Nanoparticle-Based Interventions for Liver Transplantation
Abstract
1. Introduction
2. Organ Preconditioning to Improve Allocation of Marginal Livers for Transplant
2.1. Expanding the Donor Pool by Inhibiting IRI with Nanotechnology
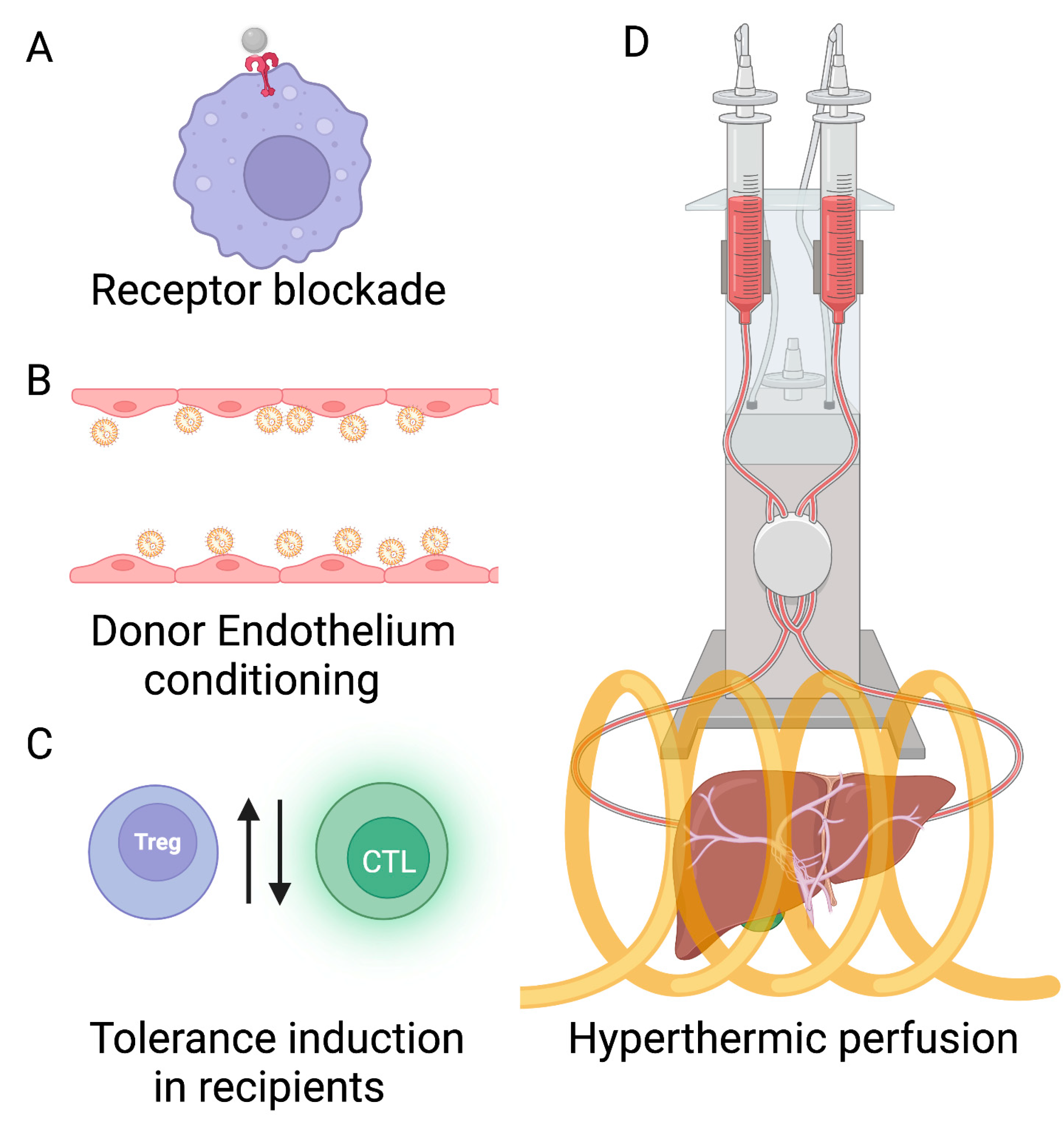
2.2. Nanoparticle-Based Tolerance Induction through Donor Graft Preconditioning
2.3. Tolerance Induction through Recipient Conditioning with Nanoparticles
2.4. Preconditioning with Hyperthermia Can Avoid Chemotherapy Toxicity
Preconditioning with Whole-Body Hyperthermia
2.5. Whole-Body vs. Local Hyperthermia: the Case for Expanding the Liver Donor Pool by Conditioning with Nanoparticle Hyperthermia
| Study | Tissue/Model | Nanoparticle | Functionality | Results |
|---|---|---|---|---|
| Tietjen et al. [48] | Human kidneys. | PLA-PEG nanoparticles, 170 nm mean diameter. | Anti-CD31 conjugated nanoparticles to target endothelial cells. | 5- to 10-fold enhancement of localization of nanoparticles vs. unconjugated nanoparticles. |
| Cui et al. [50] | Human umbilical vein endothelial cells (HUVECs), arterial allografts. | Poly (amine co-ester) nanoparticles, 288 nm mean diameter. | Loaded with non-self MHC II specific siRNA. | Attenuation of MHC II molecules, reduced T cell infiltration and T cell-mediated inflammation and improved allograft histology. |
| Zhu et al. [56] | Mouse aortic and tracheal allografts. | Polyethylene glycol micelles, 15.3 nm mean diameter. | Rapamycin (tolerogenic drug) loaded | Reduced the secretion of inflammatory cytokines and prevented allograft rejection post-transplantation with a 10-fold lower rapamycin dose vs. free Rapamycin. |
| Stead et al. [72] | Murine and non-human primate (marmosets) dendritic cells in vivo targeting. | Porous silicon nanoparticles, 21 nm mean diameter. | Nanoparticles coated with DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN), monoclonal antibody CD11c, ovalbumin (OVA) and loaded with Rapamycin. | Upregulated donor-specific regulatory Treg populations in the spleen. |
| Zhang et al. [73] | Hemophilia A C57BL/6 mice in vivo therapy. | PLGA nanoparticles. | Nanoparticles containing rapamycin and blood clotting factor FVIII | Tolerized B cells against FVIII on the nanoparticles, thereby more effective vs. free FVIII. |
| Shahzad et al. [76] | Single MHC-mismatched murine model of skin transplantation | PLGA nanoparticles, 80 and 200 nm. | Nanoparticles coated with target donor alloantigen H-2Kb-Ig dimer, modulators anti-Fas mAb, PD-L1-Fc, TGF-β (to induce apoptosis, inhibit activation and proliferation of targeted cells and induce Tregs) and CD47-Fc to inhibit phagocytosis from macrophages. | Nanoparticles could specifically target and deplete donor antigen-specific CD8+ T cells in the graft, spleen and peripheral blood (>90% reduction compared to blank nanoparticles), thereby increasing the survival of previously implanted skin allograft. |
| Hlavaty et al. [77] | Sex-mismatched murine model of bone marrow transplant. | Poly(lactide-co-glycolide; PLG) nanoparticle, 500 nm mean diameter. | Donor Hy peptide antigens CD4 epitope Dby, grafter nanoparticles. | Coated nanoparticles provide a 200-fold dose enhancement vs. free peptide in inducing tolerance to male bone marrow. |
3. Advantages and Disadvantages of Nanotechnology
4. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serracino-Inglott, F.; Habib, N.A.; Mathie, R.T. Hepatic Ischemia-Reperfusion Injury. Am. J. Surg. 2001, 181, 160–166. [Google Scholar] [CrossRef]
- Chatauret, N.; Badet, L.; Barrou, B.; Hauet, T. Ischemia-Reperfusion: From Cell Biology to Acute Kidney Injury. Prog. Urol. 2014, 24 (Suppl. S1), S4–S12. [Google Scholar] [CrossRef]
- Lisa, F.D.; Canton, M.; Menabò, R.; Kaludercic, N.; Bernardi, P. Mitochondria and Cardioprotection. Heart Fail Rev. 2007, 12, 249–260. [Google Scholar] [CrossRef]
- Joung, J.; Cho, J.; Kim, Y.; Choi, S.; Son, C. A Literature Review for the Mechanisms of Stress-induced Liver Injury. Brain Behav. 2019, 9, e01235. [Google Scholar] [CrossRef]
- Wertheim, J.A.; Petrowsky, H.; Saab, S.; Kupiec-Weglinski, J.W.; Busuttil, R.W. Major Challenges Limiting Liver Transplantation in the United States. Am. J. Transpl. 2011, 11, 1773–1784. [Google Scholar] [CrossRef]
- Vodkin, I.; Kuo, A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017, 21, 289–301. [Google Scholar] [CrossRef]
- Lentsch, A.B.; Kato, A.; Yoshidome, H.; McMasters, K.M.; Edwards, M.J. Inflammatory Mechanisms and Therapeutic Strategies for Warm Hepatic Ischemia/Reperfusion Injury. Hepatology 2000, 32, 169–173. [Google Scholar] [CrossRef]
- Zhai, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Liver Ischemia and Reperfusion Injury: New Insights into Mechanisms of Innate-Adaptive Immune-Mediated Tissue Inflammation. Am. J. Transpl. 2011, 11, 1563–1569. [Google Scholar] [CrossRef]
- Vollmar, B.; Glasz, J.; Leiderer, R.; Post, S.; Menger, M.D. Hepatic Microcirculatory Perfusion Failure Is a Determinant of Liver Dysfunction in Warm Ischemia-Reperfusion. Am. J. Pathol. 1994, 145, 1421–1431. [Google Scholar]
- Marzi, I.; Rücker, M.; Walcher, F.; Takei, Y. Endothelin-1 Is Involved in Hepatic Sinusoidal Vasoconstriction after Ischemia and Reperfusion. Transpl. Int. 1994, 7, 503–506. [Google Scholar] [CrossRef]
- Cywes, R.; Packham, M.A.; Tietze, L.; Sanabria, J.R.; Harvey, P.R.C.; Phillips, M.J.; Strasberg, S.M. Role of Platelets in Hepatic Allograft Preservation Injury in the Rat. Hepatology 1993, 18, 635–647. [Google Scholar] [CrossRef]
- Ikeda, T.; Yanaga, K.; Kishikawa, K.; Kakizoe, S.; Shimada, M.; Sugimachi, K. Ischemic Injury in Liver Transplantation: Difference in Injury Sites between Warm and Cold Ischemia in Rats. Hepatology 1992, 16, 454–461. [Google Scholar] [CrossRef]
- Tsung, A.; Klune, J.R.; Zhang, X.; Jeyabalan, G.; Cao, Z.; Peng, X.; Stolz, D.B.; Geller, D.A.; Rosengart, M.R.; Billiar, T.R. HMGB1 Release Induced by Liver Ischemia Involves Toll-like Receptor 4 Dependent Reactive Oxygen Species Production and Calcium-Mediated Signaling. J. Exp. Med. 2007, 204, 2913–2923. [Google Scholar] [CrossRef]
- Shen, X.D.; Ke, B.; Ji, H.; Gao, F.; Freitas, M.C.; Chang, W.W.; Lee, C.; Zhai, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Disruption of Type-I IFN Pathway Ameliorates Preservation Damage in Mouse Orthotopic Liver Transplantation via HO-1 Dependent Mechanism. Am. J. Transpl. 2012, 12, 1730–1739. [Google Scholar] [CrossRef]
- Lakkis, F.G.; Li, X.C. Innate Allorecognition by Monocytic Cells and Its Role in Graft Rejection. Am. J. Transpl. 2018, 18, 289–292. [Google Scholar] [CrossRef]
- Oberbarnscheidt, M.H.; Zeng, Q.; Li, Q.; Dai, H.; Williams, A.L.; Shlomchik, W.D.; Rothstein, D.M.; Lakkis, F.G. Non-Self Recognition by Monocytes Initiates Allograft Rejection. J. Clin. Investig. 2014, 124, 3579–3589. [Google Scholar] [CrossRef]
- Zecher, D.; van Rooijen, N.; Rothstein, D.M.; Shlomchik, W.D.; Lakkis, F.G. An Innate Response to Allogeneic Nonself Mediated by Monocytes. J. Immunol. 2009, 183, 7810–7816. [Google Scholar] [CrossRef]
- Cai, J.; Terasaki, P.I. Induction Immunosuppression Improves Long-Term Graft and Patient Outcome in Organ Transplantation: An Analysis of United Network for Organ Sharing Registry Data. Transplantation 2010, 90, 1511–1515. [Google Scholar] [CrossRef]
- Maira, T.D.; Little, E.C.; Berenguer, M. Immunosuppression in Liver Transplant. Best Pract. Res. Clin. Gastroenterol. 2020, 46, 101681. [Google Scholar] [CrossRef]
- Uemura, T.; Schaefer, E.; Hollenbeak, C.S.; Khan, A.; Kadry, Z. Outcome of Induction Immunosuppression for Liver Transplantation Comparing Anti-Thymocyte Globulin, Daclizumab, and Corticosteroid. Transpl. Int. 2011, 24, 640–650. [Google Scholar] [CrossRef]
- Gojo, S.; Niwaya, K.; Taniguchi, S.; Nishizaki, K.; Kitamura, S. Gene Transfer into the Donor Heart During Cold Preservation for Heart Transplantation. Ann. Thorac. Surg. 1998, 65, 647–652. [Google Scholar] [CrossRef]
- Sandovici, M.; Henning, R.H.; van Goor, H.; Helfrich, W.; de Zeeuw, D.; Deelman, L.E. Systemic Gene Therapy with Interleukin-13 Attenuates Renal Ischemia–Reperfusion Injury. Kidney Int. 2008, 73, 1364–1373. [Google Scholar] [CrossRef]
- Vassalli, G.; Roehrich, M.-E.; Vogt, P.; Pedrazzini, G.B.; Siclari, F.; Moccetti, T.; Segesser, L.K. von Modalities and Future Prospects of Gene Therapy in Heart Transplantation. Eur. J. Cardio-Thorac. 2009, 35, 1036–1044. [Google Scholar] [CrossRef]
- Zheng, X.; Zang, G.; Jiang, J.; He, W.; Johnston, N.J.; Ling, H.; Chen, R.; Zhang, X.; Liu, Y.; Haig, A.; et al. Attenuating Ischemia-Reperfusion Injury in Kidney Transplantation by Perfusing Donor Organs with SiRNA Cocktail Solution. Transplantation 2016, 100, 743–752. [Google Scholar] [CrossRef]
- Carini, R.; Albano, E. Recent Insights on the Mechanisms of Liver Preconditioning. Gastroenterology 2003, 125, 1480–1491. [Google Scholar] [CrossRef]
- Kume, M.; Yamamoto, Y.; Saad, S.; Gomi, T.; Kimoto, S.; Shimabukuro, T.; Yagi, T.; Nakagami, M.; Takada, Y.; Morimoto, T.; et al. Ischemic Preconditioning of the Liver in Rats: Implications of Heat Shock Protein Induction to Increase Tolerance of Ischemia-Reperfusion Injury. J. Lab Clin. Med. 1996, 128, 251–258. [Google Scholar] [CrossRef]
- Fudaba, Y.; Ohdan, H.; Tashiro, H.; Ito, H.; Fukuda, Y.; Dohi, K.; Asahara, T. Geranylgeranylacetone, A Heat Shock Protein Inducer, Prevents Primary Graft Nonfunction In Rat Liver Transplantation. Transplantation 2001, 72, 184–189. [Google Scholar] [CrossRef]
- Tashiro, S.; Miyake, H.; Rokutan, K. Role of Geranylgeranylacetone as Non-toxic HSP70 Inducer in Liver Surgery: Clinical Application. J. Hepato Biliary Pancreat. Sci. 2018, 25, 269–274. [Google Scholar] [CrossRef]
- Latchman, D.S. Heat Shock Proteins and Cardiac Protection. Cardiovasc. Res. 2001, 51, 637–646. [Google Scholar] [CrossRef]
- Sõti, C.; Nagy, E.; Giricz, Z.; Vígh, L.; Csermely, P.; Ferdinandy, P. Heat Shock Proteins as Emerging Therapeutic Targets. Brit. J. Pharm. 2005, 146, 769–780. [Google Scholar] [CrossRef]
- Overgaard, J.; Nielsen, O.S. The Importance of Thermotolerance for the Clinical Treatment with Hyperthermia. Radiother. Oncol. 1983, 1, 167–178. [Google Scholar] [CrossRef]
- Land, W.G. The Role of Postischemic Reperfusion Injury and Other Nonantigen-Dependent Inflammatory Pathways in Transplantation. Transplantation 2005, 79, 505–514. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Baccala, R.; Gonzalez-Quintial, R.; Lawson, B.R.; Stern, M.E.; Kono, D.H.; Beutler, B.; Theofilopoulos, A.N. Sensors of the Innate Immune System: Their Mode of Action. Nat. Rev. Rheumatol. 2009, 5, 448–456. [Google Scholar] [CrossRef]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like Receptor Signals in Innate Immune Defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef]
- Tsung, A.; Hoffman, R.A.; Izuishi, K.; Critchlow, N.D.; Nakao, A.; Chan, M.H.; Lotze, M.T.; Geller, D.A.; Billiar, T.R. Hepatic Ischemia/Reperfusion Injury Involves Functional TLR4 Signaling in Nonparenchymal Cells. J. Immunol. 2005, 175, 7661–7668. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, X.D.; O’Connell, R.; Gao, F.; Lassman, C.; Busuttil, R.W.; Cheng, G.; Kupiec-Weglinski, J.W. Cutting Edge: TLR4 Activation Mediates Liver Ischemia/Reperfusion Inflammatory Response via IFN Regulatory Factor 3-Dependent MyD88-Independent Pathway. J. Immunol. 2004, 173, 7115–7119. [Google Scholar] [CrossRef]
- Hui, W.; Jinxiang, Z.; Heshui, W.; Zhuoya, L.; Qichang, Z. Bone Marrow and Non-Bone Marrow TLR4 Regulates Hepatic Ischemia/Reperfusion Injury. Biochem. Biophys. Res. Commun. 2009, 389, 328–332. [Google Scholar] [CrossRef]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The Nuclear Factor HMGB1 Mediates Hepatic Injury after Murine Liver Ischemia-Reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Huang, H.; Tohme, S.; Al-Khafaji, A.B.; Tai, S.; Loughran, P.; Chen, L.; Wang, S.; Kim, J.; Billiar, T.; Wang, Y.; et al. Damage-associated Molecular Pattern–Activated Neutrophil Extracellular Trap Exacerbates Sterile Inflammatory Liver Injury. Hepatology 2015, 62, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Dun, H.; Ippagunta, N.; Rosario, R.; Zhang, Q.Y.; Lefkowitch, J.; Yan, S.F.; Schmidt, A.M.; Emond, J.C. Receptor for Advanced Glycation End Product (RAGE)-Dependent Modulation of Early Growth Response-1 in Hepatic Ischemia/Reperfusion Injury. J. Hepatol. 2009, 50, 929–936. [Google Scholar] [CrossRef]
- Takada, M.; Chandraker, A.; Nadeau, K.C.; Sayegh, M.H.; Tilney, N.L. The Role of the B7 Costimulatory Pathway in Experimental Cold Ischemia/Reperfusion Injury. J. Clin. Investig. 1997, 100, 1199–1203. [Google Scholar] [CrossRef]
- Caldwell, C.C.; Tschoep, J.; Lentsch, A.B. Lymphocyte Function during Hepatic Ischemia/Reperfusion Injury. J. Leukoc. Biol. 2007, 82, 457–464. [Google Scholar] [CrossRef]
- Dangi, A.; Yu, S.; Luo, X. Emerging Approaches and Technologies in Transplantation: The Potential Game Changers. Cell Mol. Immunol. 2019, 16, 334–342. [Google Scholar] [CrossRef]
- Piotti, G.; Palmisano, A.; Maggiore, U.; Buzio, C. Vascular Endothelium as a Target of Immune Response in Renal Transplant Rejection. Front. Immunol. 2014, 5, 505. [Google Scholar] [CrossRef]
- Pober, J.S.; Tellides, G. Participation of Blood Vessel Cells in Human Adaptive Immune Responses. Trends Immunol. 2012, 33, 49–57. [Google Scholar] [CrossRef]
- Tietjen, G.T.; Hosgood, S.A.; DiRito, J.; Cui, J.; Deep, D.; Song, E.; Kraehling, J.R.; Piotrowski-Daspit, A.S.; Kirkiles-Smith, N.C.; Al-Lamki, R.; et al. Nanoparticle Targeting to the Endothelium during Normothermic Machine Perfusion of Human Kidneys. Sci. Transl. Med. 2017, 9, eaam6764. [Google Scholar] [CrossRef]
- Muro, S.; Dziubla, T.; Qiu, W.; Leferovich, J.; Cui, X.; Berk, E.; Muzykantov, V.R. Endothelial Targeting of High-Affinity Multivalent Polymer Nanocarriers Directed to Intercellular Adhesion Molecule 1. J. Pharm. Exp. 2006, 317, 1161–1169. [Google Scholar] [CrossRef]
- Cui, J.; Qin, L.; Zhang, J.; Abrahimi, P.; Li, H.; Li, G.; Tietjen, G.T.; Tellides, G.; Pober, J.S.; Saltzman, W.M. Ex Vivo Pretreatment of Human Vessels with SiRNA Nanoparticles Provides Protein Silencing in Endothelial Cells. Nat. Commun. 2017, 8, 191. [Google Scholar] [CrossRef]
- Sharma, A.; Cressman, E.; Attaluri, A.; Kraitchman, D.L.; Ivkov, R. Current Challenges in Image-Guided Magnetic Hyperthermia Therapy for Liver Cancer. Nanomaterials 2022, 12, 2768. [Google Scholar] [CrossRef] [PubMed]
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical Magnetic Hyperthermia Requires Integrated Magnetic Particle Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1779. [Google Scholar] [CrossRef]
- Attaluri, A.; Seshadri, M.; Mirpour, S.; Wabler, M.; Marinho, T.; Furqan, M.; Zhou, H.; Paoli, S.D.; Gruettner, C.; Gilson, W.; et al. Image-Guided Thermal Therapy with a Dual-Contrast Magnetic Nanoparticle Formulation: A Feasibility Study. Int. J. Hyperther. 2016, 32, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.; Moon, S.H.; Lee, Y.; Park, T.G.; Cheon, J. All-in-One Target-Cell-Specific Magnetic Nanoparticles for Simultaneous Molecular Imaging and SiRNA Delivery. Angewandte Chemie 2009, 48, 4174–4179. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, C.Y.; Namsrai, B.-E.; Han, Z.; Tobolt, D.; Rao, J.S.; Gao, Z.; Etheridge, M.L.; Garwood, M.; Clemens, M.G.; et al. Cryopreservation of Whole Rat Livers by Vitrification and Nanowarming. Ann. Biomed. Eng. 2023, 51, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Atkinson, C.; Dixit, S.; Cheng, Q.; Tran, D.; Patel, K.; Jiang, Y.-L.; Esckilsen, S.; Miller, K.; Bazzle, G.; et al. Organ Preservation with Targeted Rapamycin Nanoparticles: A Pre-Treatment Strategy Preventing Chronic Rejection in Vivo. Rsc. Adv. 2018, 8, 25909–25919. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Noguchi, H.; Saito, H.; Yukawa, H.; Miyamoto, Y.; Ono, K.; Murase, K.; Sawada, M.; Hayashi, S. Novel Positive-Charged Nanoparticles for Efficient Magnetic Resonance Imaging of Islet Transplantation. Cell Med. 2012, 3, 43–49. [Google Scholar] [CrossRef]
- Hwang, J.H.; Noh, Y.; Choi, J.; Noh, J.; Kim, Y.; Gang, G.; Kim, K.; Park, H.S.; Lim, Y.T.; Moon, H.; et al. In Vivo Imaging of Islet Transplantation Using PLGA Nanoparticles Containing Iron Oxide and Indocyanine Green. Magn. Reson. Med. 2014, 71, 1054–1063. [Google Scholar] [CrossRef]
- Zheng, X.X.; Sanchez-Fueyo, A.; Domenig, C.; Strom, T.B. The Balance of Deletion and Regulation in Allograft Tolerance. Immunol. Rev. 2003, 196, 75–84. [Google Scholar] [CrossRef]
- Lechler, R.I.; Garden, O.A.; Turka, L.A. The Complementary Roles of Deletion and Regulation in Transplantation Tolerance. Nat. Rev. Immunol. 2003, 3, 147–158. [Google Scholar] [CrossRef]
- Wood, K.J.; Sakaguchi, S. Regulatory Lymphocytes: Regulatory T Cells in Transplantation Tolerance. Nat. Rev. Immunol. 2003, 3, nri1027. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, C.I.; Karim, M.; Bushell, A.R.; Wood, K.J. CD25+CD4+ Regulatory T Cells Prevent Graft Rejection: CTLA-4- and IL-10-Dependent Immunoregulation of Alloresponses. J. Immunol. 2002, 168, 1080–1086. [Google Scholar] [CrossRef]
- Zheng, X.X.; Sánchez-Fueyo, A.; Sho, M.; Domenig, C.; Sayegh, M.H.; Strom, T.B. Favorably Tipping the Balance between Cytopathic and Regulatory T Cells to Create Transplantation Tolerance. Immunity 2003, 19, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fueyo, A.; Strom, T.B. Immunological Tolerance and Liver Transplantation. J. Hepatol. 2004, 41, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Calne, R.Y.; Sells, R.A.; Pena, J.R.; Davis, D.R.; Millard, P.R.; Herbertson, B.M.; Binns, R.M.; Davies, D.A.L. Induction of Immunological Tolerance By Porcine Liver Allografts. Nature 1969, 223, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Demetris, A.J.; Murase, N.; Rao, A.S.; Fung, J.J.; Starzl, T.E. Murine Liver Allograft Transplantation: Tolerance and Donor Cell Chimerism. Hepatology 1994, 19, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Starzl, T.E.; Demetris, A.J.; Trucco, M.; Murase, N.; Ricordi, C.; Ildstad, S.; Ramos, H.; Todo, S.; Tzakis, A.; Fung, J.J.; et al. Cell Migration and Chimerism after Whole-organ Transplantation: The Basis of Graft Acceptance. Hepatology 1993, 17, 1127–1152. [Google Scholar] [CrossRef]
- Jiang, X.; Morita, M.; Sugioka, A.; Harada, M.; Kojo, S.; Wakao, H.; Watarai, H.; Ohkohchi, N.; Taniguchi, M.; Seino, K. The Importance of CD25+CD4+ Regulatory T Cells in Mouse Hepatic Allograft Tolerance. Liver Transpl. 2006, 12, 1112–1118. [Google Scholar] [CrossRef]
- Gassel, H.-J.; Hutchinson, I.V.; Engemann, R.; Morris, P.J. The Role Of T Suppressor Cells In The Maintenance Of Spontaneously Accepted Orthotopic Rat Liver Allografts. Transplantation 1992, 54, 1048–1052. [Google Scholar] [CrossRef]
- Gomes, A.C.; Mohsen, M.; Bachmann, M.F. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines 2017, 5, 6. [Google Scholar] [CrossRef]
- Kishimoto, T.K.; Maldonado, R.A. Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance. Front. Immunol. 2018, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.O.; Kireta, S.; McInnes, S.J.P.; Kette, F.D.; Sivanathan, K.N.; Kim, J.; Cueto-Diaz, E.J.; Cunin, F.; Durand, J.-O.; Drogemuller, C.J.; et al. Murine and Non-Human Primate Dendritic Cell Targeting Nanoparticles for in Vivo Generation of Regulatory T-Cells. ACS Nano 2018, 12, 6637–6647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-H.; Rossi, R.J.; Yoon, J.; Wang, H.; Scott, D.W. Tolerogenic Nanoparticles to Induce Immunologic Tolerance: Prevention and Reversal of FVIII Inhibitor Formation. Cell Immunol. 2016, 301, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, G.; Saleh, M.B.; Leleu-Chavain, N.; Ningarhari, M.; Gantier, E.; Carpentier, R.; Artru, F.; Gnemmi, V.; Bertin, B.; Maboudou, P.; et al. Nucleotide-Binding Oligomerization Domain 1 (NOD1) Modulates Liver Ischemia Reperfusion through the Expression Adhesion Molecules. J. Hepatol. 2019, 70, 1159–1169. [Google Scholar] [CrossRef]
- Shirali, A.C.; Look, M.; Du, W.; Kassis, E.; Stout-Delgado, H.W.; Fahmy, T.M.; Goldstein, D.R. Nanoparticle Delivery of Mycophenolic Acid Upregulates PD-L1 on Dendritic Cells to Prolong Murine Allograft Survival. Am. J. Transpl. 2011, 11, 2582–2592. [Google Scholar] [CrossRef]
- Shahzad, K.A.; Wan, X.; Zhang, L.; Pei, W.; Zhang, A.; Younis, M.; Wang, W.; Shen, C. On-Target and Direct Modulation of Alloreactive T Cells by a Nanoparticle Carrying MHC Alloantigen, Regulatory Molecules and CD47 in a Murine Model of Alloskin Transplantation. Drug Deliv. 2018, 25, 703–715. [Google Scholar] [CrossRef]
- Hlavaty, K.A.; McCarthy, D.P.; Saito, E.; Yap, W.T.; Miller, S.D.; Shea, L.D. Tolerance Induction Using Nanoparticles Bearing HY Peptides in Bone Marrow Transplantation. Biomaterials 2016, 76, 1–10. [Google Scholar] [CrossRef]
- Martinez, J.O.; Evangelopoulos, M.; Bhavane, R.; Acciardo, S.; Salvatore, F.; Liu, X.; Ferrari, M.; Tasciotti, E. Multistage Nanovectors Enhance the Delivery of Free and Encapsulated Drugs. Curr. Drug Targets 2014, 16, 1582–1590. [Google Scholar] [CrossRef]
- Corbo, C.; Parodi, A.; Evangelopoulos, M.; Engler, D.; Matsunami, R.; Engler, A.; Molinaro, R.; Scaria, S.; Salvatore, F.; Tasciotti, E. Proteomic Profiling of a Biomimetic Drug Delivery Platform. Curr. Drug Targets 2015, 16, 1540–1547. [Google Scholar] [CrossRef]
- Evangelopoulos, M.; Parodi, A.; Martinez, J.O.; Yazdi, I.K.; Cevenini, A.; van de Ven, A.L.; Quattrocchi, N.; Boada, C.; Taghipour, N.; Corbo, C.; et al. Cell Source Determines the Immunological Impact of Biomimetic Nanoparticles. Biomaterials 2016, 82, 168–177. [Google Scholar] [CrossRef]
- Parodi, A.; Quattrocchi, N.; van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V.; et al. Synthetic Nanoparticles Functionalized with Biomimetic Leukocyte Membranes Possess Cell-like Functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Hlavaty, K.A.; Zhang, X.; Yap, W.-T.; Zhang, L.; Shea, L.D.; Luo, X. Nanoparticle Delivery of Donor Antigens for Transplant Tolerance in Allogeneic Islet Transplantation. Biomaterials 2014, 35, 8887–8894. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, K.A.; Luo, X.; Shea, L.D.; Miller, S.D. Cellular and Molecular Targeting for Nanotherapeutics in Transplantation Tolerance. Clin. Immunol. 2015, 160, 14–23. [Google Scholar] [CrossRef]
- Jayant, K.; Reccia, I.; Virdis, F.; Shapiro, A.M.J. The Role of Normothermic Perfusion in Liver Transplantation (TRaNsIT Study): A Systematic Review of Preliminary Studies. HPB Surg. 2018, 2018, 6360423. [Google Scholar] [CrossRef] [PubMed]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Karimian, N.; Matton, A.P.M.; Westerkamp, A.C.; Burlage, L.C.; op den Dries, S.; Leuvenink, H.G.D.; Lisman, T.; Uygun, K.; Markmann, J.F.; Porte, R.J. Ex Situ Normothermic Machine Perfusion of Donor Livers. J. Vis. Exp. 2015, e52688. [Google Scholar] [CrossRef]
- Mosbah, I.B.; Roselló-Catafau, J.; Alfany-Fernandez, I.; Rimola, A.; Parellada, P.P.; Mitjavila, M.T.; Lojek, A.; Abdennebi, H.B.; Boillot, O.; Rodés, J.; et al. Addition of Carvedilol to University Wisconsin Solution Improves Rat Steatotic and Nonsteatotic Liver Preservation. Liver Transpl. 2010, 16, 163–171. [Google Scholar] [CrossRef]
- Serafín, A.; Roselló-Catafau, J.; Prats, N.; Xaus, C.; Gelpí, E.; Peralta, C. Ischemic Preconditioning Increases the Tolerance of Fatty Liver to Hepatic Ischemia-Reperfusion Injury in the Rat. Am. J. Pathol. 2002, 161, 587–601. [Google Scholar] [CrossRef]
- Berthiaume, F.; Barbe, L.; Mokuno, Y.; MacDonald, A.D.; Jindal, R.; Yarmush, M.L. Steatosis Reversibly Increases Hepatocyte Sensitivity to Hypoxia-Reoxygenation Injury. J. Surg. Res. 2009, 152, 54–60. [Google Scholar] [CrossRef]
- Vairetti, M.; Ferrigno, A.; Carlucci, F.; Tabucchi, A.; Rizzo, V.; Boncompagni, E.; Neri, D.; Gringeri, E.; Freitas, I.; Cillo, U. Subnormothermic Machine Perfusion Protects Steatotic Livers against Preservation Injury: A Potential for Donor Pool Increase? Liver Transpl. 2009, 15, 20–29. [Google Scholar] [CrossRef]
- Selzner, M.; Clavien, P.A. Fatty Liver in Liver Transplantation and Surgery. Semin Liver Dis. 2001, 21, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nagasaki, H.; Barama, A.; Boudjema, K.; Jaeck, D.; Kumada, K.; Tatsuno, M.; Baek, Y.; Kitamura, N.; Suzuki, T.; et al. The Effects of N-Acetylcysteine and Anti-Intercellular Adhesion Molecule-1 Monoclonal Antibody against Ischemia-Reperfusion Injury of the Rat Steatotic Liver Produced by a Choline-Methionine-Deficient Diet. Hepatology 1997, 26, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Mokuno, Y.; Berthiaume, F.; Tompkins, R.G.; Balis, U.J.; Yarmush, M.L. Technique for Expanding the Donor Liver Pool: Heat Shock Preconditioning in a Rat Fatty Liver Model. Liver Transpl. 2004, 10, 264–272. [Google Scholar] [CrossRef]
- Yamagami, K.; Yamamoto, Y.; Kume, M.; Kimoto, S.; Yamamoto, H.; Ozaki, N.; Yamamoto, M.; Shimahara, Y.; Toyokuni, S.; Yamaoka, Y. Heat Shock Preconditioning Ameliorates Liver Injury Following Normothermic Ischemia–Reperfusion in Steatotic Rat Livers. J. Surg. Res. 1998, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.H.; Bhatti, T.R.; Fouraschen, S.; Chakravorty, S.; Wang, L.; Kurian, S.; Salomon, D.; Olthoff, K.M.; Hancock, W.W.; Levine, M.H. Heat Shock Protein 70 Is Required for Optimal Liver Regeneration after Partial Hepatectomy in Mice. Liver Transpl. 2014, 20, 376–385. [Google Scholar] [CrossRef]
- Deckers, R.; Debeissat, C.; Fortin, P.-Y.; Moonen, C.T.W.; Couillaud, F. Arrhenius Analysis of the Relationship between Hyperthermia and Hsp70 Promoter Activation: A Comparison between Ex Vivo and in Vivo Data. Int. J. Hyperther. 2012, 28, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Honda, K.; Kobayashi, N. Protective effect of heat preconditioning of rat liver graft resulting in improved transplant survival. Transplantation 2001, 71, 862–868. [Google Scholar] [CrossRef]
- Thorne, A.M.; Ubbink, R.; Brüggenwirth, I.M.A.; Nijsten, M.W.; Porte, R.J.; de Meijer, V.E. Hyperthermia-Induced Changes in Liver Physiology and Metabolism: A Rationale for Hyperthermic Machine Perfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G43–G50. [Google Scholar] [CrossRef]
- Lucke, J.N. Liver metabolism during malignant hyperthermia in the pietrain pig. Vet Anaesth. Analg. 1978, 8, 70–72. [Google Scholar] [CrossRef]
- Glehen, O.; Cotte, E.; Kusamura, S.; Deraco, M.; Baratti, D.; Passot, G.; Beaujard, A.; Noel, G.F. Hyperthermic Intraperitoneal Chemotherapy: Nomenclature and Modalities of Perfusion. J. Surg. Oncol. 2008, 98, 242–246. [Google Scholar] [CrossRef]
- Lehmann, K.; Rickenbacher, A.; Jang, J.-H.; Oberkofler, C.E.; Vonlanthen, R.; von Boehmer, L.; Humar, B.; Graf, R.; Gertsch, P.; Clavien, P.-A. New Insight Into Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. 2012, 256, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Horn, C.; Minor, T. Transient Hyperthermia during Oxygenated Rewarming of Isolated Rat Livers. Transpl. Int. 2020, 33, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Rylander, M.N.; Feng, Y.; Zimmermann, K.; Diller, K.R. Measurement and Mathematical Modeling of Thermally Induced Injury and Heat Shock Protein Expression Kinetics in Normal and Cancerous Prostate Cells. Int. J. Hyperther. 2010, 26, 748–764. [Google Scholar] [CrossRef]
- Morano, W.F.; Khalili, M.; Chi, D.S.; Bowne, W.B.; Esquivel, J. Clinical Studies in CRS and HIPEC: Trials, Tribulations, and Future Directions—A Systematic Review. J. Surg. Oncol. 2018, 117, 245–259. [Google Scholar] [CrossRef]
- Neuwirth, M.G.; Alexander, H.R.; Karakousis, G.C. Then and Now: Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (HIPEC), a Historical Perspective. J. Gastrointest. Oncol. 2016, 7, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliver Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Simamora, P.; Alvarez, J.M.; Yalkowsky, S.H. Solubilization of Rapamycin. Int. J. Pharm. 2001, 213, 25–29. [Google Scholar] [CrossRef]
- Grattoni, A.; Shen, H.; Fine, D.; Ziemys, A.; Gill, J.S.; Hudson, L.; Hosali, S.; Goodall, R.; Liu, X.; Ferrari, M. Nanochannel Technology for Constant Delivery of Chemotherapeutics: Beyond Metronomic Administration. Pharm. Res. 2011, 28, 292–300. [Google Scholar] [CrossRef]
- Ferrati, S.; Nicolov, E.; Zabre, E.; Geninatti, T.; Shirkey, B.A.; Hudson, L.; Hosali, S.; Crawley, M.; Khera, M.; Palapattu, G.; et al. The Nanochannel Delivery System for Constant Testosterone Replacement Therapy. J. Sex. Med. 2015, 12, 1375–1380. [Google Scholar] [CrossRef]
- Ananta, J.S.; Godin, B.; Sethi, R.; Moriggi, L.; Liu, X.; Serda, R.E.; Krishnamurthy, R.; Muthupillai, R.; Bolskar, R.D.; Helm, L.; et al. Geometrical Confinement of Gadolinium-Based Contrast Agents in Nanoporous Particles Enhances T1 Contrast. Nat. Nanotechnol. 2010, 5, 815–821. [Google Scholar] [CrossRef]
- Misra, R.D.K. Magnetic Nanoparticle Carrier for Targeted Drug Delivery: Perspective, Outlook and Design. Mater. Sci. Tech. Ser. 2013, 24, 1011–1019. [Google Scholar] [CrossRef]
- Hom, C.; Lu, J.; Liong, M.; Luo, H.; Li, Z.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles Facilitate Delivery of SiRNA to Shutdown Signaling Pathways in Mammalian Cells. Small 2010, 6, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; Henegouwen, P.M. van B. en Nanobody-Based Cancer Therapy of Solid Tumors. Nanomed. Lond. Engl. 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Li, T.Z.; Gong, F.; Zhang, B.Y.; Sun, J.D.; Zhang, T.; Kong, L.; Xue, Y.Y.; Tang, M. Acute Toxicity and Bio-Distribution of Silver Nitrate and Nano-Silver with Different Particle Diameters in Rats. Zhonghua Shao Shang Za Zhi Zhonghua Shaoshang Zazhi Chin. J. Burn. 2016, 32, 606–612. [Google Scholar] [CrossRef]
- Chinde, S.; Grover, P. Toxicological Assessment of Nano and Micron-Sized Tungsten Oxide after 28days Repeated Oral Administration to Wistar Rats. Mutat. Res. Genet. Toxicol. Env. Mutagen 2017, 819, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chang, X.; Tian, M.; Zhu, A.; Zou, L.; Han, A.; Su, L.; Li, S.; Sun, Y. Nano NiO Induced Liver Toxicity via Activating the NF-ΚB Signaling Pathway in Rats. Toxicol. Res. 2017, 6, 242–250. [Google Scholar] [CrossRef]
- Sha, B.; Gao, W.; Wang, S.; Gou, X.; Li, W.; Liang, X.; Qu, Z.; Xu, F.; Lu, T.J. Oxidative Stress Increased Hepatotoxicity Induced by Nano-titanium Dioxide in BRL-3A Cells and Sprague–Dawley Rats. J. Appl. Toxicol. 2014, 34, 345–356. [Google Scholar] [CrossRef]
- Magaye, R.R.; Yue, X.; Zou, B.; Shi, H.; Yu, H.; Liu, K.; Lin, X.; Xu, J.; Yang, C.; Wu, A.; et al. Acute Toxicity of Nickel Nanoparticles in Rats after Intravenous Injection. Int. J. Nanomed. 2014, 9, 1393–1402. [Google Scholar] [CrossRef]
- Recordati, C.; Maglie, M.D.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue Distribution and Acute Toxicity of Silver after Single Intravenous Administration in Mice: Nano-Specific and Size-Dependent Effects. Part Fibre Toxicol. 2016, 13, 12. [Google Scholar] [CrossRef]
- Suker, D.K.; Jasim, F.A. Liver Histopathological Alteration after Repeated Intra-Tracheal Instillation of Titanium Dioxide in Male Rats. Gastroenterol. Hepatol. Bed Bench 2018, 11, 159–168. [Google Scholar] [PubMed]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N.; et al. Peptide-Functionalized Gold Nanorods Increase Liver Injury in Hepatitis. ACS Nano 2012, 6, 8767–8777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Rippstein, P.; Tayabali, A.F.; Willmore, W.G. Mitochondrial Toxicity of Cadmium Telluride Quantum Dot Nanoparticles in Mammalian Hepatocytes. Toxicol. Sci. 2015, 146, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.L.; Meyer, J.N. A Systematic Review of Evidence for Silver Nanoparticle-Induced Mitochondrial Toxicity. Env. Sci. Nano 2016, 3, 311–322. [Google Scholar] [CrossRef]
- Kuang, H.; Yang, P.; Yang, L.; Aguilar, Z.P.; Xu, H. Size Dependent Effect of ZnO Nanoparticles on Endoplasmic Reticulum Stress Signaling Pathway in Murine Liver. J. Hazard. Mater. 2016, 317, 119–126. [Google Scholar] [CrossRef]
- Yu, K.-N.; Sung, J.H.; Lee, S.; Kim, J.-E.; Kim, S.; Cho, W.-Y.; Lee, A.Y.; Park, S.J.; Lim, J.; Park, C.; et al. Inhalation of Titanium Dioxide Induces Endoplasmic Reticulum Stress-Mediated Autophagy and Inflammation in Mice. Food Chem. Toxicol. 2015, 85, 106–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, J.S.; Ivkov, R.; Sharma, A. Nanoparticle-Based Interventions for Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 7496. https://doi.org/10.3390/ijms24087496
Rao JS, Ivkov R, Sharma A. Nanoparticle-Based Interventions for Liver Transplantation. International Journal of Molecular Sciences. 2023; 24(8):7496. https://doi.org/10.3390/ijms24087496
Chicago/Turabian StyleRao, Joseph Sushil, Robert Ivkov, and Anirudh Sharma. 2023. "Nanoparticle-Based Interventions for Liver Transplantation" International Journal of Molecular Sciences 24, no. 8: 7496. https://doi.org/10.3390/ijms24087496
APA StyleRao, J. S., Ivkov, R., & Sharma, A. (2023). Nanoparticle-Based Interventions for Liver Transplantation. International Journal of Molecular Sciences, 24(8), 7496. https://doi.org/10.3390/ijms24087496






