Thyroid Autoimmunity in CSU: A Potential Marker of Omalizumab Response?
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Statistical Analysis
4.4. Ethics
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Schmetzer, O.; Lakin, E.; Topal, F.A.; Preusse, P.; Freier, D.; Church, M.K.; Maurer, M. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2018, 142, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Grattan, C.E.; Francis, D.M.; Hide, M.; Greaves, M.W. Detection of circulating histamine releasing autoantibodies with functional properties of anti-IgE in chronic urticaria. Clin. Exp. Allergy 1991, 21, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Hide, M.; Francis, D.M.; Grattan, C.E.; Hakimi, J.; Kochan, J.P.; Greaves, M.W. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 1993, 328, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Gericke, J.; Metz, M.; Ohanyan, T.; Weller, K.; Altrichter, S.; Skov, P.S.; Falkencrone, S.; Brand, J.; Kromminga, A.; Hawro, T.; et al. Serum autoreactivity predicts time to response to omalizumab therapy in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2017, 139, 1059–1061.e1. [Google Scholar] [CrossRef]
- Endo, T.; Toyoshima, S.; Hayama, K.; Tagui, M.; Niwa, Y.; Ito, M.; Terui, T.; Okayama, Y. Relationship between changes in the 7-day urticaria activity score after treatment with omalizumab and the responsiveness of basophils to FcεRI stimulation in patients with chronic spontaneous urticaria. Asia Pac. Allergy 2020, 10, e12. [Google Scholar] [CrossRef]
- Schoepke, N.; Asero, R.; Ellrich, A.; Ferrer, M.; Gimenez-Arnau, A.; Grattan, C.E.H.; Jakob, T.; Konstantinou, G.N.; Raap, U.; Skov, P.S.; et al. Biomarkers and clinical characteristics of autoimmune chronic spontaneous urticaria: Results of the PURIST Study. Allergy 2019, 74, 2427–2436. [Google Scholar] [CrossRef]
- Ertaş, R.; Hawro, T.; Altrichter, S.; Özyurt, K.; Erol, K.; Ertaş, K.; Maurer, M. Antinuclear antibodies are common and linked to poor response to omalizumab treatment in patients with CSU. Allergy 2019, 75, 468–470. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Casazza, G.; Fierro, M.T.; Dapavo, P.; Crimi, N.; Ferrucci, S.; Pepe, P.; Liberati, S.; Pigatto, P.D.; et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: A study of 470 patients. J. Eur. Acad. Dermatol. Venereol. 2018, 33, 918–924. [Google Scholar] [CrossRef]
- Ertas, R.; Ozyurt, K.; Atasoy, M.; Hawro, T.; Maurer, M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy 2017, 73, 705–712. [Google Scholar] [CrossRef]
- Weller, K.; Ohanyan, T.; Hawro, T.; Ellrich, A.; Sussman, G.; Koplowitz, J.; Gimenez-Arnau, A.M.; Peveling-Oberhag, A.; Staubach, P.; Metz, M.; et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy 2018, 73, 2406–2408. [Google Scholar] [CrossRef]
- Straesser, M.D.; Oliver, E.; Palacios, T.; Kyin, T.; Patrie, J.; Borish, L.; Saini, S.S.; Lawrence, M.G. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J. Allergy Clin. Immunol. Pract. 2017, 6, 1386–1388.e1. [Google Scholar] [CrossRef]
- Nettis, E.; Cegolon, L.; Di Leo, E.; Lodi Rizzini, F.; Detoraki, A.; Canonica, G.W. Italian OCUReL study group. Omalizumab in chronic spontaneous urticaria: Efficacy, safety, predictors of treatment outcome, and time to response. Ann. Allergy Asthma. Immunol. 2018, 121, 474–478. [Google Scholar] [CrossRef]
- Asero, R. Chronic spontaneous urticaria treated with omalizumab: What differentiates early from late responders? Eur. Ann. Allergy Clin. Immunol. 2021, 53, 47–48. [Google Scholar] [CrossRef]
- Asero, R.; Ferrucci, S.; Casazza, G.; Marzano, A.V.; Cugno, M. Total IgE and atopic status in patients with severe chronic spontaneous urticaria unresponsive to omalizumab treatment. Allergy 2019, 74, 1561–1563. [Google Scholar] [CrossRef]
- Cugno, M.; Genovese, G.; Ferrucci, S.; Casazza, G.; Asero, R.; Marzano, A. IgE and D-dimer baseline levels are higher in responders than nonresponders to omalizumab in chronic spontaneous urticaria. Br. J. Dermatol. 2018, 179, 776–777. [Google Scholar] [CrossRef]
- Leznoff, A.; Josse, R.G.; Denburg, J.; Dolovich, J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch. Derm. 1983, 119, 636–640. [Google Scholar] [CrossRef]
- Kolkhir, P.; Borzova, E.; Grattan, C.; Asero, R.; Pogorelov, D.; Maurer, M. Autoimmune comorbidity in chronic spontaneous urticaria: A systematic review. Autoimmun. Rev. 2017, 16, 1196–1208. [Google Scholar] [CrossRef]
- Confino-Cohen, R.; Chodick, G.; Shalev, V.; Leshno, M.; Kimhi, O.; Goldberg, A. Chronic urticaria and autoimmunity: Associations found in a large population study. J. Allergy Clin. Immunol. 2012, 129, 1307–1313. [Google Scholar] [CrossRef]
- Kolkhir, P.; Altrichter, S.; Asero, R.; Daschner, A.; Ferrer, M.; Gimenez-Arnau, A.; Hawro, T.; Jakob, T.; Kinaciyan, T.; Kromminga, A.; et al. Autoimmune Diseases Are Linked to Type IIb Autoimmune Chronic Spontaneous Urticaria. Allergy Asthma. Immunol. Res. 2021, 13, 545–559. [Google Scholar] [CrossRef]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.K.; Maurer, M. Autoimmune chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Asero, R. Clinical variables of severe chronic spontaneous urticaria from total IgE standpoint: A retrospective study. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 30. [Google Scholar] [CrossRef] [PubMed]
- Leznoff, A.; Sussman, G. Syndrome of idiopathic chronic urticaria and angioedema with thyroid autoimmunity: A study of 90 patients. J. Allergy Clin. Immunol. 1989, 84, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Metz, M.; Altrichter, S.; Maurer, M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: A systematic review. Allergy 2017, 72, 1440–1460. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Francis, D.M.; Swana, G.T.; Seed, P.T.; Kobza Black, A.; Greaves, M.W. Thyroid autoimmunity in chronic urticarial. Br. J. Dermatol. 2005, 153, 331–335. [Google Scholar] [CrossRef]
- Kolkhir, P.; Kovalkova, E.; Chernov, A.; Danilycheva, I.; Krause, K.; Sauer, M.; Shulzhenko, A.; Fomina, D.; Maurer, M. Autoimmune Chronic Spontaneous Urticaria Detection with IgG Anti-TPO and Total IgE. J. Allergy Clin. Immunol. Pract. 2021, 9, 4138–4146. [Google Scholar] [CrossRef]

| Study Population (n = 385) | Thyroid + (n = 92) | Thyroid − (n = 293) |
|---|---|---|
| Non-response (n = 39) | 8 (9%) | 31 (11%) |
| Partial response (n = 62) | 17 (18%) | 45 (15%) |
| Late response (n = 84) | 22 (24%) | 62 (21%) |
| Early response (n = 200) | 45 (49%) | 155 (53%) |
| Patients (n = 44) | Weak Thyroid Autoimmunity (60–500 IU/mL; n = 22) | Strong Thyroid Autoimmunity (>500 IU/mL; n = 22) | p |
|---|---|---|---|
| Early response | 14 | 8 | NS |
| Late response | 5 | 6 | NS |
| Non-response | 0 | 3 | NS |
| Partial response | 3 | 5 | NS |
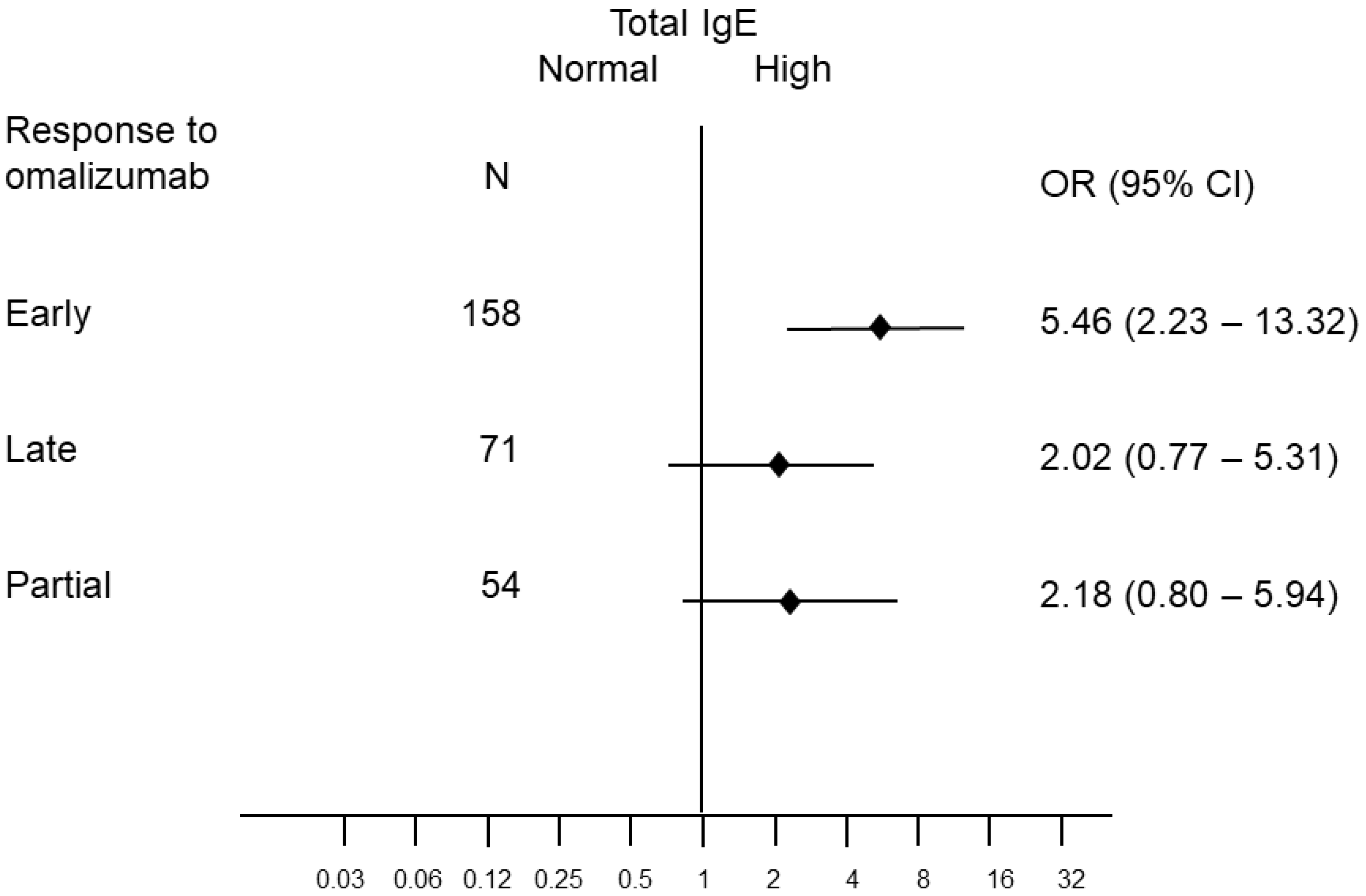
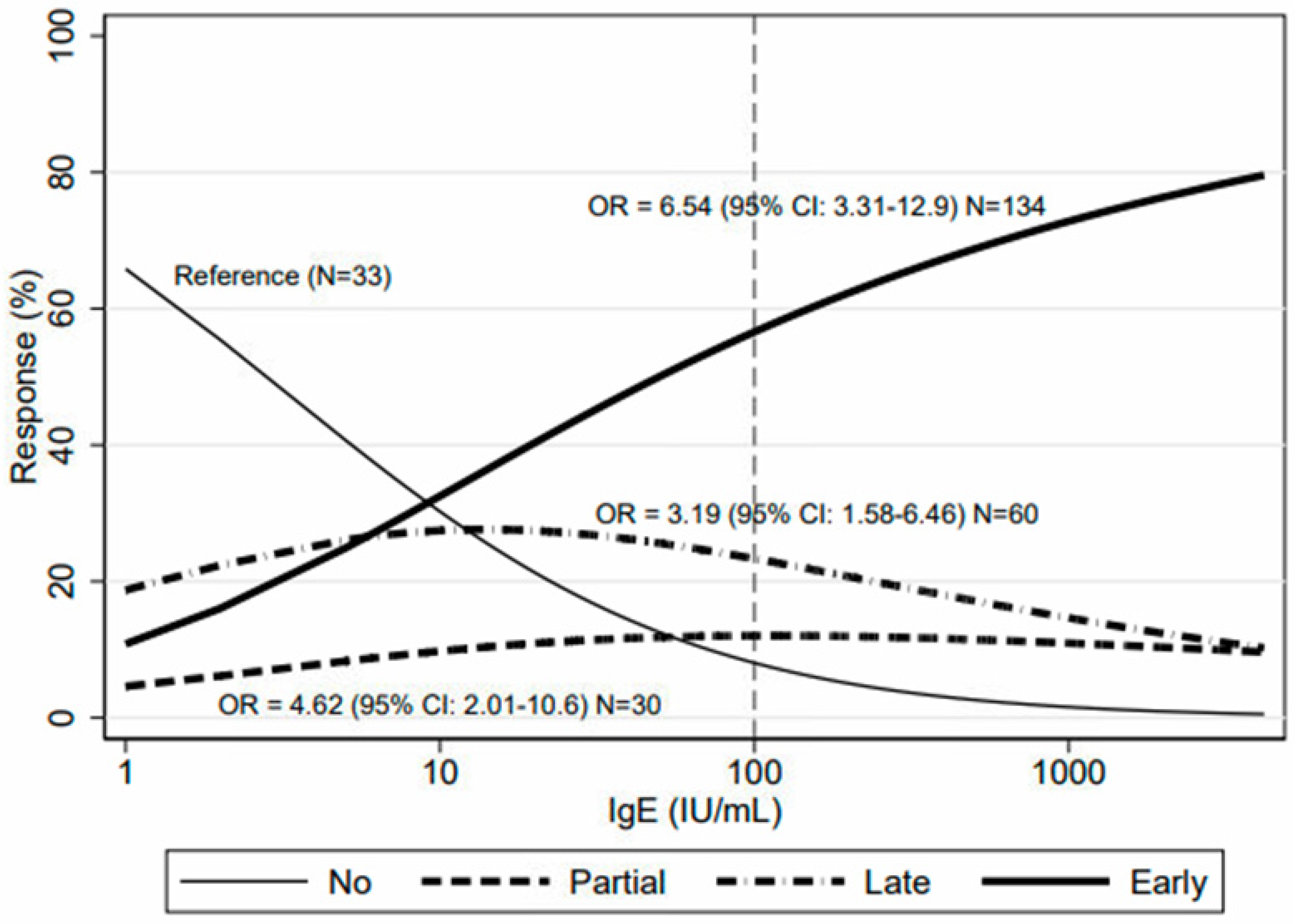
| Study Population (n = 316) | IgE >= 100 (n = 146) | IgE < 100 (n = 170) |
|---|---|---|
| Non-response (n = 33) | 7 (5%) | 26 (15%) |
| Partial response (n = 54) | 20 (14%) | 34 (20%) |
| Late response (n = 71) | 25 (17%) | 46 (27%) |
| Early response (n = 158) | 94 (64%) | 64 (38%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asero, R.; Ferrucci, S.M.; Calzari, P.; Consonni, D.; Cugno, M. Thyroid Autoimmunity in CSU: A Potential Marker of Omalizumab Response? Int. J. Mol. Sci. 2023, 24, 7491. https://doi.org/10.3390/ijms24087491
Asero R, Ferrucci SM, Calzari P, Consonni D, Cugno M. Thyroid Autoimmunity in CSU: A Potential Marker of Omalizumab Response? International Journal of Molecular Sciences. 2023; 24(8):7491. https://doi.org/10.3390/ijms24087491
Chicago/Turabian StyleAsero, Riccardo, Silvia Mariel Ferrucci, Paolo Calzari, Dario Consonni, and Massimo Cugno. 2023. "Thyroid Autoimmunity in CSU: A Potential Marker of Omalizumab Response?" International Journal of Molecular Sciences 24, no. 8: 7491. https://doi.org/10.3390/ijms24087491
APA StyleAsero, R., Ferrucci, S. M., Calzari, P., Consonni, D., & Cugno, M. (2023). Thyroid Autoimmunity in CSU: A Potential Marker of Omalizumab Response? International Journal of Molecular Sciences, 24(8), 7491. https://doi.org/10.3390/ijms24087491







