MKK4 Inhibitors—Recent Development Status and Therapeutic Potential
Abstract
1. Introduction
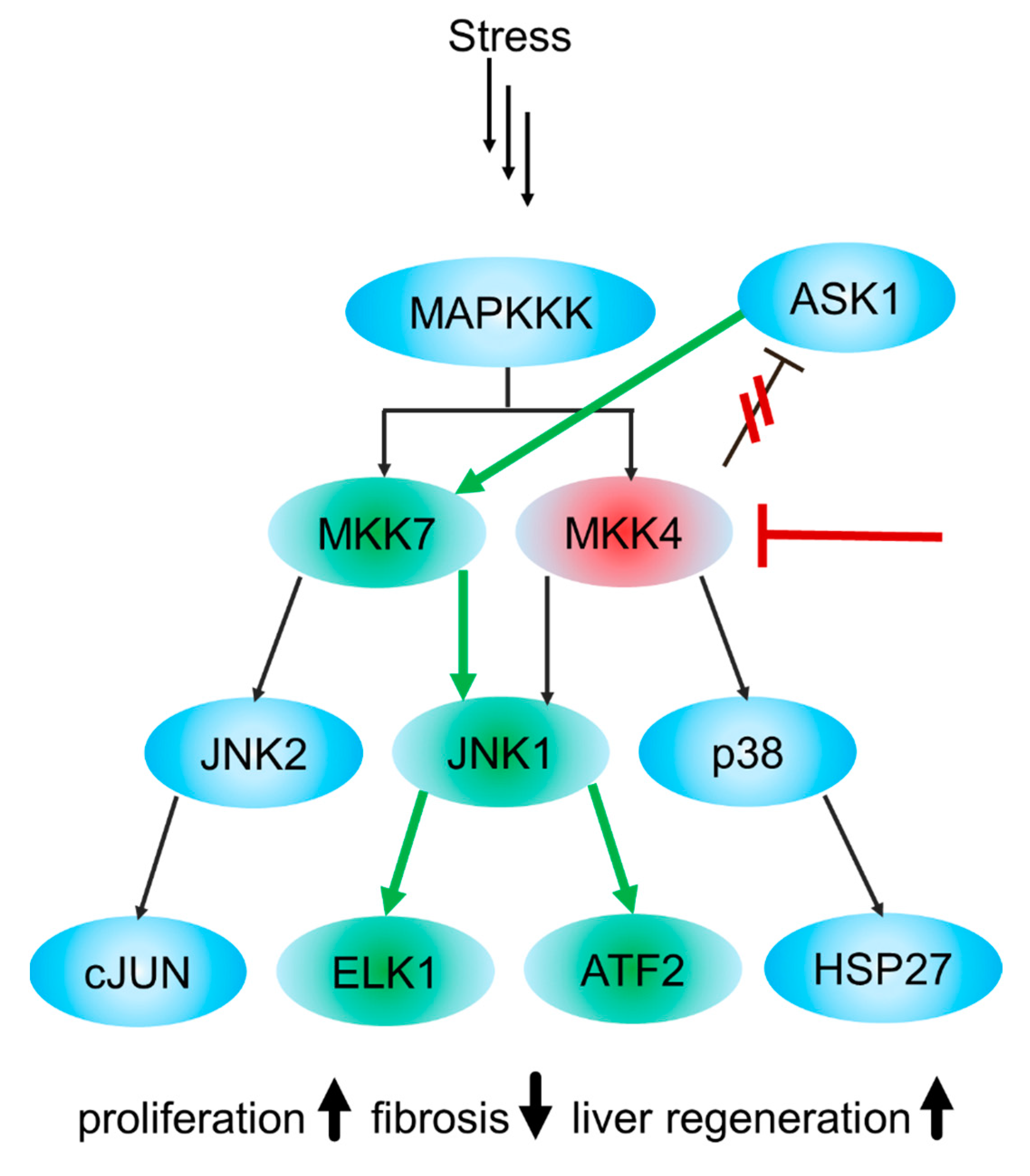
2. Classification, Structure and Biological Function of MKK4
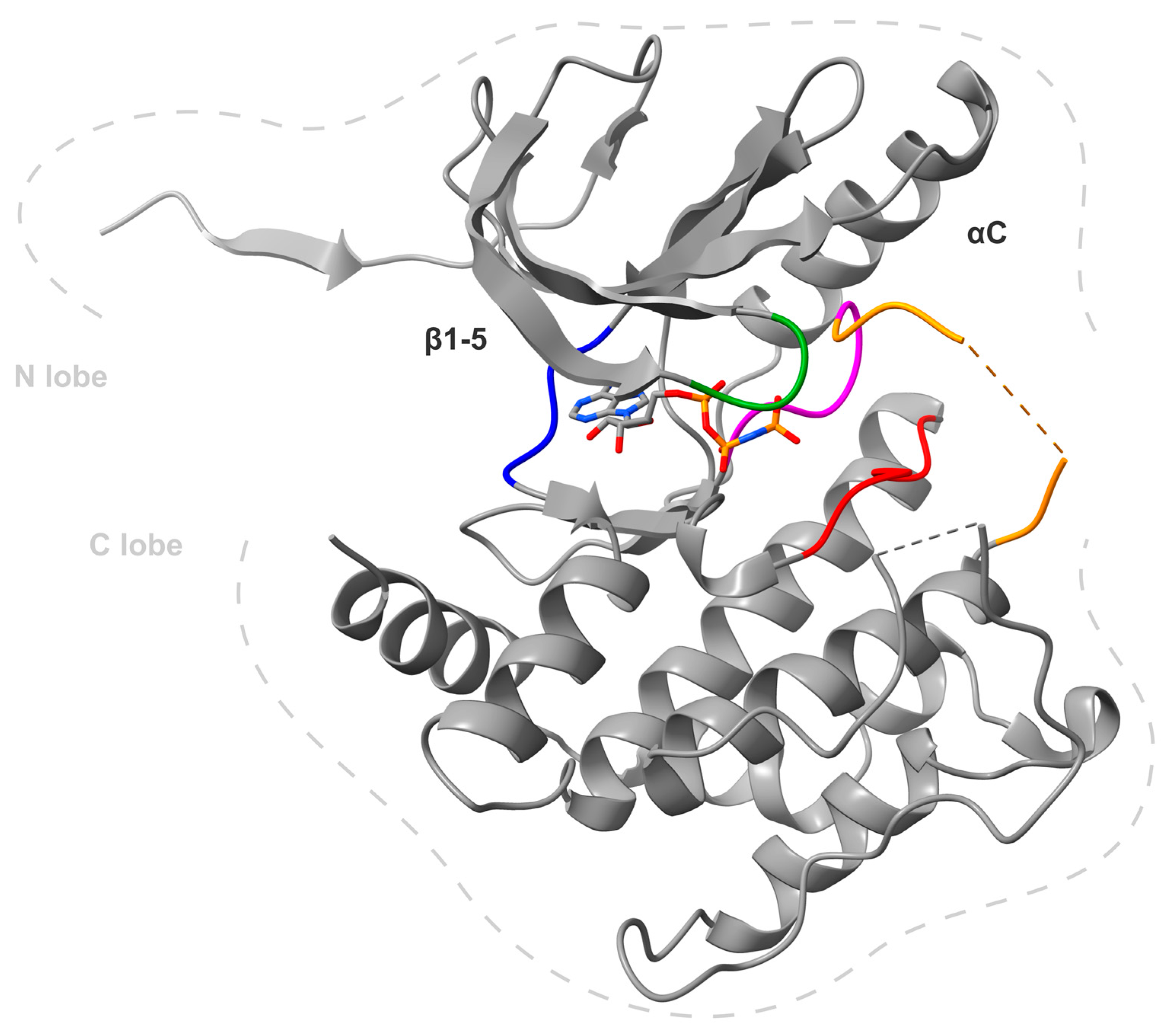
3. MKK4 Inhibitors
3.1. Role of MKK4 Inhibitors in Cancer Therapies
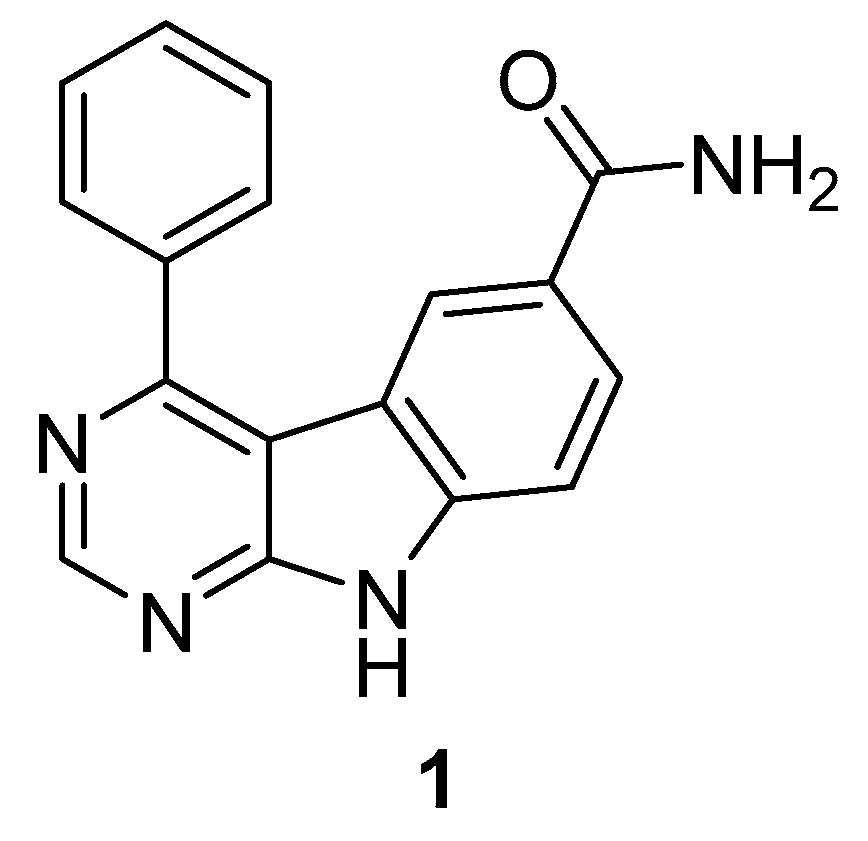
3.1.1. 9-H-pyrimido [4,5-b]ind-6-ol-scaffold
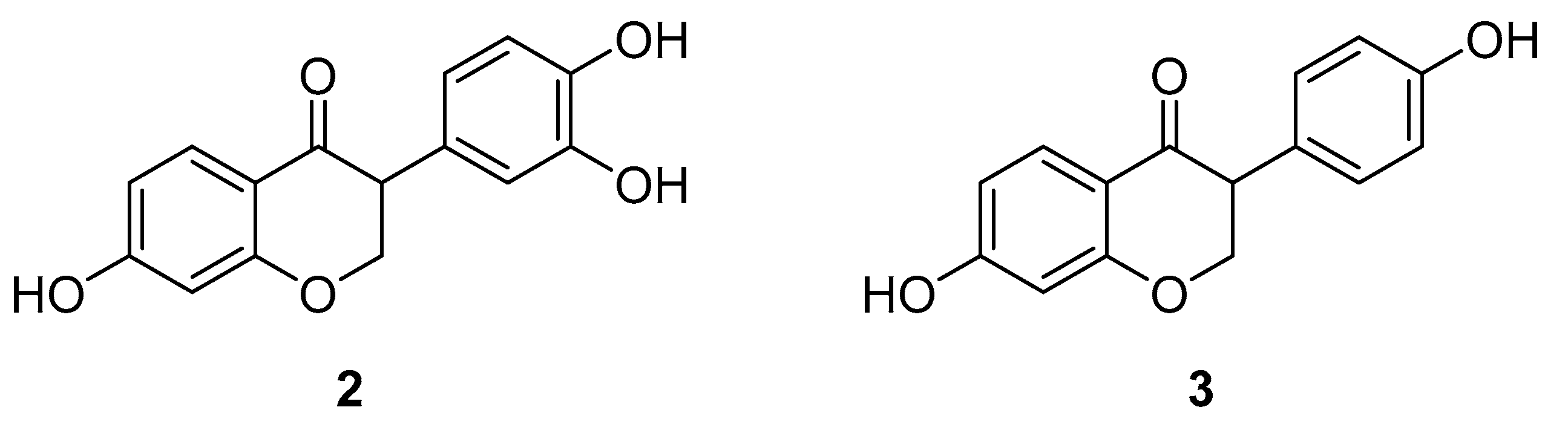
3.1.2. 7,3′,4′-Trihydroxyisoflavone (THIF)
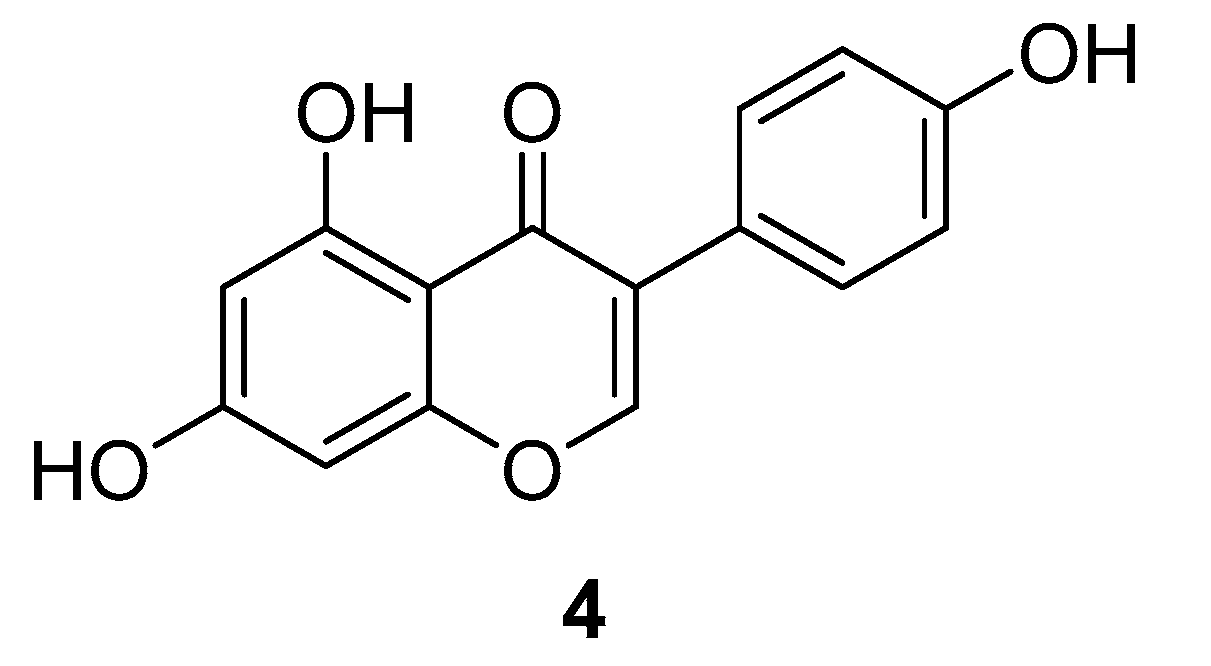
3.1.3. Genistein
3.1.4. Dehydroglyasperin C
3.1.5. HWY336
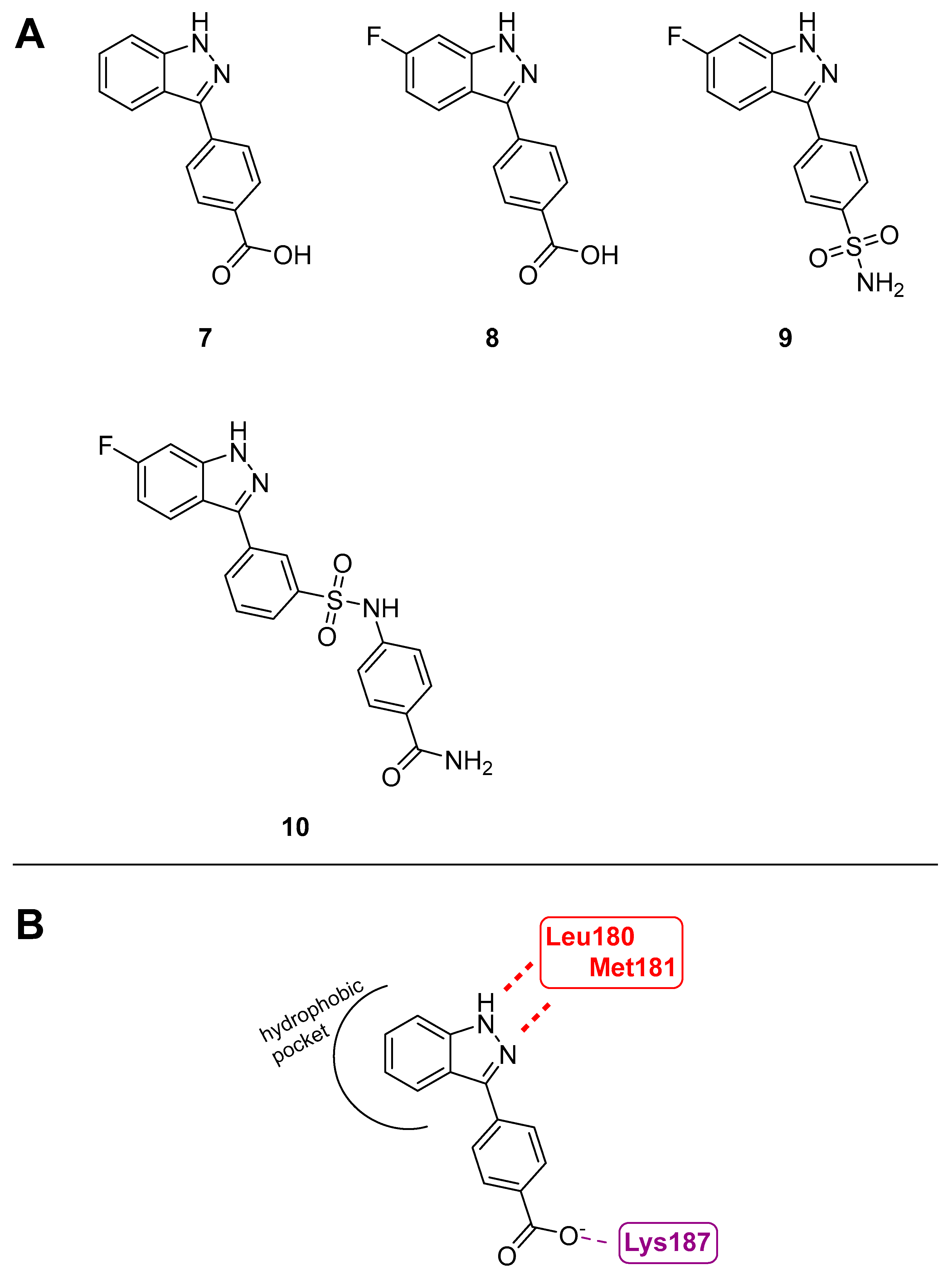
3.1.6. 3-Arylindazoles
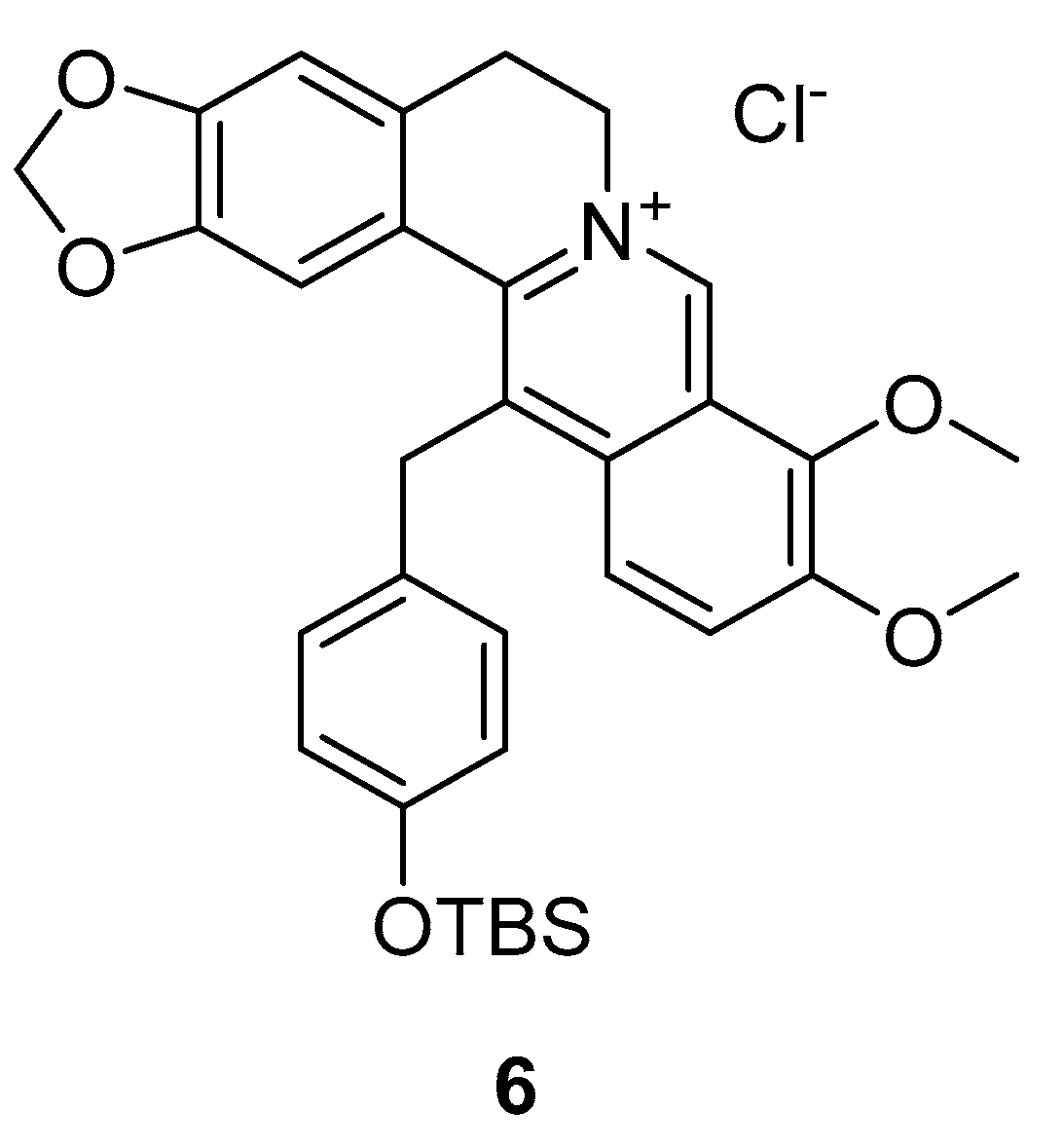
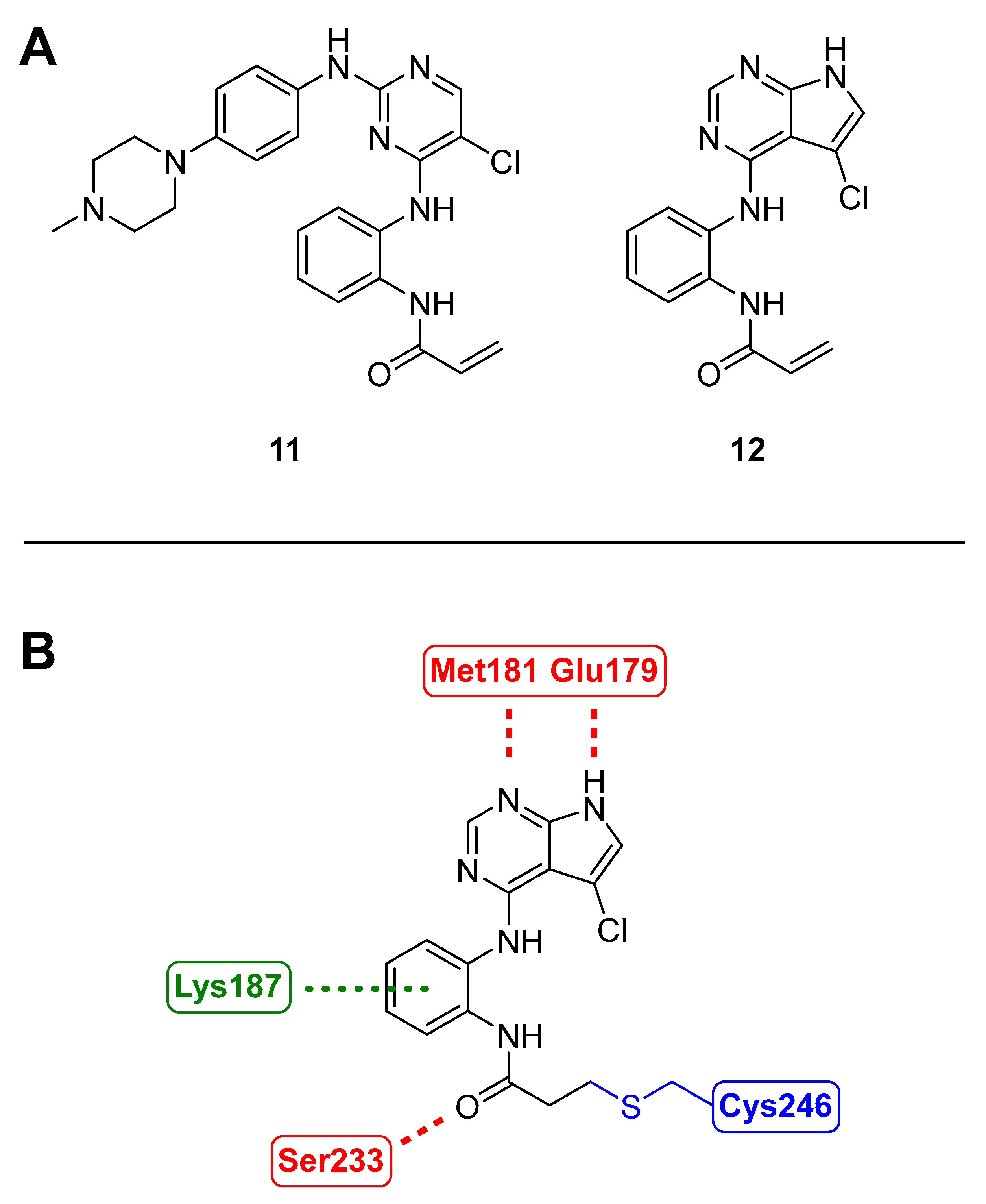
3.1.7. BSJ-04-122
3.2. Role of MKK4 Inhibitors in Liver Regeneration
3.2.1. Azaindoles
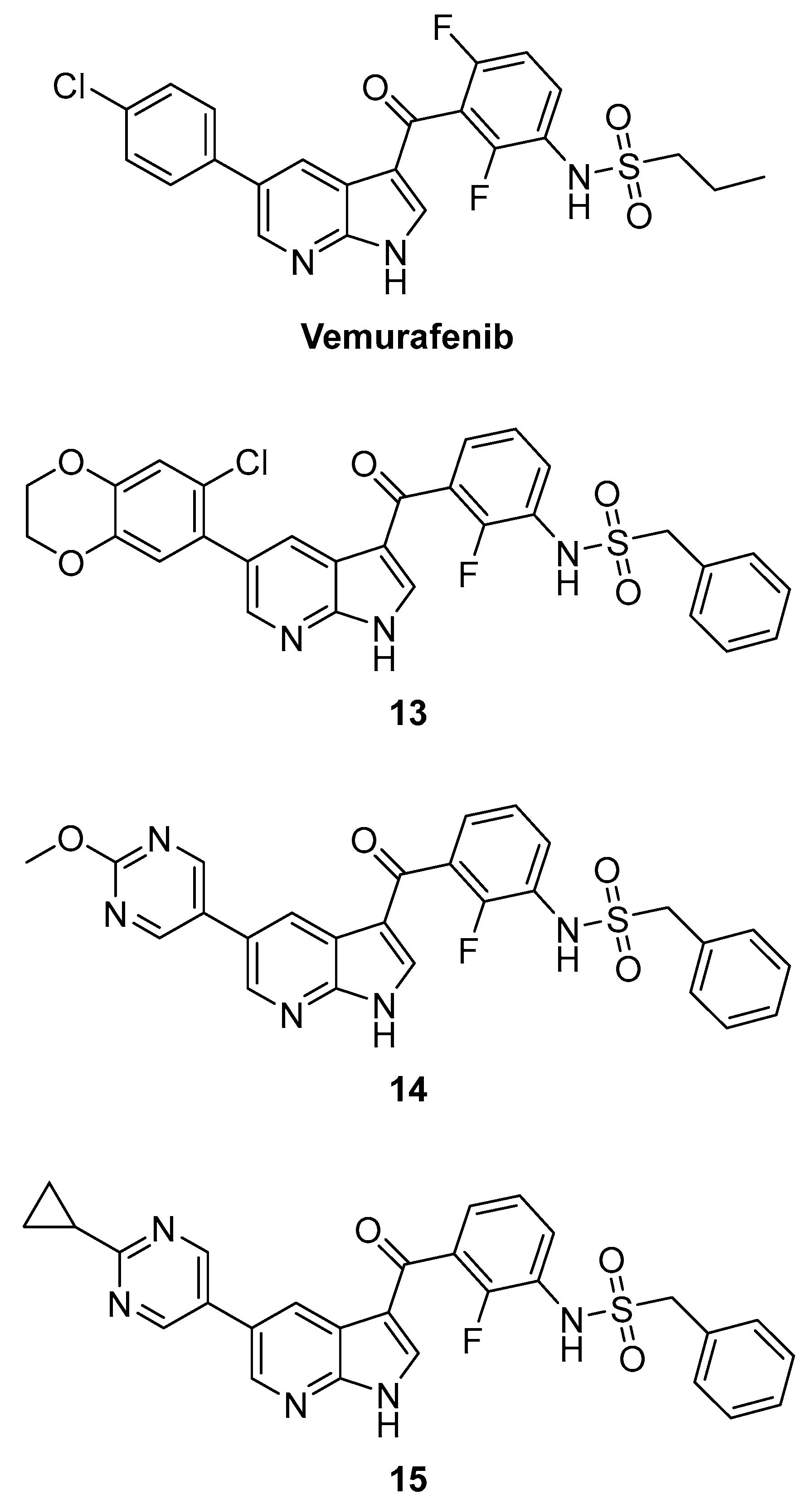
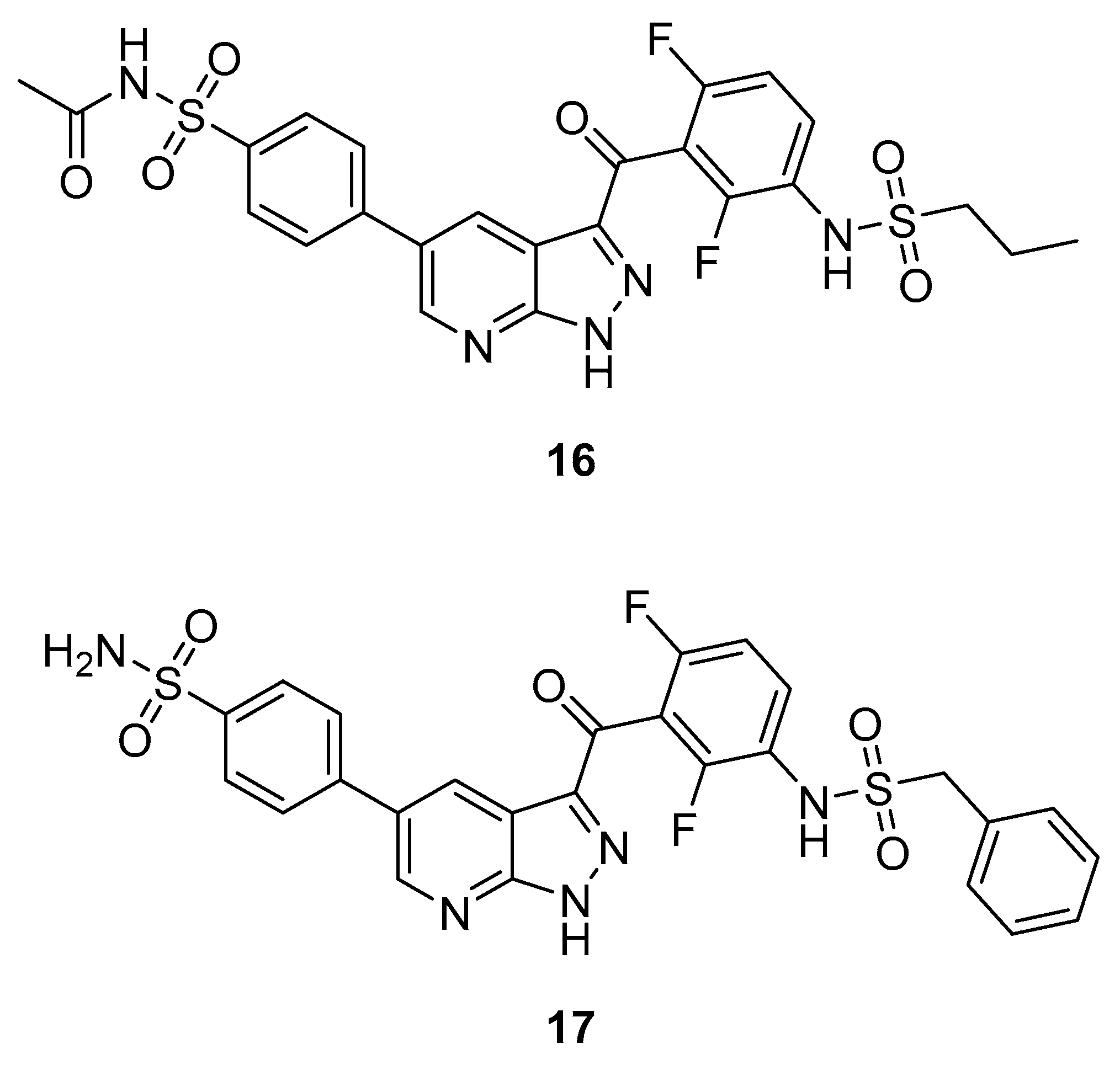
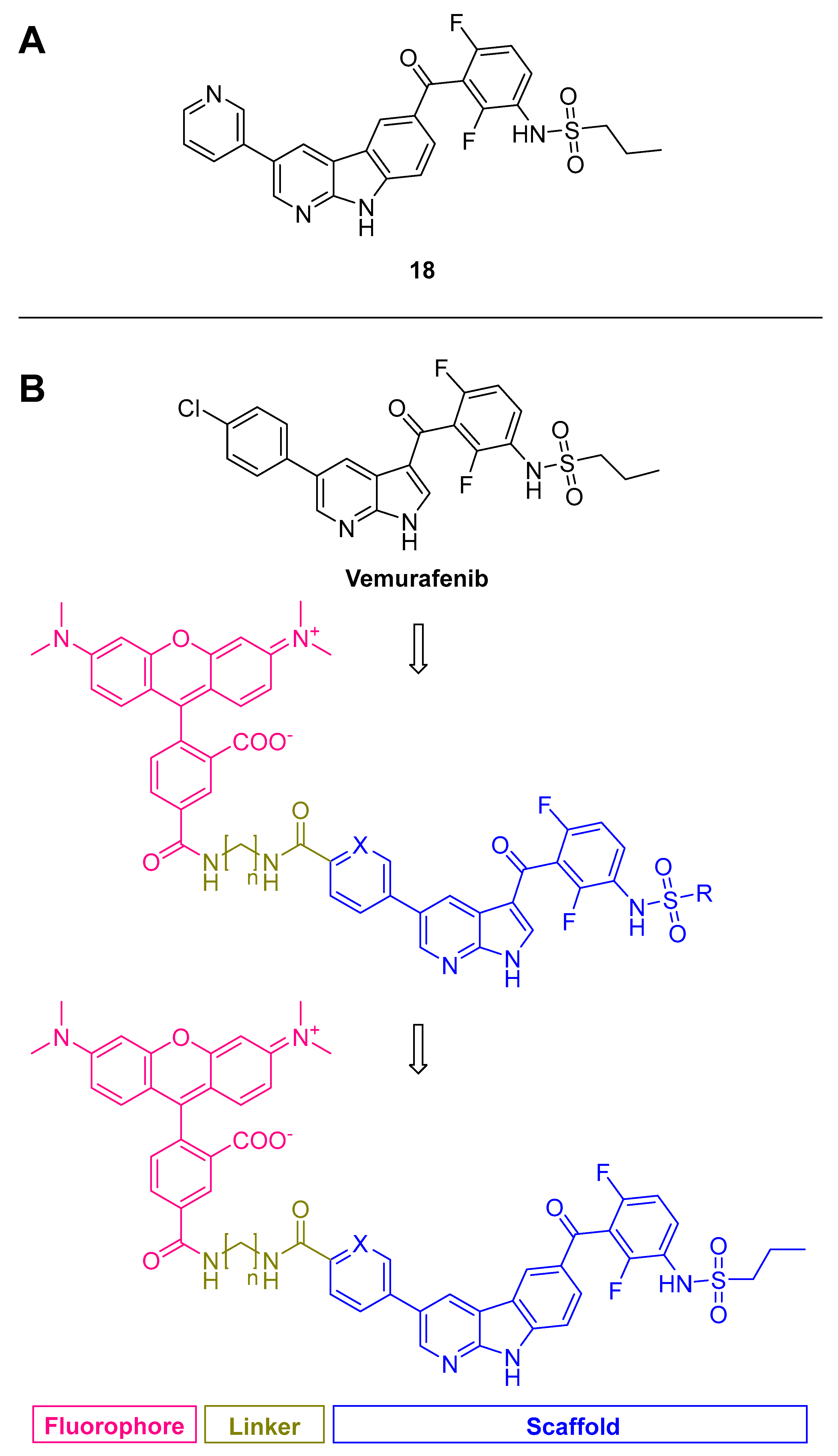
3.2.2. Pyrazolopyridines
3.2.3. Carbolines
3.3. Role of MKK4 Inhibitors in Other Diseases
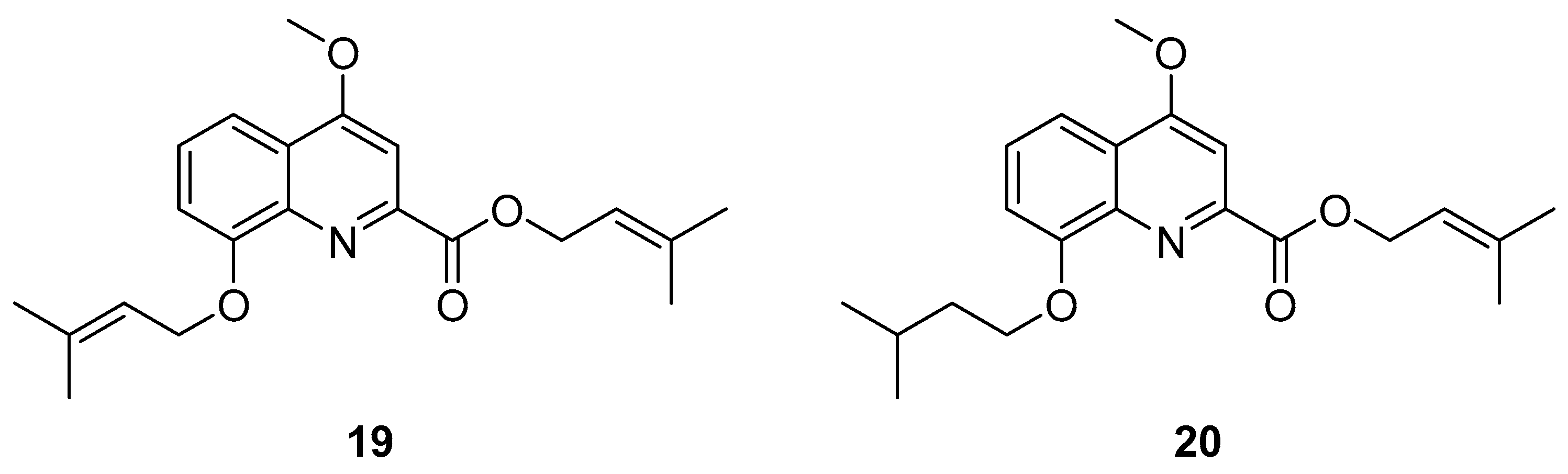
Prenylated Quinoline Carboxylic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Bajorath, J.; Gehringer, M.; Gray, N.; Frye, S.; Lindsley, C.W. Publication Criteria and Requirements for Studies on Protein Kinase Inhibitors─What Is Expected? J. Med. Chem. 2022, 65, 6973–6974. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.-e.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions *. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, H. Current Development Status of MEK Inhibitors. Molecules 2017, 22, 1551. [Google Scholar] [CrossRef]
- Burotto, M.; Chiou, V.L.; Lee, J.-M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- Akinleye, A.; Furqan, M.; Mukhi, N.; Ravella, P.; Liu, D. MEK and the inhibitors: From bench to bedside. J. Hematol. Oncol. 2013, 6, 27. [Google Scholar] [CrossRef]
- Sanchez, J.N.; Wang, T.; Cohen, M.S. BRAF and MEK inhibitors: Use and resistance in BRAF-mutated cancers. Drugs 2018, 78, 549–566. [Google Scholar] [CrossRef]
- Kwong, A.J.; Scheidt, K.A. Non-‘classical’MEKs: A review of MEK3-7 inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127203. [Google Scholar] [CrossRef]
- Albrecht, W. HepaRegeniX Reports Positive Topline Results of Its Phase 1 Clinical Trial of HRX-0215, a First-in-Class MKK4 Inhibitor. Available online: https://www.heparegenix.com/heparegenix-reports-positive-topline-results-of-its-phase-1-clinical-trial-of-hrx-0215-a-first-in-class-mkk4-inhibitor/ (accessed on 5 August 2022).
- Cuenda, A. Mitogen-Activated Protein Kinase Kinase 4 (MKK4). Int. J. Biochem. Cell Biol. 2000, 32, 581–587. [Google Scholar] [CrossRef]
- Sánchez, I.; Hughes, R.T.; Mayer, B.J.; Yee, K.; Woodgett, J.R.; Avruch, J.; Kyriakls, J.M.; Zon, L.I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 1994, 372, 794–798. [Google Scholar] [CrossRef]
- Dérijard, B.; Raingeaud, J.; Barrett, T.; Wu, I.-H.; Han, J.; Ulevitch, R.J.; Davis, R.J. Independent Human MAP-Kinase Signal Transduction Pathways Defined by MEK and MKK Isoforms. Science 1995, 267, 682–685. [Google Scholar] [CrossRef]
- Lin, A.; Minden, A.; Martinetto, H.; Claret, F.-X.; Lange-Carter, C.; Mercurio, F.; Johnson, G.L.; Karin, M. Identification of a Dual Specificity Kinase That Activates the Jun Kinases and p38-Mpk2. Science 1995, 268, 286–290. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kinoshita, T.; Kirii, Y.; Yokota, K.; Hamada, K.; Tada, T. Crystal structures of MKK4 kinase domain reveal that substrate peptide binds to an allosteric site and induces an auto-inhibition state. Biochem. Biophys. Res. Commun. 2010, 400, 369–373. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kinoshita, T.; Kirii, Y.; Tada, T.; Yamano, A. Crystal and solution structures disclose a putative transient state of mitogen-activated protein kinase kinase 4. Biochem. Biophys. Res. Commun. 2012, 425, 195–200. [Google Scholar] [CrossRef]
- Hanks, S.K.; Quinn, A.M.; Hunter, T. The Protein Kinase Family: Conserved Features and Deduced Phylogeny of the Catalytic Domains. Science 1988, 241, 42–52. [Google Scholar] [CrossRef]
- Yang, J.; New, L.; Jiang, Y.; Han, J.; Su, B. Molecular cloning and characterization of a human protein kinase that specifically activates c-Jun N-terminal kinase. Gene 1998, 212, 95–102. [Google Scholar] [CrossRef]
- Yan, M.; Dai, T.; Deak, J.C.; Kyriakis, J.M.; Zon, L.I.; Woodgett, J.R.; Templeton, D.J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature 1994, 372, 798–800. [Google Scholar] [CrossRef]
- Deacon, K.; Blank, J.L. Characterization of the Mitogen-activated Protein Kinase Kinase 4 (MKK4)/c-Jun NH2-terminal kinase 1 and MKK3/p38 Pathways Regulated by MEK Kinases 2 and 3: MEK Kinase 3 Activates MKK3 but does not Cause Activation OF p38 kinase in vivo *. J. Biol. Chem. 1997, 272, 14489–14496. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Miyano, N.; Nakai, R.; Yokota, K.; Ishiguro, H.; Tada, T. Protein purification and preliminary crystallographic analysis of human Lyn tyrosine kinase. Protein Expr. Purif. 2008, 58, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.; Poso, A.; Pantsar, T. The autoinhibited state of MKK4: Phosphorylation, putative dimerization and R134W mutant studied by molecular dynamics simulations. Comput. Struct. Biotechnol. J. 2020, 18, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Deibler, K.K.; Mishra, R.K.; Clutter, M.R.; Antanasijevic, A.; Bergan, R.; Caffrey, M.; Scheidt, K.A. A Chemical Probe Strategy for Interrogating Inhibitor Selectivity Across the MEK Kinase Family. ACS Chem. Biol. 2017, 12, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jiang, B.; He, Z.; Ficarro, S.B.; Che, J.; Marto, J.A.; Gao, Y.; Zhang, T.; Gray, N.S. Discovery of covalent MKK4/7 dual inhibitor. Cell Chem. Biol. 2020, 27, 1553–1560.e8. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Gurbani, D.; Du, G.; Everley, R.A.; Browne, C.M.; Chaikuad, A.; Tan, L.; Schröder, M.; Gondi, S.; Ficarro, S.B.; et al. Leveraging compound promiscuity to identify targetable cysteines within the kinome. Cell Chem. Biol. 2019, 26, 818–829.e9. [Google Scholar] [CrossRef]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef]
- Tournier, C.; Whitmarsh, A.J.; Cavanagh, J.; Barrett, T.; Davis, R.J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 7337–7342. [Google Scholar] [CrossRef]
- Lu, X.; Nemoto, S.; Lin, A. Identification of c-Jun NH2-terminal Protein Kinase (JNK)-activating Kinase 2 as an Activator of JNK but Not p38 *. J. Biol. Chem. 1997, 272, 24751–24754. [Google Scholar] [CrossRef]
- Holland, P.M.; Suzanne, M.; Campbell, J.S.; Noselli, S.; Cooper, J.A. MKK7 Is A Stress-activated Mitogen-activated Protein Kinase Kinase Functionally Related to hemipterous. J. Biol. Chem. 1997, 272, 24994–24998. [Google Scholar] [CrossRef]
- Lawler, S.; Fleming, Y.; Goedert, M.; Cohen, P. Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr. Biol. 1998, 8, 1387–1391. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, E.J.; Kim, H.-S.; Kurie, J.M.; Ahn, Y.-H. MKK4 activates non-canonical NFκB signaling by promoting NFκB2-p100 processing. Biochem. Biophys. Res. Commun. 2017, 491, 337–342. [Google Scholar] [CrossRef]
- Takekawa, M.; Tatebayashi, K.; Saito, H. Conserved Docking Site Is Essential for Activation of Mammalian MAP Kinase Kinases by Specific MAP Kinase Kinase Kinases. Mol. Cell 2005, 18, 295–306. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, Z.; Su, B.; Murray, B.; Karin, M. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 1998, 12, 3369–3381. [Google Scholar] [CrossRef]
- Ho, D.T.; Bardwell, A.J.; Abdollahi, M.; Bardwell, L. A docking site in MKK4 mediates high affinity binding to JNK MAPKs and competes with similar docking sites in JNK substrates. J. Biol. Chem. 2003, 278, 32662–32672. [Google Scholar] [CrossRef]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. In Inflammatory Processes; Birkhäuser: Basel, Switzerland, 2000; pp. 13–21. [Google Scholar]
- Fleming, Y.; Armstrong, C.G.; Morrice, N.; Paterson, A.; Goedert, M.; Cohen, P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem. J. 2000, 352, 145–154. [Google Scholar] [CrossRef]
- Tournier, C.; Dong, C.; Turner, T.K.; Jones, S.N.; Flavell, R.A.; Davis, R.J. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001, 15, 1419–1426. [Google Scholar] [CrossRef]
- Doza, Y.N.; Cuenda, A.; Thomas, G.M.; Cohen, P.; Nebreda, A.R. Activation of the MAP kinase homologue RK requires the phosphorylation of Thr-180 and Tyr-182 and both residues are phosphorylated in chemically stressed KB cells. FEBS Lett. 1995, 364, 223–228. [Google Scholar]
- Oltmanns, U.; Issa, R.; Sukkar, M.B.; John, M.; Chung, K.F. Role of c-jun N-terminal kinase in the induced release of GM-CSF, RANTES and IL-8 from human airway smooth muscle cells. Br. J. Pharmacol. 2003, 139, 1228–1234. [Google Scholar] [CrossRef]
- Shi, Y.; Gaestel, M. In the cellular garden of forking paths: How p38 MAPKs signal for downstream assistance. Biol. Chem. 2002, 383, 1519–1536. [Google Scholar] [CrossRef]
- Wang, X.; Destrument, A.; Tournier, C. Physiological roles of MKK4 and MKK7: Insights from animal models. Biochim. Biophys. Acta 2007, 1773, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Hwang, W.-S.; Lee, Y.-D.; Han, P.-L. Dynamic expression of SEK1 suggests multiple roles of the gene during embryogenesis and in adult brain of mice. Mol. Brain Res. 1999, 66, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ganiatsas, S.; Kwee, L.; Fujiwara, Y.; Perkins, A.; Ikeda, T.; Labow, M.A.; Zon, L.I. SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 6881–6886. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nadarajah, B.; Robinson, A.C.; McColl, B.W.; Jin, J.-W.; Dajas-Bailador, F.; Boot-Handford, R.P.; Tournier, C. Targeted deletion of the mitogen-activated protein kinase kinase 4 gene in the nervous system causes severe brain developmental defects and premature death. Mol. Cell. Biol. 2007, 27, 7935–7946. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kawasaki, H.; Arakawa, S.; Shimizu, K.; Shimizu, S.; Reiner, O.; Okano, H.; Nishina, S.; Azuma, N.; Penninger, J.M. Stress-activated protein kinase MKK7 regulates axon elongation in the developing cerebral cortex. J. Neurosci. 2011, 31, 16872–16883. [Google Scholar] [CrossRef]
- Castro-Torres, R.D.; Olloquequi, J.; Etchetto, M.; Caruana, P.; Steele, L.; Leighton, K.-M.; Ureña, J.; Beas-Zarate, C.; Camins, A.; Verdaguer, E.; et al. Dual Mkk4 and Mkk7 Gene Deletion in Adult Mouse Causes an Impairment of Hippocampal Immature Granule Cells. Int. J. Mol. Sci. 2021, 22, 9545. [Google Scholar] [CrossRef]
- Syc-Mazurek, S.B.; Rausch, R.L.; Fernandes, K.A.; Wilson, M.P.; Libby, R.T. Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death. Cell Death Dis. 2018, 9, 1095. [Google Scholar] [CrossRef]
- Nishina, H.; Bachmann, M.; Oliveira-dos-Santos, A.J.; Kozieradzki, I.; Fischer, K.D.; Odermatt, B.; Wakeham, A.; Shahinian, A.; Takimoto, H.; Bernstein, A.; et al. Impaired CD28-mediated interleukin 2 production and proliferation in stress kinase SAPK/ERK1 kinase (SEK1)/mitogen-activated protein kinase kinase 4 (MKK4)-deficient T lymphocytes. J. Exp. Med. 1997, 186, 941–953. [Google Scholar] [CrossRef]
- Nishina, H.; Radvanyi, L.; Raju, K.; Sasaki, T.; Kozieradzki, I.; Penninger, J.M. Impaired TCR-mediated apoptosis and Bcl-XL expression in T cells lacking the stress kinase activator SEK1/MKK4. J. Immunol. 1998, 161, 3416–3420. [Google Scholar] [CrossRef]
- Swat, W.; Fujikawa, K.; Ganiatsas, S.; Yang, D.; Xavier, R.J.; Harris, N.L.; Davidson, L.; Ferrini, R.; Davis, R.J.; Labow, M.A. SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Immunity 1998, 8, 625–634. [Google Scholar] [CrossRef]
- Preston, S.P.; Doerflinger, M.; Scott, H.W.; Allison, C.C.; Horton, M.; Cooney, J.; Pellegrini, M. The role of MKK4 in T-cell development and immunity to viral infections. Immunol. Cell Biol. 2021, 99, 428–435. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Su, G.H.; Hilgers, W.; Shekher, M.C.; Tang, D.J.; Yeo, C.J.; Hruban, R.H.; Kern, S.E. Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res. 1998, 58, 2339–2342. [Google Scholar]
- Nakayama, K.; Nakayama, N.; Davidson, B.; Katabuchi, H.; Kurman, R.J.; Velculescu, V.E.; Shih, l.-M.; Wang, T.-L. Homozygous deletion of MKK4 in ovarian serous carcinoma. Cancer Biol. Ther. 2006, 5, 630–634. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Liu, X.-D.; Zhang, Z.-W.; Wu, H.-W.; Liang, Z.-Y. A new prognosis prediction model combining TNM stage with MAP2K4 and JNK in postoperative pancreatic cancer patients. Pathol. Res. Pract. 2021, 217, 153313. [Google Scholar] [CrossRef]
- Parsons, D.W.; Wang, T.-L.; Samuels, Y.; Bardelli, A.; Cummins, J.M.; DeLong, L.; Silliman, N.; Ptak, J.; Szabo, S.; Willson, J.K.V.; et al. Mutations in a signalling pathway. Nature 2005, 436, 792. [Google Scholar] [CrossRef]
- Davies, H.; Hunter, C.; Smith, R.; Stephens, P.; Greenman, C.; Bignell, G.; Teague, J.; Butler, A.; Edkins, S.; Stevens, C. Somatic mutations of the protein kinase gene family in human lung cancer. Cancer Res. 2005, 65, 7591–7595. [Google Scholar] [CrossRef]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; Yang, Y.; Gibbons, D.L.; Creighton, C.J.; Yang, F.; Wistuba, I.I.; Lin, W.; Thilaganathan, N.; Alvarez, C.A.; Roybal, J. Map2k4 functions as a tumor suppressor in lung adenocarcinoma and inhibits tumor cell invasion by decreasing peroxisome proliferator-activated receptor γ2 expression. Mol. Cell. Biol. 2011, 31, 4270–4285. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Dai, J.L. Evidence of MKK4 pro-oncogenic activity in breast and pancreatic tumors. Oncogene 2004, 23, 5978–5985. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Vander Griend, D.J.; Yang, X.; Benson, D.A.; Dubauskas, Z.; Yoshida, B.A.; Chekmareva, M.A.; Ichikawa, Y.; Sokoloff, M.H.; Zhan, P. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001, 61, 2833–2837. [Google Scholar] [PubMed]
- Yamada, S.D.; Hickson, J.A.; Hrobowski, Y.; Vander Griend, D.J.; Benson, D.; Montag, A.; Karrison, T.; Huo, D.; Rutgers, J.; Adams, S. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res. 2002, 62, 6717–6723. [Google Scholar]
- Pavese, J.M.; Ogden, I.M.; Voll, E.A.; Huang, X.; Xu, L.; Jovanovic, B.; Bergan, R.C. Mitogen-activated protein kinase kinase 4 (MAP2K4) promotes human prostate cancer metastasis. PLoS ONE 2014, 9, e102289. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results (SEER) Program. 2022. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 17 April 2023).
- Xu, L.; Ding, Y.; Catalona, W.J.; Yang, X.J.; Anderson, W.F.; Jovanovic, B.; Wellman, K.; Killmer, J.; Huang, X.; Scheidt, K.A.; et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J. Natl. Cancer Inst. 2009, 101, 1141–1155. [Google Scholar] [CrossRef]
- Lakshman, M.; Xu, L.; Ananthanarayanan, V.; Cooper, J.; Takimoto, C.H.; Helenowski, I.; Pelling, J.C.; Bergan, R.C. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008, 68, 2024–2032. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, S.; Pan, H.; Cheng, Z.; Rui, X. MicroRNA-136 inhibits prostate cancer cell proliferation and invasion by directly targeting mitogen-activated protein kinase kinase 4. Mol. Med. Rep. 2018, 17, 4803–4810. [Google Scholar] [CrossRef]
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Radic. Biol. Med. 2006, 40, 1603–1614. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, K.W.; Byun, S.; Jung, S.K.; Song, N.; Lim, S.H.; Heo, Y.-S.; Kim, J.E.; Kang, N.J.; Kim, B.Y.; et al. 7,3′,4′-Trihydroxyisoflavone, a Metabolite of the Soy Isoflavone Daidzein, Suppresses Ultraviolet B-induced Skin Cancer by Targeting Cot and MKK4 *. J. Biol. Chem. 2011, 286, 14246–14256. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Oh, S.-H.; Suh, Y.-A.; Baek, J.H.; Papadimitrakopoulou, V.; Huang, S.; Hong, W.K. Response of Non–Small Cell Lung Cancer Cells to the Inhibitors of Phosphatidylinositol 3-Kinase/Akt- and MAPK Kinase 4/c-Jun NH2-Terminal Kinase Pathways: An Effective Therapeutic Strategy for Lung Cancer. Clin. Cancer Res. 2005, 11, 6065–6074. [Google Scholar] [CrossRef]
- An, E.; Brognard, J. Orange is the new black: Kinases are the new master regulators of tumor suppression. IUBMB Life 2019, 71, 738–748. [Google Scholar] [CrossRef]
- Lowinger, T.; Shimazaki, M.; Sato, H.; Tanaka, K.; Tsuno, N.; Marx, K.; Yamamoto, M.; Urbahns, K.; Gantner, F.; Okigami, H.; et al. Preparation of Pyrimido [4,5-b]indole Derivatives as Inhibitors of MKK7 and MKK4. WO2003037898, 8 May 2003. [Google Scholar]
- Sato, H.; Inoue, T.; Ly, T.-w.; Muramatsu, A.; Shimazaki, M.; Urbahns, K.; Gantner, F.; Okigami, H.; Bacon, K.B.; Komura, H.; et al. Preparation of 4-Phenyl-Pyrimido [4,5-b]indoles as Inhibitors of MKK7, MKK4 and Treatment of Related Diseases. WO2004058764, 15 July 2004. [Google Scholar]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Kennedy, N.J.; Davis, R.J. Role of JNK in tumor development. Cell Cycle 2003, 2, 199–201. [Google Scholar] [PubMed]
- Franke, A.A.; Custer, L.J.; Cerna, C.M.; Narala, K.K. Quantitation of phytoestrogens in legumes by HPLC. J. Agric. Food Chem. 1994, 42, 1905–1913. [Google Scholar] [CrossRef]
- Chang, T.-S. Isolation, Bioactivity, and Production of ortho-Hydroxydaidzein and ortho-Hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699–5716. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Chen, N.; Scarpa, R.; Zhang, L.; Seiberg, M.; Lin, C.B. Nondenatured Soy Extracts Reduce UVB-induced Skin Damage via Multiple Mechanisms. Photochem. Photobiol. 2008, 84, 1551–1559. [Google Scholar] [CrossRef]
- Rundhaug, J.E.; Simper, M.S.; Surh, I.; Fischer, S.M. The role of the EP receptors for prostaglandin E2 in skin and skin cancer. Cancer Metastasis Rev. 2011, 30, 465–480. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Byun, S.; Kang, N.J.; Lim, S.H.; Heo, Y.-S.; Bode, A.M.; Bowden, G.T.; Lee, H.J.; Dong, Z. Myricetin Suppresses UVB-Induced Skin Cancer by Targeting Fyn. Cancer Res. 2008, 68, 6021–6029. [Google Scholar] [CrossRef]
- Record, I.R.; Dreosti, I.E.; McInerney, J.K. The antioxidant activity of genistein in vitro. J. Nutr. Biochem. 1995, 6, 481–485. [Google Scholar] [CrossRef]
- Severson, R.K.; Nomura, A.M.; Grove, J.S.; Stemmermann, G.N. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989, 49, 1857–1860. [Google Scholar]
- Huang, X.; Chen, S.; Xu, L.; Liu, Y.; Deb, D.K.; Platanias, L.C.; Bergan, R.C. Genistein Inhibits p38 Map Kinase Activation, Matrix Metalloproteinase Type 2, and Cell Invasion in Human Prostate Epithelial Cells. Cancer Res. 2005, 65, 3470–3478. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Markkanen, H.; Watanabe, S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 1993, 342, 1209–1210. [Google Scholar] [CrossRef]
- Xu, L.; Chen, S.; Bergan, R.C. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 2006, 25, 2987–2998. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G.; Yu, A.E. Proteases in invasion: Matrix metalloproteinases. Semin. Cancer Biol. 2001, 11, 143–152. [Google Scholar] [CrossRef]
- Zhang, H.; Gordon, R.; Li, W.; Yang, X.; Pattanayak, A.; Fowler, G.; Zhang, L.; Catalona, W.J.; Ding, Y.; Xu, L.; et al. Genistein treatment duration effects biomarkers of cell motility in human prostate. PLoS ONE 2019, 14, e0214078. [Google Scholar] [CrossRef]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.-i.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [CrossRef]
- Peterson, G.; Barnes, S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells. Cell Growth Differ. 1996, 7, 1345–1352. [Google Scholar]
- Spinozzi, F.; Pagliacci, M.C.; Migliorati, G.; Moraca, R.; Grignani, F.; Riccardi, C.; Nicoletti, I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk. Res. 1994, 18, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Zhang, L.; Lee, W.; Park, K. Genistein-induced G2/M arrest is associated with the inhibition of cyclin B1 and the induction of p21 in human breast carcinoma cells. Int. J. Oncol. 1998, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-L.; Kung, M.-L.; Chow, N.-H.; Su, S.-J. Genistein arrests hepatoma cells at G2/M phase: Involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochem. Pharmacol. 2004, 67, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Kim, B.S.; Chun, S.Y.; Park, Y.K.; Kang, K.S.; Kwon, T.G. Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J. Korean Med. Sci. 2011, 26, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.B.; Woo, S.U.; Chin, Y.W.; Jang, Y.J.; Yim, H. Sensitivity of TP53-mutated cancer cells to the phytoestrogen genistein is associated with direct inhibition of Plk1 activity. J. Cell. Physiol. 2017, 232, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef] [PubMed]
- Mae, T.; Kishida, H.; Nishiyama, T.; Tsukagawa, M.; Konishi, E.; Kuroda, M.; Mimaki, Y.; Sashida, Y.; Takahashi, K.; Kawada, T. A licorice ethanolic extract with peroxisome proliferator-activated receptor-γ ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats. J. Nutr. 2003, 133, 3369–3377. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.-Y. Antibacterial Compounds from Glycyrrhiza u ralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, J.-Y.; Suh, H.-J.; Lim, S.S.; Kim, J.-S. Antioxidant activities of licorice-derived prenylflavonoids. Nutr Res Pract. 2012, 6, 491–498. [Google Scholar] [CrossRef]
- Kim, J.-E.; Son, J.E.; Jung, S.K.; Kang, N.J.; Lee, C.Y.; Lee, K.W.; Lee, H.J. Cocoa polyphenols suppress TNF-α-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br. J. Nutr. 2010, 104, 957–964. [Google Scholar] [CrossRef]
- Lee, J.R.; Park, S.J.; Lee, H.-S.; Jee, S.Y.; Seo, J.; Kwon, Y.K.; Kwon, T.K.; Kim, S.C. Hepatoprotective activity of licorice water extract against Cadmium-induced toxicity in rats. Evid. Based Complement. Altern. Med. 2009, 6, 195–201. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Dong, X.-W.; Wu, X.-M.; Yan, X.-F.; Xie, Q.-M. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int. Immunopharmacol. 2009, 9, 194–200. [Google Scholar] [CrossRef]
- Yo, Y.-T.; Shieh, G.-S.; Hsu, K.-F.; Wu, C.-L.; Shiau, A.-L. Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J. Agric. Food Chem. 2009, 57, 8266–8273. [Google Scholar] [CrossRef]
- Niedel, J.E.; Kuhn, L.J.; Vandenbark, G.R. Phorbol diester receptor copurifies with protein kinase C. Proc. Natl. Acad. Sci. USA 1983, 80, 36–40. [Google Scholar] [CrossRef]
- Castagna, M.; Takai, Y.; Kaibuchi, K.; Sano, K.; Kikkawa, U.; Nishizuka, Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982, 257, 7847–7851. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.E.; Jang, Y.J.; Lee, C.C.; Lim, T.G.; Jung, S.K.; Lee, E.; Lim, S.S.; Heo, Y.S.; Seo, S.G. Dehydroglyasperin C suppresses TPA-induced cell transformation through direct inhibition of MKK4 and PI3K. Mol. Carcinog. 2016, 55, 552–562. [Google Scholar] [CrossRef]
- Weiß, D. Protoberberin-Alkaloide; Georg Thieme Verlag: Stuttgart, Germany, 2019. [Google Scholar]
- Wang, L.; Liu, L.; Shi, Y.; Cao, H.; Chaturvedi, R.; Calcutt, M.W.; Hu, T.; Ren, X.; Wilson, K.T.; Polk, D.B.; et al. Berberine Induces Caspase-Independent Cell Death in Colon Tumor Cells through Activation of Apoptosis-Inducing Factor. PLoS ONE 2012, 7, e36418. [Google Scholar] [CrossRef]
- Goto, H.; Kariya, R.; Shimamoto, M.; Kudo, E.; Taura, M.; Katano, H.; Okada, S. Antitumor effect of berberine against primary effusion lymphoma via inhibition of NF-κB pathway. Cancer Sci. 2012, 103, 775–781. [Google Scholar] [CrossRef]
- Kim, N.; Park, J.; Gadhe, C.G.; Cho, S.J.; Oh, Y.; Kim, D.; Song, K. A Protoberberine Derivative HWY336 Selectively Inhibits MKK4 and MKK7 in Mammalian Cells: The Importance of Activation Loop on Selectivity. PLoS ONE 2014, 9, e91037. [Google Scholar] [CrossRef]
- Deibler, K.K.; Schiltz, G.E.; Clutter, M.R.; Mishra, R.K.; Vagadia, P.P.; O’Connor, M.; George, M.D.; Gordon, R.; Fowler, G.; Bergan, R.; et al. Synthesis and Biological Evaluation of 3-Arylindazoles as Selective MEK4 Inhibitors. ChemMedChem 2019, 14, 615–620. [Google Scholar] [CrossRef]
- Kwong, A.J.; Pham, T.N.D.; Oelschlager, H.E.; Munshi, H.G.; Scheidt, K.A. Rational Design, Optimization, and Biological Evaluation of Novel MEK4 Inhibitors against Pancreatic Adenocarcinoma. ACS Med. Chem. Lett. 2021, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Ohren, J.F.; Chen, H.; Pavlovsky, A.; Whitehead, C.; Zhang, E.; Kuffa, P.; Yan, C.; McConnell, P.; Spessard, C.; Banotai, C.; et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004, 11, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Wuestefeld, T.; Pesic, M.; Rudalska, R.; Dauch, D.; Longerich, T.; Kang, T.-W.; Yevsa, T.; Heinzmann, F.; Hoenicke, L.; Hohmeyer, A. A Direct in vivo RNAi screen identifies MKK4 as a key regulator of liver regeneration. Cell 2013, 153, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Fingas, C.D.; Best, J.; Sowa, J.-P.; Canbay, A. Epidemiology of nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin. Liver Dis. 2016, 8, 119–122. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Ooshio, T.; Yamamoto, M.; Fujii, K.; Xin, B.; Watanabe, K.; Goto, M.; Okada, Y.; Suzuki, A.; Penninger, J.M.; Nishina, H.; et al. Hepatocyte Mitogen-Activated Protein Kinase Kinase 7 Contributes to Restoration of the Liver Parenchyma Following Injury in Mice. Hepatology 2021, 73, 2510–2526. [Google Scholar] [CrossRef]
- Kloevekorn, P.; Pfaffenrot, B.; Juchum, M.; Selig, R.; Albrecht, W.; Zender, L.; Laufer, S.A. From off-to on-target: New BRAF-inhibitor-template-derived compounds selectively targeting mitogen activated protein kinase kinase 4 (MKK4). Eur. J. Med. Chem. 2021, 210, 112963. [Google Scholar] [CrossRef]
- Pfaffenrot, B.; Klövekorn, P.; Juchum, M.; Selig, R.; Albrecht, W.; Zender, L.; Laufer, S.A. Design and synthesis of 1H-pyrazolo [3, 4-b] pyridines targeting mitogen-activated protein kinase kinase 4 (MKK4)-A promising target for liver regeneration. Eur. J. Med. Chem. 2021, 218, 113371. [Google Scholar] [CrossRef]
- Juchum, M.; Pfaffenrot, B.; Klövekorn, P.; Selig, R.; Albrecht, W.; Zender, L.; Laufer, S.A. Scaffold modified Vemurafenib analogues as highly selective mitogen activated protein kinase kinase 4 (MKK4) inhibitors. Eur. J. Med. Chem. 2022, 240, 114584. [Google Scholar] [CrossRef]
- Fabian, M.A.; Biggs, W.H.; Treiber, D.K.; Atteridge, C.E.; Azimioara, M.D.; Benedetti, M.G.; Carter, T.A.; Ciceri, P.; Edeen, P.T.; Floyd, M.; et al. A small molecule–kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 2005, 23, 329–336. [Google Scholar] [CrossRef]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Kircher, T.; Pantsar, T.; Oder, A.; Peter von Kries, J.; Juchum, M.; Pfaffenrot, B.; Kloevekorn, P.; Albrecht, W.; Selig, R.; Laufer, S. Design and synthesis of novel fluorescently labeled analogs of vemurafenib targeting MKK4. Eur. J. Med. Chem. 2021, 209, 112901. [Google Scholar] [CrossRef]
- Karoulia, Z.; Gavathiotis, E.; Poulikakos, P.I. New perspectives for targeting RAF kinase in human cancer. Nat. Rev. Cancer 2017, 17, 676–691. [Google Scholar] [CrossRef]
- Davies, L.; Jin, J.; Shen, W.; Tsui, H.; Shi, Y.; Wang, Y.; Zhang, Y.; Hao, G.; Wu, J.; Chen, S. Mkk4 Is a Negative Regulator of the Transforming Growth Factor Beta 1 S ignaling Associated with Atrial Remodeling and Arrhythmogenesis with Age. J. Am. Heart Assoc. 2014, 3, e000340. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef]
- Bozyczko-Coyne, D.; Saporito, S.M.; Hudkins, L.R. Targeting the JNK Pathway for Therapeutic Benefit in CNS Disease. Curr. Drug Targets-CNS Neurol. Disord. 2002, 1, 31–49. [Google Scholar] [CrossRef]
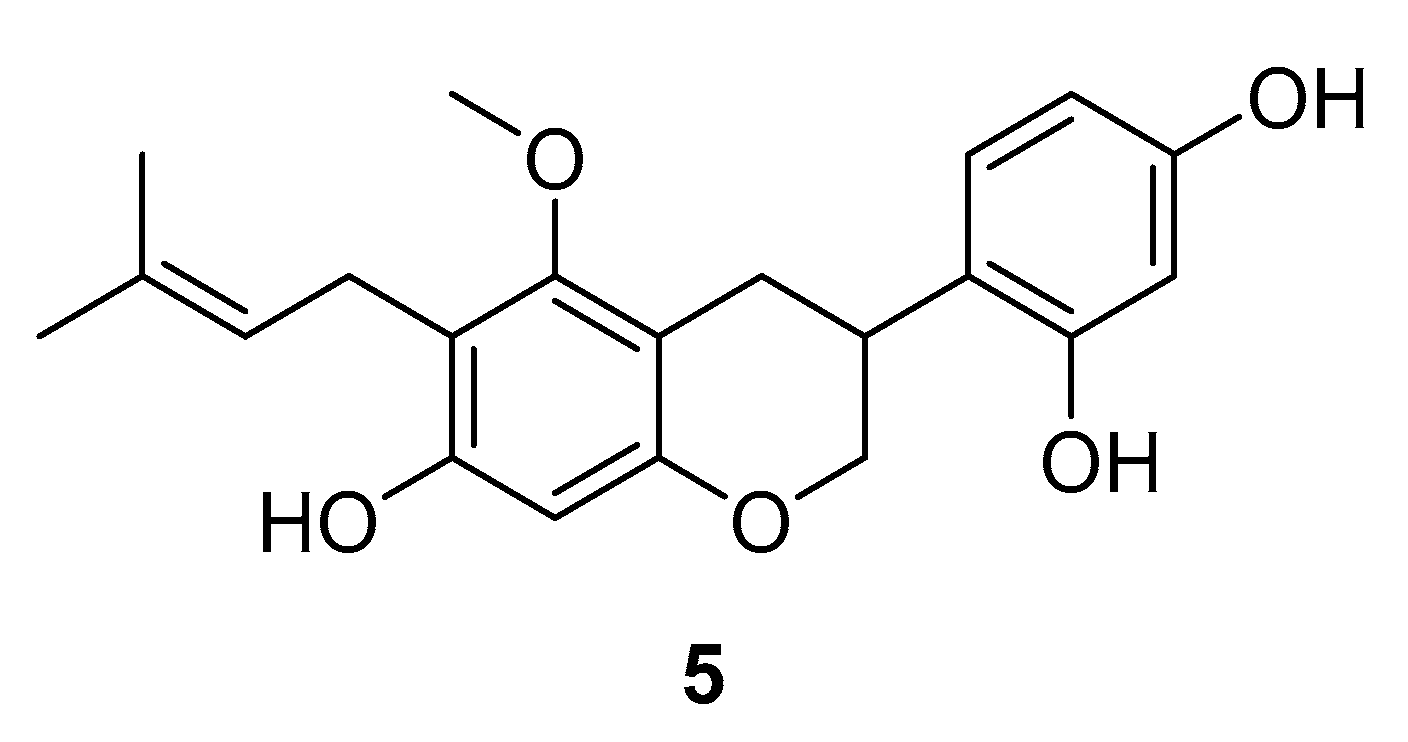
- Ogura, M.; Kikuchi, H.; Shakespear, N.; Suzuki, T.; Yamaki, J.; Homma, M.K.; Oshima, Y.; Homma, Y. Prenylated quinolinecarboxylic acid derivative prevents neuronal cell death through inhibition of MKK4. Biochem. Pharmacol. 2019, 162, 109–122. [Google Scholar] [CrossRef]
- Young, M.M.; Kester, M.; Wang, H.-G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzengruber, L.; Sander, P.; Laufer, S. MKK4 Inhibitors—Recent Development Status and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 7495. https://doi.org/10.3390/ijms24087495
Katzengruber L, Sander P, Laufer S. MKK4 Inhibitors—Recent Development Status and Therapeutic Potential. International Journal of Molecular Sciences. 2023; 24(8):7495. https://doi.org/10.3390/ijms24087495
Chicago/Turabian StyleKatzengruber, Leon, Pascal Sander, and Stefan Laufer. 2023. "MKK4 Inhibitors—Recent Development Status and Therapeutic Potential" International Journal of Molecular Sciences 24, no. 8: 7495. https://doi.org/10.3390/ijms24087495
APA StyleKatzengruber, L., Sander, P., & Laufer, S. (2023). MKK4 Inhibitors—Recent Development Status and Therapeutic Potential. International Journal of Molecular Sciences, 24(8), 7495. https://doi.org/10.3390/ijms24087495





