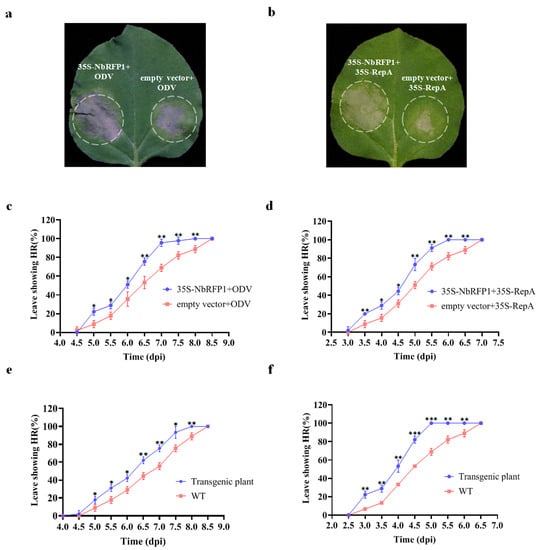
Figure 4.
The effects of overexpression of NbRFP1 on ODV RepA-induced HR. (a,b) Representative leaves in N.benthamiana plants agroinoculated using 35S-NbRFP1 and ODV infectious clones or 35S-RepA together. Photographs were taken at 3.5 dpi (a) or 6.0 dpi (b). (c,d) The percentage of exhibiting cell death in co-agroinfiltrated leaves using 35S-NbRFP1 and ODV infectious clones (c), or 35S-RepA (d) together. (e,f) The percentage of exhibiting cell death in wild-type leaves and NbRFP1-overexpressing transgenic N. benthamiana leaves agroinfiltrated with ODV infectious clones (e), or 35S-RepA (f). The data represent the means ± the SD of the three biological replicates. Statistical analysis was performed using two-way ANOVA followed by Student’s t test, and significant difference between treatments was indicated by asterisk (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
To further clarify the role of NbRFP1 in the induction of HR symptoms in N. benthamiana, we generated NbRFP1-overexpressing transgenic plants through agrobacterium-mediated genetic transformations. The positive transgenic plants were confirmed by Western blotting analysis and were used for further examination. When the transgenic plants were inoculated with ODV infectious clones, approximately 50% of the inoculated leaves showed HR at 6 dpi and 100% of the inoculated leaves showed a HR phenotype at 8 dpi. A significant positive shift of 0.5 day was observed when compared with the control plants (Figure 4e). Similarly, as shown in Figure 4f, approximately 50% of the transgenic N. benthamiana plants showed HR in their infiltrated leaves at 4 dpi after 35S-RepA inoculation; all inoculated leaves displayed HR symptoms at 5 dpi. In contrast, approximately 50% of the wild-type N. benthamiana showed HR symptoms until 4.5 dpi after 35S-RepA inoculation, and all inoculated plants displayed HR symptoms at 6.5 dpi (Figure 4f). Collectively, these results suggest that the overexpression of NbRFP1 can remarkably promote ODV RepA-induced HR in N. benthamiana.
2.5. ODV RepA Interacts with NbRFP1
To examine the interaction between ODV RepA and NbRFP1, bimolecular fluorescence complementation (BiFC) and coimmunoprecipitation (CoIP) assays were individually performed in N. benthamiana. The full-length sequence of RepA or NbRFP1 was inserted into a p2YC or p2YN vector. As anticipated, no fluorescence could be observed in the leaf epidermal cells of N. benthamiana that were co-agroinfiltrated with p2YN and p2YC:RepA, or with p2YN:NbRFP1 and p2YC, respectively (Figure 5a). In contrast, when p2YC:RepA and p2YN:NbRFP1 were co-expressed in the leaf epidermal cells of N. benthamiana, their noticeable YFP fluorescence was detected (Figure 5a), thus indicating that ODV RepA can interact with full-length NbRFP1 in vivo. The CoIP assay showed that RepA-Flag specifically pulled down the NbRFP1-GFP, and that RepA-Flag could not pull down GFP (Figure 5b), further indicating that RepA interacts with full-length NbRFP1 in vivo. Taken together, these results suggest that ODV RepA interacts with full-length NbRFP1 in vivo.
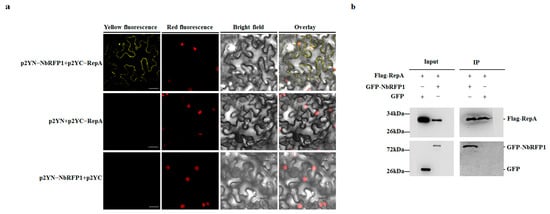
Figure 5.
ODV RepA interacts with NbRFP1. (a) The interaction between RepA and NbRFP1 was examined using a BiFC assay in RFP-H2B transgenic N. benthamiana leaves, which were co-agroinfiltrated with the indicated combination. Fluorescence was observed via confocal microscopy at 48 h post-infiltration (hpi). Bar scale = 20 μm. (b) The interaction between RepA and NbRFP1 was examined using a CoIP assay. Leaves from different combinations were extracted before (input) and after immunoprecipitation (IP). Then, samples were individually analyzed by Western blotting using specific anti-Flag or anti-GFP antibodies.
Next, we asked whether NbRFP1 affects the protein stability of ODV RepA in the process of RepA-induced HR. To this end, we examined the RepA protein abundance in control and NbRFP1-overexpressing transgenic N. benthamiana plants, which were inoculated with ODV infectious clones or 35S-RepA through Western blot analysis. As shown in Figure 6a,b, the overexpression of NbRFP1 could not increase or decrease the ODV RepA protein abundance during ODV infection, nor could it affect the transient expression of 35S-RepA when compared to the controls. Together, these results suggest the interaction between NbRFP1 and RepA does not affect the stability of the ODV RepA protein.
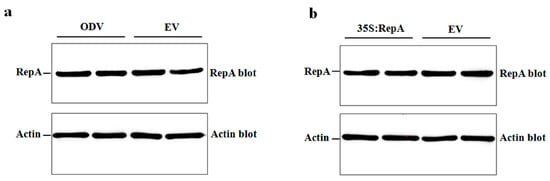
Figure 6.
The assay of the stability of the ODV RepA protein in NbRFP1-overexpressing transgenic N. benthamiana. The detection of the accumulation levels of RepA in transgenic plants that were inoculated with ODV infectious clones (a) or 35S-RepA (b) by Western blotting with polyclonal antibodies that are specific for the RepA or Actin protein.
2.6. The RING-Finger Domain in NbRFP1 Was Involved in the Interaction with RepA and Positively Regulated RepA-Induced HR
In order to determine the domain of NbRFP1 that is involved in modulating the interaction with ODV RepA, we constructed truncated mutants of NbRFP1. As shown in Figure 7a, NbRFP1-M1 deleted 315 to 483 amino acids in the C-terminus, while NbRFP1-M2, which contains a RING-finger domain, deleted N-terminal 1 to 420 amino acids. In vivo BiFC assays indicated that the co-expression of p2YN-RepA and p2YC-NbRFP1:M2 produced yellow fluorescence signals, specifically mainly in the cytoplasm of tobacco cells, whereas the combinations of p2YN-RepA and p2YC-NbRFP1:M1 or p2YN-RepA and p2YC both failed to produce fluorescence signals (Figure 7b). These results suggest that the RING-finger domain may play an important role in modulating the interaction between NbRFP1 and RepA.
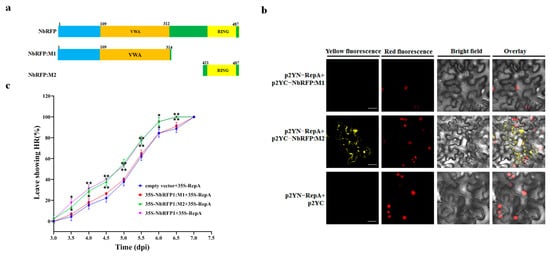
Figure 7.
The assay of the effect of the mutation produced in NbRFP1 following the interaction with ODV-RepA and RepA-induced HR. (a) The schematic representation of the truncated mutants of NbRFP1. (b) The interaction between the mutants of NbRFP1 and RepA was examined using a BiFC assay in RFP-H2B transgenic N. benthamiana leaves. Bar scale = 20 μm. (c) The percentage of cell death in N. benthamiana leaves were co-agroinfiltrated with 35S-NbRFP1:M1 or 35S-NbRFP1:M2 together with 35S-RepA over time. The data represent the means ± the SD of the three biological replicates. Statistical analysis was performed using two-way ANOVA followed by Student’s t test, and significant difference between treatments was indicated by asterisk (* p ≤ 0.05; ** p ≤ 0.01).
Previous reports revealed that the RING domain is necessary for E3 ligase enzyme activity, resulting in interference with the plant immune response [17,18]. To investigate whether the RING-finger domain can mediate RepA-induced HR-like cell death, the co-expression of the mutants of NbRFP1 and RepA were performed in N. benthamiana plants. As shown in Figure 7c, approximately 50% of the leaves were infiltrated with combinations of 35S-NbRFP1:M2 and 35S-RepA, or with 35S-NbRFP1 and 35S-RepA demonstrated cell death at 5 dpi and all infiltrated leaves showed HR phenotypes at 6.5 dpi. In contrast, a remarkable delay was observed in the leaves that were infiltrated with the combinations of 35S-NbRFP1:M1 and 35S-RepA or empty vectors and 35S-RepA (Figure 7c). These results suggest that the RING-finger domain might be involved in positively modulating ODV RepA-induced HR.
3. Discussion
Emerging reports confirm that particular pathogen proteins can elicit robust HR-type immune responses [29,30,31]. Previously, we have demonstrated that ODV RepA is an elicitor that induces HR during ODV infection [9]. However, little is known about the molecular mechanism regarding RepA-induced HR. In this study, we identified that the expression of NbRFP1 was highly induced by ODV infection and was targeted by ODV RepA. A conserved domain search revealed that NbRFP1 contained a central conserved vWFA domain and a C-terminal RING-finger domain. Further sequence alignment revealed that NbRFP1 shared a 98.1% similarity with NtRFP1 from N. tabacum. Additionally, NtRFP1 has been confirmed as a RING-finger E3 ligase [17]. These cases of evidence strongly imply that NbRFP1 is a RING-type E3 ligase.
In plants, many RING-type E3 ligases have been widely reported to be involved in plant immune responses to abiotic and biotic stresses [32,33,34,35]. For example, Kim et al. reports the expression level of rice OsRFPHC-13, a RING-type E3 ligase, is sharply induced under salt stress. Accordingly, overexpression of OsRFPHC-13 in rice improves salinity resistance via ABA dependent manner [36]. Similar findings reveal the expression levels of Arabidopsis RING-type E3 ubiquitin ligases AtRDUF1 and AtRDUF2 are highly induced by flagellin 22 (flg22). Loss-of-function mutants of AtRDUF1 and AtRDUF2 result in inhibiting the PTI response elicited by flg22 and displaying susceptibility to Pseudomonas syringae (P. syringae) 3000 [37]. Evidence shows two closely related RPM1-interacting proteins, RIN2 and RIN3, that encode E3 ligase, and contribute positively to RPM1- and RPS2-dependent HR elicited by P. syringae strains expressing either the AvrRpm1 or AvrB type III effector proteins [38]. Recently, two rice RING-type E3 ligases, OsAPIP6 and OSPIP1, are discovered to positively regulate plant immunity by interacting with OSROD1 and reducing OSROD1 protein levels through UPS to interfere with Ca2+ sensor-mediated ROS scavenging [39]. ROD1 disruption results in broad-spectrum disease resistance to multiple pathogens, including M. oryzae, Xanthomonas oryzae pv. oryzae (Xoo), and Rhizoctonia solani (R. solani) [39]. In contrast, RIP1 or APIP6 knockout shows compromised resistance to M. oryzae, Xoo, and R. solani [39].
In line with these previous findings, our results demonstrate that NbRFP1 plays a positive role in regulating ODV RepA-elicited HR in N. benthamiana. The overexpression of NbRFP1 could promote RepA-induced cell death, whereas the silencing of NbRFP1 produced the opposite effect. It is worth noting that previous studies also revealed that the silencing of NtRING1, which is a RING-finger E3 ligase gene, can delay tobacco mosaic virus (TMV)-induced HR [40]. Several cases showed that an intact RING domain is essential for the enzyme activity of RING-type E3 ligases [17,41,42]. As expected, our investigation of the NbRFP1 mutants showed that the RING domain was required for NbRFP1 to positively regulate RepA-elicited HR.
In the past two decades, several effectors from fungi and bacterium have been reported to directly target RING-type E3 ligases [24,25,43]. Currently, several RING-finger E3 ligases have been demonstrated to positively interact with viral proteins in regulating viral infections. For example, OsRFPH2-10, a RING-Finger E3 ligase from rice, can interact and promote the degradation of the P2 that is encoded by the rice dwarf virus (RDV). In addition, it plays a critical antiviral function at the early stages of RDV infection in rice [44]. In N. tabacum, NtRFP1 could interact and mediate the degradation of the βC1 protein that is encoded by TYLVVNB (i.e., the satellite DNAβ that is associated with tomato yellow leaf curl China virus, TYLCCNB) resulting in the overexpression of NtRFP1, which attenuates viral symptoms [17]. It is well known that RING-type E3 ligase plays a crucial role in regulating plant immunity in various ways [12,45,46,47,48]. Recently, a microtubule-associated E3 ligase MEL from N. benthamiana and rice was reported to elicit a broad-spectrum host resistance to the rice stripe virus (RSV) and to a variety of other destructive pathogens by mediating the degradation of negative immune regulators [18]. In contrast, a functional RING-finger E3 ligase (AtRKP) was discovered to be induced by the C4 protein from the beet severe curly top virus (BSCTV), which played a negative role in the host resistance to BSCTV through degrading the protein ICK2/KRP2 and the mutation of AtRKP, which resulted in a reduced susceptibility to BSCTV [49].
It is widely acknowledged that many ubiquitin E3 ligases act as either positive or negative regulators of immunity by promoting the degradation of various substrates [12]. Here, our BiFC and Co-IP experiments demonstrated that NbRFP1 could function as a novel ODV RepA-interacting host factor. However, unlike the module of NtRFP1 and βC1, the analysis of the accumulation level of RepA in NbRFP1 transgenic plants demonstrated that the overexpression of NbRFP1 could not affect the stability of RepA. Hence, it still remains to be addressed how the interaction of NbRFP1 and RepA contribute to RepA-induced HR. Interestingly, our BiFC experiments identified that the mutant of NbRFP1, with the deletion of the RING domain, failed to interact with RepA. The RING domain has been revealed to serve as a Ub-E2 docking site [16]. Recent reports have also revealed that Avr1d, an Avr effector from Phytophthora sojae, could act as an E2 competitor by occupying the site on GmPUB13 that is for binding the E2 ubiquitin conjugating enzyme [50]. Hence, this might suggest the possibility of RepA as an unknown E2 competitor by occupying its binding site. On the other hand, Li et al. discovered that the interaction between Avr1d and E3 ligase GmPUB15 is required for Avr1d recognition by R protein Rps1-b and Rps1-k [51]. Whether NbRFP1 mimics the similar modulating mode to be involved in regulating the plant immunity that is specific for RepA remains to be elucidated.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Wild-type and NbRFP1-overexpressing transgenic N. benthamiana plants were used in this study. Transgenic plants with a constitutive expression of NbRFP1 using a recombinant 3× Flag-pCAMBIA binary vector carrying the full coding sequence of NbRFP1 were generated through agrobacterium-mediated genetic transformation. These plant materials were grown in a growth chamber at 25 ± 1 °C under a 16/8 h (light/dark) photoperiod and in 70 ± 5% relative humidity.
4.2. RNA Extraction, RT-PCR, and qPCR Analyses
Total RNA extraction from N. benthamiana leaves was performed using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA), as was described previously [9]. The cDNA was reverse transcribed from 1 μg of the total RNA using a PrimeScriptTM RT Master Mix (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. RT-PCR and RT-qPCR analyses were carried out as described previously [9]. For RT-PCR analysis, the relative expression levels were calculated using a 2−ΔΔCt method [52] and N. benthamiana glyceraldehyde-3-phosphate dehydrogenase (NbGADPH) was used as the internal reference.
4.3. Subcellular Localization
The full-length open reading frame (ORF) of NbRFP1 was RT-PCR-amplified (the primers are listed in Table S1) from the N. benthamiana leaf’s cDNA and then introduced into the binary pCHF3 vector containing a GFP insertion to produce 35S-NbRFP1:GFP. The resulting 35S-NbRFP1:GFP and empty vector (35S-GFP) were electroporated individually into A. tumefaciens (C58C1). The transformed A. tumefaciens cultures were adjusted to an optical density at 600 nm (OD600) of 1.0 and infiltrated into leaves of four-leaf-stage RFP-H2B transgenic N. benthamiana seedlings. GFP fluorescence was observed and photographed using confocal microscopy (Leica TCS SP5, Leica, Mannheim, Germany) at 48 hpi.
4.4. BiFC Assay
The full-length ORFs of NbRFP1 and ODV RepA were cloned individually into p2YN and p2YC to produce p2YN-NbRFP1 and p2YC-ODV RepA. The truncated mutants of NbRFP1 were also inserted into p2YN to produce p2YN-NbRFP1:M1 p2YN-NbRFP1:M2. Additionally, the recombinant plasmids were then individually transformed into the A. tumefaciens (C58C1) and were co-agroinfiltrated into the leaves of four-leaf-stage N. benthamiana seedlings according to different inoculation combinations. The agroinfiltrated leaves were observed and photographed using confocal microscopy (Leica TCS SP5, Leica, Mannheim, Germany) at 48 hpi, as described by Li et al. [53].
4.5. Protein Extraction and Western Blotting Analysis
The total protein extraction from N. benthamiana leaves was performed using an extraction buffer, and the subsequently carried out Western blotting analysis was conducted as described previously [9]. The membranes were probed with specific polyclonal antibodies against Actin (ABclonal, Wuhan, China) or with specific polyclonal antibodies that were implemented against the ODV RepA produced by our lab.
4.6. CoIP Assay
The full-length ORFs of the ODV RepA were cloned into a 3×Flag-pCAMBIA vector to produce a Flag-RepA construct. Four-week-old N. benthamiana leaves were infiltrated, according to a 1.1 ratio, with A. tumefaciens containing Flag-RepA or GFP-NbRFP1 together. Then, the total protein was extracted at 2 days after agroinoculation with the IP buffer as described by Li et al. [54]. Immunoprecipitation was performed using an antibody against the Flag (α-Flag, Merck Sigma-Aldrich, Milwaukee, WI, USA). In addition, the immunoprecipitated proteins were analyzed by Western blotting analysis using an anti-GFP (α-GFP, Merck Sigma-Aldrich, Milwaukee, WI, USA) antibody. The immunoblot signals were detected as described previously [53].
4.7. Silencing of Endogenous NbRFP1
To induce the silencing of NbRFP1 in the N. benthamiana plant, a TRV-based VIGS system was used. The 421 bp fragment from NbRFP1 was cloned into a TRV-RNA2 vector. The N. benthamiana seedlings (5–6 leaf stage) were co-inoculated with equal amounts of agrobacterium cultures carrying TRV-RNA 1 or recombinant TRV-RNA2. At 10 dpi, the VIGS efficiency was evaluated by RT-qPCR using GADPH as the reference gene. Then, all silenced plants were subjected to HR assay through infiltration using agrobacterium cultures that contained 35S-RepA or ODV infectious clones. The cell death in the infiltrated leaves was observed over time.
4.8. Transient Co-Expression of RepA and NbRFP1
To transiently overexpress NbRFP1, or its derived mutant, the full ORF of NbRFP1 or the truncated mutants were individually cloned into the pCHF3 vector in order to generate the constructs of 35S-NbRFP1, 35S-NbRFP1:M1, and 35S-NbRFP1:M2. The co-expression of NbRFP1 and RepA, or the mutants of NbRFP1 and RepA by agrobacterium inoculation in N. benthamiana was carried out. Subsequently, the typical necrosis phenotype was observed over time.
4.9. Phylogenetic Analysis
Multiple sequence alignment was performed using MUSCLE (v3.8.31, a multiple sequence alignment method with reduced time and space complexity) with the default parameters [54]. The phylogenetic tree was constructed using the neighbor-joining (NJ) method via the use of MEGA11 software, and was evaluated using a bootstrap test with 1000 replicates.
4.10. Statistical Analysis
The data were shown as the means ± the standard deviation (SD) of the three independent biological replicates. Differences in the mean values were assessed using the Data Processing System (DPS, v15.10) [55], followed by the LSD test. The values were considered to be significantly different at a p-value of <0.05.
5. Conclusions
ODV RepA was previously reported to induce HR-type cell death during viral infection. However, little was known about the host factor(s) that are involved in modulating RepA-induced HR. In this study, our results indicate that the up-regulated abundance of NbRFP1 was identified in response to ODV infection or in ODV RepA transient expression. In addition, NbRFP1 plays a positive role in regulating RepA-induced HR by interacting with ODV RepA. To our knowledge, this is the first report of RING-type E3 ligase serving as a collaborator modulating ODV RepA-elicited HR. Our results will provide new insights for a better understanding of the molecular mechanism of the viral proteins involved in eliciting plant HR-type immune responses.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24097697/s1.
Author Contributions
Conceptualization, X.Z. and Y.Q.; methodology, Z.W., X.Z. and Y.Q.; formal analysis, Z.W., Y.L. and Q.W.; investigation, Y.L. and Q.W.; writing—original draft preparation, Y.L. and Z.W.; writing—review and editing, Y.Q.; visualization, Y.L. and Q.W.; supervision and project administration, Y.Q.; funding acquisition, Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31871929).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this manuscript.
Acknowledgments
We thank J. Schubert from the Federal Research Institute for Cultivated Plants, Germany for his generous gift of infectious clone ODV-Hak14, M. M. Goodin from University of Kentucky for providing N. benthamiana RFP-H2B seed.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Li, P.; Day, B. Battlefield cytoskeleton: Turning the tide on plant immunity. Mol. Plant Microbe Interact. 2019, 32, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Davies, L.J.; Brown, C.R.; Elling, A.A. Calcium is involved in the RMc1(blb)-mediated hypersensitive response against Meloidogyne chitwoodi in potato. Plant Cell Rep. 2015, 34, 167–177. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef]
- Kunstler, A.; Bacso, R.; Gullner, G.; Hafez, Y.M.; Kiraly, L. Staying alive-is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- Chowdhury, R.N.; Lasky, D.; Karki, H.; Zhang, Z.Y.; Goyer, A.; Halterman, D.; Rakotondrafara, A.M. HCPro suppression of callose deposition contributes to strain-specific resistance against potato virus Y. Phytopathology 2020, 110, 164–173. [Google Scholar] [CrossRef]
- Gamage, S.; McGrath, D.J.; Persley, D.M.; Dietzgen, R.G. Transcriptome analysis of capsicum chlorosis virus-induced hypersensitive resistance response in bell capsicum. PLoS ONE 2016, 11, e0159085. [Google Scholar]
- Lukan, T.; Zupanic, A.; Povalej, T.M.; Brunkard, J.O.; Kmetic, M.; Jutersek, M.; Baebler, S.; Gruden, K. Chloroplast redox state changes mark cell-to-cell signaling in the hypersensitive response. New Phytol. 2023, 237, 548–562. [Google Scholar] [CrossRef]
- Qian, Y.; Hou, H.; Shen, Q.; Cai, X.; Sunter, G.; Zhou, X. RepA protein encoded by oat dwarf virus elicits a temperature-sensitive hypersensitive response-type cell death that involves jasmonic acid-dependent signaling. Mol. Plant Microbe Interact. 2016, 29, 5–21. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, C.; Li, Z.C.; Wang, Z.R.; Jiang, X.X.; Shi, Y.F.; Tian, S.N.; Braun, E.; Mei, Y.; Qiu, W.L.; et al. The MAPK kinase kinase GmMEKK1 regulates cell death and defense responses. Plant Physiol. 2018, 178, 907–922. [Google Scholar] [CrossRef]
- Chen, Y.M.; Song, Y.Y.; Liu, J.; Xu, G.Y.; Dou, D.L. Ubiquitination of receptorsomes, frontline of plant immunity. Int. J. Mol. Sci. 2022, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.Y.; Tang, D.Z.; Wang, W. The role of ubiquitination in plant immunity: Fine-tuning immune signaling and beyond. Plant Cell Physiol. 2022, 63, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, D.P.; Shan, L.B. Ubiquitination of pattern recognition receptors in plant innate immunity. Mol. Plant Pathol. 2014, 15, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta 2004, 1695, 55–72. [Google Scholar] [CrossRef]
- Bueso, E.; Rodriguez, L.; Lorenzo-Orts, L.; Gonzalez-Guzman, M.; Sayas, E.; Munoz-Bertomeu, J.; Ibanez, C.; Serrano, R.; Rodriguez, P.L. The single-subunit RING-type E3 ubiquitin ligase RSL1 targets PYL4 and PYR1 ABA receptors in plasma membrane to modulate abscisic acid signaling. Plant J. 2014, 80, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Smalle, J.; Vierstra, R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004, 55, 555–590. [Google Scholar] [CrossRef]
- Shen, Q.T.; Hu, T.; Bao, M.; Cao, L.G.; Zhang, H.W.; Song, F.M.; Xie, Q.; Zhou, X.P. Tobacco RING E3 ligase NtRFP1 mediates ubiquitination and proteasomal degradation of a geminivirus-encoded beta C1. Mol. Plant 2016, 9, 911–925. [Google Scholar] [CrossRef]
- Fu, S.; Wang, K.; Ma, T.; Liang, Y.; Ma, Z.; Wu, J.; Xu, Y.; Zhou, X. An evolutionarily conserved C4HC3-type E3 ligase regulates plant broad-spectrum resistance against pathogens. Plant Cell 2022, 34, 1822–1843. [Google Scholar] [CrossRef]
- Deshaies, R.J.; Joazeiro, C.A.P. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009, 78, 399–434. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Belloni, S.; Marone, D.; De Leonardis, A.M.; Guerra, D.; Fonzo, N.; Cattivelli, L.; Mastrangelo, A.M. The E3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zheng, D.W.; Song, F.M.; Jiang, M. Expression patterns and functional analysis of 11 E3 ubiquitin ligase genes in rice. Front. Plant Sci. 2022, 13, 840360. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Choi, H.W.; Hwang, B.K. The pepper E3 ubiquitin ligase RING1 gene, CaRING1, is required for cell death and the salicylic acid-dependent defense response. Plant Physiol. 2011, 156, 2011–2025. [Google Scholar] [CrossRef]
- Li, W.; Zhong, S.; Li, G.; Li, Q.; Mao, B.; Deng, Y.; Zhang, H.; Zeng, L.; Song, F.; He, Z. Rice RING protein OsBBI1 with E3 ligase activity confers broad-spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res. 2011, 21, 835–848. [Google Scholar] [CrossRef]
- Yu, G.; Derkacheva, M.; Rufian, J.S.; Brillada, C.; Kowarschik, K.; Jiang, S.S.; Derbyshire, P.; Ma, M.M.; DeFalco, T.A.; Morcillo, R.J.L.; et al. The Arabidopsis E3 ubiquitin ligase PUB4 regulates BIK1 and is targeted by a bacterial type-III effector. EMBO J. 2022, 41, e107257. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chen, S.; Shirsekar, G.; Zhou, B.; Khang, C.H.; Songkumarn, P.; Afzal, A.J.; Ning, Y.; Wang, R.; Bellizzi, M.; et al. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell 2012, 24, 4748–4762. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olive, E.; Lett, J.M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J.; Consortium, I.R. ICTV virus taxonomy profile: Geminiviridae 2021. J. Gen. Virol. 2021, 102, 001696. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Habekuss, A.; Kazmaier, K.; Jeske, H. Surveying cereal-infecting geminiviruses in Germany-diagnostics and direct sequencing using rolling circle amplification. Virus Res. 2007, 127, 61–70. [Google Scholar] [CrossRef]
- Hou, H.; Hu, Y.; Wang, Q.; Xu, X.; Qian, Y.; Zhou, X. Gene expression profiling shows that NbFDN1 is involved in modulating the hypersensitive response-like cell death induced by the oat dwarf virus RepA protein. Mol. Plant-Microbe Interact. 2018, 31, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, S.; Nishimura, T.; Noutoshi, Y.; Yamamoto, M.; Toyoda, K.; Ichinose, Y.; Matsui, H. HopAZ1, a type III effector of Pseudomonas amygdali pv. tabaci, induces a hypersensitive response in tobacco wildfire-resistant Nicotiana tabacum ‘N509’. Mol. Plant Pathol. 2022, 23, 885–894. [Google Scholar] [CrossRef]
- Matic, S.; Pegoraro, M.; Noris, E. The C2 protein of tomato yellow leaf curl Sardinia virus acts as a pathogenicity determinant and a 16-amino acid domain is responsible for inducing a hypersensitive response in plants. Virus Res. 2016, 215, 12–19. [Google Scholar] [CrossRef]
- Mesarich, C.H.; Stergiopoulos, I.; Beenen, H.G.; Cordovez, V.; Guo, Y.N.; Jashni, M.K.; Bradshaw, R.E.; de Wit, P. A conserved proline residue in Dothideomycete Avr4 effector proteins is required to trigger a Cf-4-dependent hypersensitive response. Mol. Plant Pathol. 2016, 17, 84–95. [Google Scholar] [CrossRef]
- Brugiere, N.; Zhang, W.; Xu, Q.; Scolaro, E.J.; Lu, C.; Kahsay, R.Y.; Kise, R.; Trecker, L.; Williams, R.W.; Hakimi, S.; et al. Overexpression of RING domain E3 ligase ZmXerico1 confers drought tolerance through regulation of ABA homeostasis. Plant Physiol. 2017, 175, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, H.L.; Park, C.; Chen, Y.C.; Yoon, G.M. Reciprocal antagonistic regulation of E3 ligases controls ACC synthase stability and responses to stress. Proc. Natl. Acad. Sci. USA. 2021, 118, e2011900118. [Google Scholar] [CrossRef]
- Liu, H.X.; Ravichandran, S.; Teh, O.K.; McVey, S.; Lilley, C.; Teresinski, H.J.; Gonzalez-Ferrer, C.; Mullen, R.T.; Hofius, D.; Prithiviraj, B.; et al. The RING-type E3 ligase XBAT35.2 is involved in cell death induction and pathogen response. Plant Physiol. 2017, 175, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Yao, W.; Wang, J.; Ma, F.; Wang, C.; Yang, Y.; Tong, W.; Zhang, J.; Xu, Y.; et al. RING-H2-type E3 gene VpRH2 from Vitis pseudoreticulata improves resistance to Powdery mildew by interacting with VpGRP2A. J. Exp. Bot. 2017, 68, 1669–1687. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.D.; Jung, K.H.; Jang, C.S. Overexpression of a C3HC4-type RING E3 ligase gene, OsRFPHC-13, improves salinity resistance in rice, Oryza sativa, by altering the expression of Na+/K+ transporter genes. Environ. Exp. Bot. 2023, 207, 925–939. [Google Scholar] [CrossRef]
- Yi, S.Y.; Lee, M.Y.J.; Kwon, S.Y.; Kim, W.T.; Lim, Y.P.; Kang, S.Y. RING-type E3 ubiquitin ligases AtRDUF1 and AtRDUF2 positively regulate the expression of PR1 gene and pattern-triggered immunity. Int. J. Mol. Sci. 2022, 23, 14525. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nam, J.; Boyes, D.C.; Holt, B.F.; Hubert, D.A.; Wiig, A.; Dangl, J.L. A duplicated pair of Arabidopsis RING-finger E3 ligases contribute to the RPM1-and RPS2-mediated hypersensitive response. Plant J. 2005, 44, 258–270. [Google Scholar] [CrossRef]
- Gao, M.J.; He, Y.; Yin, X.; Zhong, X.B.; Yan, B.X.; Wu, Y.; Chen, J.; Li, X.Y.; Zhai, K.R.; Huang, Y.F.; et al. Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 2021, 184, 5391. [Google Scholar] [CrossRef]
- Ghannam, A.; Jacques, A.; de Ruffray, P.; Kauffmann, S. NtRING1, putative RING-finger E3 ligase protein, is a positive regulator of the early stages of elicitin-induced HR in tobacco. Plant Cell Rep. 2016, 35, 415–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Li, Y.; Zheng, N.; Chen, H.; Zhao, Q.; Gao, T.; Guo, H.; Xie, Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 2007, 19, 1912–1929. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Williams, L.A.; Farmer, L.M.; Vierstra, R.D.; Callis, J. Keep on going, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 2006, 18, 3415–3428. [Google Scholar] [CrossRef]
- Park, C.H.; Shirsekar, G.; Bellizzi, M.; Chen, S.B.; Songkumarn, P.; Xie, X.; Shi, X.T.; Ning, Y.S.; Zhou, B.; Suttiviriya, P.; et al. The E3 ligase APIP10 connects the effector AvrPiz-t to the NLR receptor Piz-t in rice. PLoS Pathog. 2016, 12, e1005529. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Jin, L.; Huang, X.H.; Geng, Y.T.; Li, F.; Qin, Q.Q.; Wang, R.; Ji, S.Y.; Zhao, S.S.; Xie, Q.; et al. OsRFPH2-10, a RING-H2 Finger E3 ubiquitin ligase, is involved in rice antiviral defense in the early stages of rice dwarf virus infection. Mol. Plant 2014, 7, 1057–1060. [Google Scholar] [CrossRef]
- Ali, M.R.M.; Uemura, T.; Ramadan, A.; Adachi, K.; Nemoto, K.; Nozawa, A.; Hoshino, R.; Abe, H.; Sawasaki, T.; Arimura, G.I. The Ring-type E3 ubiquitin ligase JUL1 targets the VQ-Motif protein JAV1 to coordinate jasmonate signaling. Plant Physiol. 2019, 179, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.S.; Shi, X.T.; Wang, R.Y.; Fan, J.B.; Park, C.H.; Zhang, C.Y.; Zhang, T.; Ouyang, X.H.; Li, S.G.; Wang, G.L. OsELF3-2, an ortholog of Arabidopsis ELF3, interacts with the E3 Ligase APIP6 and negatively regulates immunity against Magnaporthe oryzae in Rice. Mol. Plant. 2015, 8, 1679–1682. [Google Scholar] [CrossRef]
- Suh, J.Y.; Kim, S.J.; Oh, T.R.; Cho, S.K.; Yang, S.W.; Kim, W.T. Arabidopsis Toxicos en Levadura 78 (AtATL78) mediates ABA-dependent ROS signaling in response to drought stress. Biochem. Biophys. Res. Commun. 2016, 469, 8–14. [Google Scholar] [CrossRef]
- Trujillo, M.; Shirasu, K. Ubiquitination in plant immunity. Curr. Opin. Plant Biol. 2010, 13, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.B.; Chen, H.; Teng, K.L.; Zhao, Q.Z.; Zhang, Z.H.; Li, Y.; Liang, L.M.; Xia, R.; Wu, Y.R.; Guo, H.S.; et al. RKP, a RING finger E3 ligase induced by BSCTV C4 protein, affects geminivirus infection by regulation of the plant cell cycle. Plant J. 2009, 57, 905–917. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Q.; Zhou, J.; Yin, W.; Yao, D.; Shao, Y.; Zhao, Y.; Guo, B.; Xia, Y.; Chen, Q.; et al. Phytophthora sojae effector Avr1d functions as an E2 competitor and inhibits ubiquitination activity of GmPUB13 to facilitate infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2018312118. [Google Scholar] [CrossRef]
- Li, S.; Hanlon, R.; Wise, H.; Pal, N.; Brar, H.; Liao, C.; Gao, H.; Perez, E.; Zhou, L.; Tyler, B.M.; et al. Interaction of Phytophthora sojae effector Avr1b with E3 ubiquitin ligase GmPUB1 is required for recognition by soybeans carrying Phytophthora resistance Rps1-b and Rps1-k genes. Front. Plant Sci. 2021, 12, 725571. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Xu, Y.; Fu, S.; Liu, Y.; Li, Z.D.; Zhang, T.Z.; Wu, J.X.; Zhou, X.P. The unfolded protein response plays dual roles in rice stripe virus infection through fine-tuning the movement protein accumulation. PLoS Pathog. 2021, 17, e1009370. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data processing system (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).