Understanding the Molecular Conformation and Viscoelasticity of Low Sol-Gel Transition Temperature Gelatin Methacryloyl Suspensions
Abstract
1. Introduction
2. Results
2.1. Amino Acid Composition of Salmon and Porcine Gelatins
2.2. Determination of Degree of Functionalization in GelMA
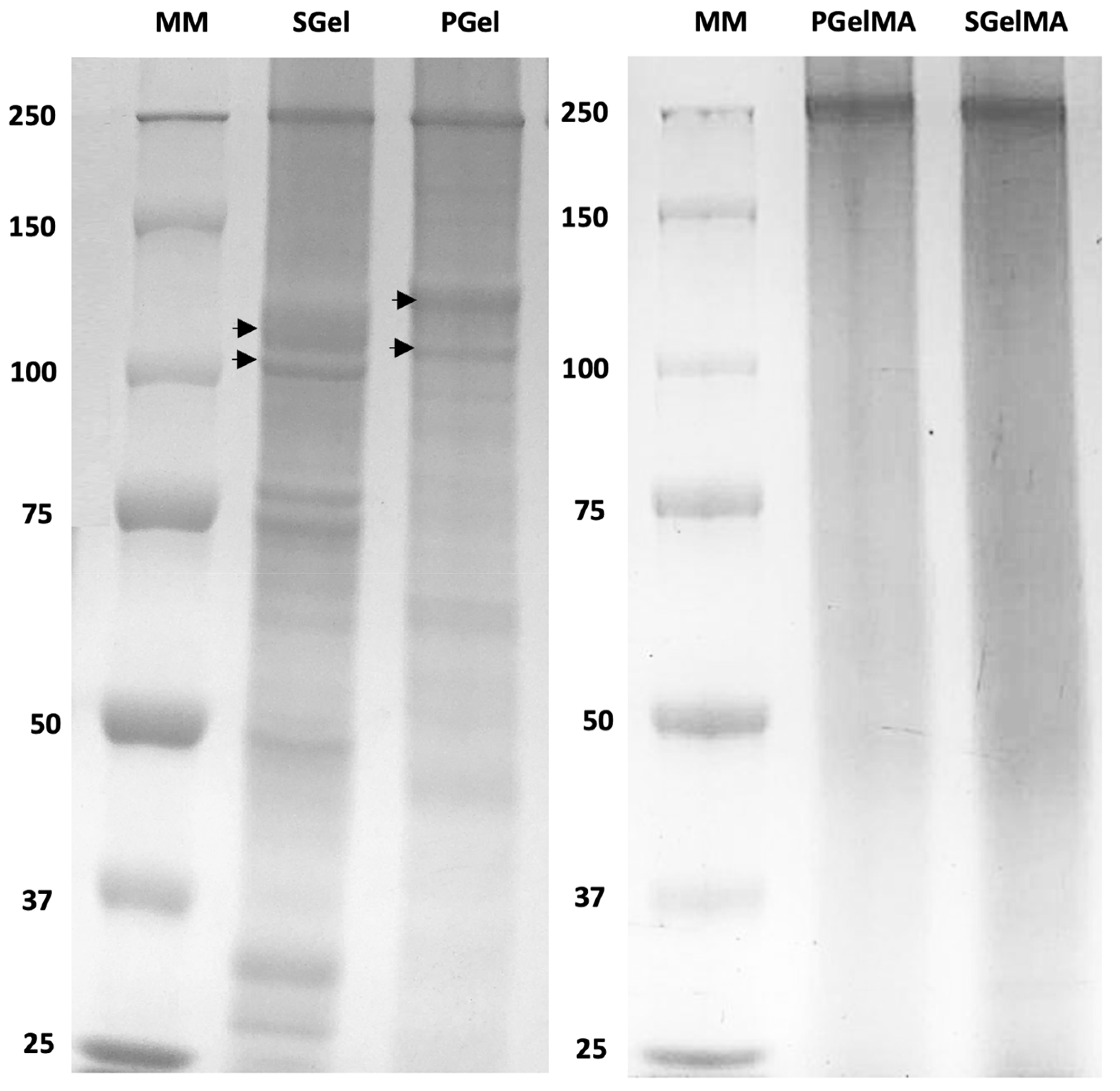
2.3. Molecular Weight and ζ-Potential of Salmon and Porcine Gelatins
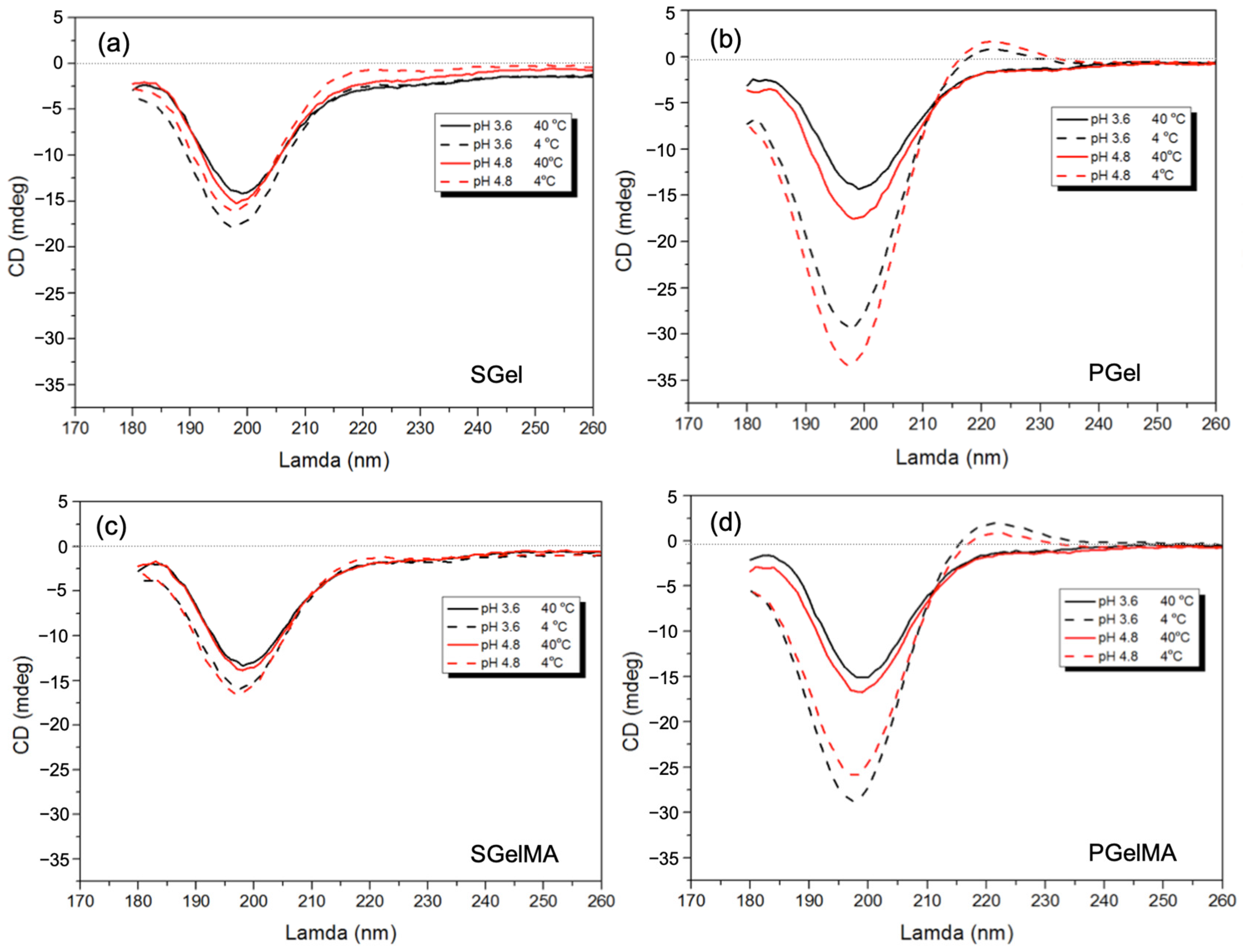
2.4. Secondary Structure Molecular Configuration
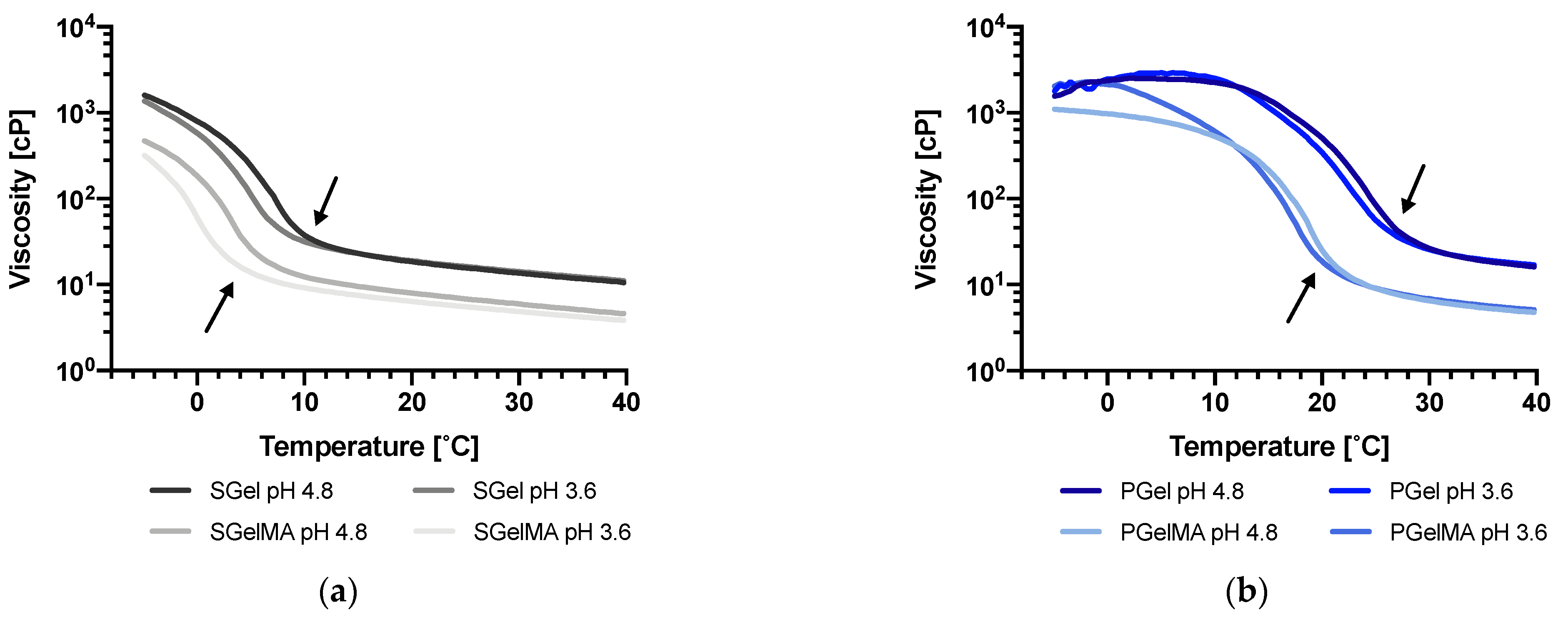
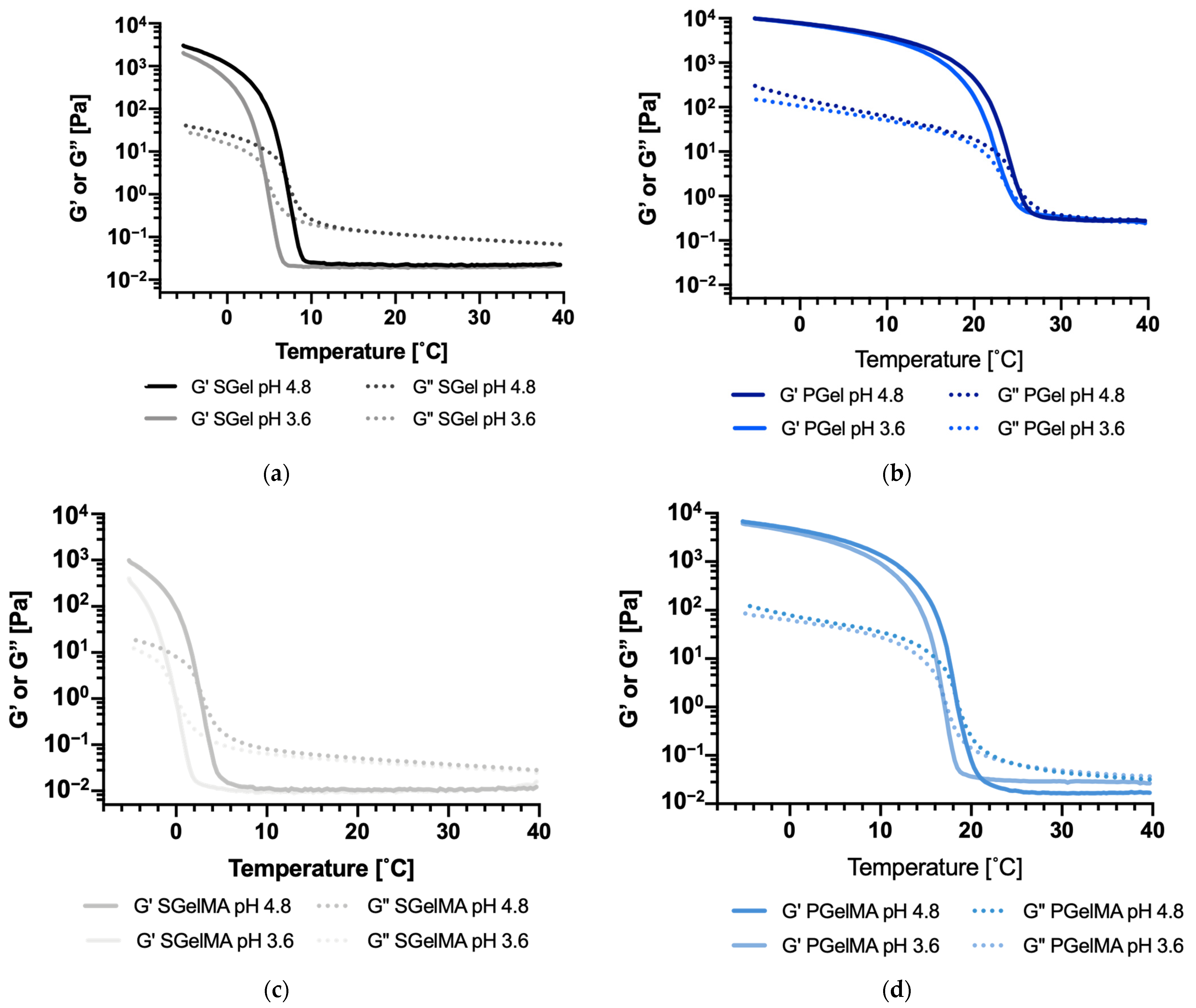
2.5. Rheological Characterization
2.6. Thermal Characterization by DSC
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Gelatin Production from Salmon Skin
4.3. Glycine, Proline and Hydroxyproline Content
4.4. Gelatin Functionalization
4.5. Determination of Degree of Functionalization in GelMA
4.5.1. O-Phtalaldehyde Method
4.5.2. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
4.6. Determination of Molecular Weight
4.6.1. SDS-PAGE
4.6.2. Capillary Viscometry
4.6.3. MALDI-TOF Mass Spectroscopy
4.7. ζ-Potential and Isoelectric Point
4.8. Secondary Structure Molecular Configuration
4.9. Rheological Measurements
4.10. Thermal Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karim, A.A.; Bhat, R. Gelatin Alternatives for the Food Industry: Recent Developments, Challenges and Prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for Developing Active Biodegradable Films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Alfaro, A.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Papon, P.; Leblond, J.; Meijer, P.H.E. Gelation and Transitions in Biopolymers. In The Physics of Phase Transitions. Advanced Texts in Physics; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Bella, J.; Brodsky, B.; Berman, H.M. Hydration Structure of a Collagen Peptide. Structure 1995, 3, 893–906. [Google Scholar] [CrossRef]
- Gornall, J.L.; Terentjev, E.M. Concentration-Temperature Superposition of Helix Folding Rates in Gelatin. Phys. Rev. Lett. 2007, 99, 1–4. [Google Scholar] [CrossRef]
- Díaz-Calderón, P.; Flores, E.; González-Muñoz, A.; Pepczynska, M.; Quero, F.; Enrione, J. Influence of Extraction Variables on the Structure and Physical Properties of Salmon Gelatin. Food Hydrocoll. 2017, 71, 118–128. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Joly-Duhamel, C.; Hellio, D.; Djabourov, M. All Gelatin Networks: 1. Biodiversity and Physical Chemistry. Langmuir 2002, 18, 7208–7217. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent Advances in the Use of Gelatin in Biomedical Research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical Tailoring of Gelatin to Adjust Its Chemical and Physical Properties for Functional Bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Wang, J. Photo-Crosslinkable Hydrogel and Its Biological Applications. Chin. Chem. Lett. 2021, 32, 1603–1614. [Google Scholar] [CrossRef]
- van den Bulcke, A.I.; Bogdanov, B.; de Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Rebers, L.; Granse, T.; Tovar, G.E.M.; Southan, A.; Borchers, K. Physical Interactions Strengthen Chemical Gelatin Methacryloyl Gels. Gels 2019, 5, 4. [Google Scholar] [CrossRef]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, E.M.; Borchers, K. Stiff Gelatin Hydrogels Can Be Photo-Chemically Synthesized from Low Viscous Gelatin Solutions Using Molecularly Functionalized Gelatin with a High Degree of Methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Peh, G.S.L.; Ang, H.P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-Crosslinked Bioactive Hydrogels as Nano-Patterned Substrates with Customizable Stiffness and Degradation for Corneal Tissue Engineering Applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef]
- Kim, C.; Young, J.L.; Holle, A.W.; Jeong, K.; Major, L.G.; Jeong, J.H.; Aman, Z.M.; Han, D.W.; Hwang, Y.; Spat, J.P.; et al. Stem Cell Mechanosensation on Gelatin Methacryloyl (GelMA) Stiffness Gradient Hydrogels. Ann. Biomed. Eng. 2020, 48, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiang, Y.; Fang, J.; Li, X.; Lin, Z.; Dai, G.; Yin, J.; Wei, P.; Zhang, D. The influence of the stiffness of GelMA substrate on the outgrowth of PC12 cells. Biosci. Rep. 2019, 39, BSR20181748. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.W.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Biomaterials Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 7, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lang, Q.; Zhang, H.; Cheng, L.; Zhang, Y.; Pan, G.; Zhao, X.; Yang, H.; Zhang, Y.; Santos, H.A.; et al. Electrospun Photocrosslinkable Hydrogel Fibrous Scaffolds for Rapid In Vivo Vascularized Skin Flap Regeneration. Adv. Funct. Mater. 2017, 27, 1604617. [Google Scholar] [CrossRef]
- Shie, M.Y.; Lee, J.J.; Ho, C.C.; Yen, S.Y.; Ng, H.Y.; Chen, Y.-W. Effects of Gelatin Methacrylate Bio-ink Concentration on Mechano-Physical Properties and Human Dermal Fibroblast Behavior. Polymers 2020, 12, 1930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Ye, P.; Gao, G.; Hubbell, K.; Cui, X. Coculture of mesenchymal stem cells and endothelial cells enhances host tissue integration and epidermis maturation through AKT activation in gelatin methacryloyl hydrogel-based skin model. Acta Biomater. 2017, 59, 317–326. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Li, M.N.; Yu, H.P.; Ke, Q.F.; Zhang, C.Q.; Gao, Y.S.; Guo, Y.P. Gelatin methacryloyl hydrogels functionalized with endothelin-1 for angiogenesis and full-thickness wound healing. J. Mater. Chem. B 2021, 9, 4700–4709. [Google Scholar] [CrossRef] [PubMed]
- Modaresifara, K.; Hadjizadeha, A.; Niknejadb, H. Design and fabrication of GelMA/chitosan nanoparticles composite hydrogel for angiogenic growth factor delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin Methacryloyl Hydrogels Control the Localized Delivery of Albumin-Bound Paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative Study of Gelatin Methacrylate Hydrogels from Different Sources for Biofabrication Applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef]
- Zaupa, A.; Byres, N.; Dal Zovo, C.; Acevedo, C.A.; Angelopoulos, I.; Terraza, C.; Nestle, N.; Abarzúa-Illanes, P.N.; Quero, F.; Díaz-Calderón, P.; et al. Cold-Adaptation of a Methacrylamide Gelatin towards the Expansion of the Biomaterial Toolbox for Specialized Functionalities in Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 373–390. [Google Scholar] [CrossRef]
- Aguirre-Alvarez, G.; Foster, T.; Hill, S.E. Impact of the Origin of Gelatins on Their Intrinsic Properties. CyTA J. Food 2012, 10, 306–312. [Google Scholar] [CrossRef]
- Diaz, P.; López, D.; Matiacevich, S.; Osorio, F.; Enrione, J. State Diagram of Salmon (Salmo Salar) Gelatin Films. J. Sci. Food Agric. 2011, 91, 2558–2565. [Google Scholar] [CrossRef]
- Elharfaoui, N.; Djabourov, M.; Babel, W. Molecular Weight Influence on Gelatin Gels: Structure, Enthalpy and Rheology. Macromol. Symp. 2007, 256, 149–157. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Gómez-Guillén, M.C. Physico-Chemical and Film-Forming Properties of Bovine-Hide and Tuna-Skin Gelatin: A Comparative Study. J. Food Eng. 2009, 90, 480–486. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and Rheological Properties of Fish Gelatin Compared to Mammalian Gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Eysturskarð, J.; Haug, I.J. Mechanical Properties of Mammalian and Fish Gelatins as a Function of the Contents of α-Chain, β-Chain, and Low and High Molecular Weight Fractions. Food Biophys. 2010, 5, 9–16. [Google Scholar] [CrossRef]
- Char, C.; Padilla, C.; Campos, V.; Pepczynska, M.; Calderón, P.D. Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings 2019, 9, 595. [Google Scholar] [CrossRef]
- Enrione, J.I.; Sáez, C.; López, D.; Skurtys, O.; Acevedo, C.; Osorio, F.; Macnaughtan, W.; Hill, S. Structural Relaxation of Salmon Gelatin Films in the Glassy State. Food Bioproc. Tech. 2012, 5, 2446–2453. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.; Kim, J.; Do, T.; Song, H.; Bae, H. Cold Water Fish Gelatin Methacryloyl Hydrogel for Tissue Engineering Application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef]
- Zaupa, A.; Terraza, C.; Abarzúa-Illanes, P.N.; Byres, N.; Zavala, G.; Cuenca, J.; Hidalgo, C.; Viafara-Garcia, S.M.; Wolf, B.; Pino-Lagos, K.; et al. A Psychrophilic GelMA: Breaking Technical and Immunological Barriers for Multimaterial High-Resolution 3D Bioprinting. Biomacromolecules 2023, 24, 150–165. [Google Scholar] [CrossRef]
- Nurul, A.G.; Sarbon, N.M. Effects of PH on Functional, Rheological and Structural Properties of Eel (Monopterus Sp.) Skin Gelatin Compared to Bovine Gelatin. Int. Food Res. J. 2015, 22, 572–583. [Google Scholar]
- Arnesen, J.A.; Gildberg, A. Extraction and Characterisation of Gelatine from Atlantic Salmon (Salmo Salar) Skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. Vol. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Chiou, B.; Avena-bustillos, R.J.; Shey, J.; Yee, E.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Rheological and Mechanical Properties of Cross-Linked Fish Gelatins. Polymer 2006, 47, 6379–6386. [Google Scholar] [CrossRef]
- Enrione, J.; Char, C.; Pepczynska, M.; Padilla, C.; González-Muñoz, A.; Olguín, Y.; Quinzio, C.; Iturriaga, L.; Díaz-Calderón, P. Rheological and Structural Study of Salmon Gelatin with Controlled Molecular Weight. Polymers 2020, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Shirahama, H.; Cho, N.-J.; Tan, L.P. Efficient and Controllable Synthesis of Highly Substituted Gelatin Methacrylamide for Mechanically Stiff Hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Yue, K.; Li, X.; Schrobback, K.; Sheikhi, A.; Annabi, N.; Leijten, J.; Zhang, W.; Zhang, Y.S.; Hutmacher, D.W.; Klein, T.J.; et al. Structural Analysis of Photocrosslinkable Methacryloyl-Modified Protein Derivatives. Biomaterials 2017, 139, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Quero, F.; Padilla, C.; Campos, V.; Luengo, J.; Caballero, L.; Melo, F.; Li, Q.; Eichhorn, S.J.; Enrione, J. Stress Transfer and Matrix-Cohesive Fracture Mechanism in Microfibrillated Cellulose-Gelatin Nanocomposite Films. Carbohydr. Polym. 2018, 195, 89–98. [Google Scholar] [CrossRef]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of Gelatin Source and Photoinitiator Type on Chondrocyte Redifferentiation in Gelatin Methacryloyl-Based Tissue-Engineered Cartilage Constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef]
- Smejkal, G.B. The Coomassie Chronicles: Past, Present and Future Perspectives in Polyacrylamide Gel Staining. Expert. Rev. Proteom. 2004, 1, 381–387. [Google Scholar] [CrossRef]
- van Hoorick, J.; Gruber, P.; Markovic, M.; Tromayer, M.; van Erps, J.; Thienpont, H.; Liska, R.; Ovsianikov, A.; Dubruel, P.; van Vlierberghe, S. Cross-Linkable Gelatins with Superior Mechanical Properties Through Carboxylic Acid Modification: Increasing the Two-Photon Polymerization Potential. Biomacromolecules 2017, 18, 3260–3272. [Google Scholar] [CrossRef]
- Sewald, L.; Claaßen, C.; Götz, T.; Claaßen, M.H.; Truffault, V.; Tovar, G.E.M.; Southan, A.; Borchers, K. Beyond the Modification Degree: Impact of Raw Material on Physicochemical Properties of Gelatin Type A and Type B Methacryloyls. Macromol. Biosci. 2018, 18, e1800168. [Google Scholar] [CrossRef]
- de Wolf, F.A.; Keller, R.C.A. Characterization of Helical Structures in Gelatin Networks and Model Polypeptides by Circular Dichroism. In Progress in Colloid & Polymer Science; Zrínyi, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 102. [Google Scholar]
- Gomez-Guillen, M.C.; Turnay, J.; Fernandez-Diaz, M.D.; Ulmo, M.; Lizarbe, M.A.; Montero, P. Structural and Physical Properties of Gelatin Extracted from Different Marine Species: A Comparative Study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Lopes, J.L.S.; Miles, A.J.; Whitmore, L.; Wallace, B.A. Distinct Circular Dichroism Spectroscopic Signatures of Polyproline II and Unordered Secondary Structures: Applications in Secondary Structure Analyses. Protein Sci. 2014, 23, 1765–1772. [Google Scholar] [CrossRef]
- Wetzel, R.; Buder, E.; Hermel, H.; Hiittner, A. Polymer Science Conformations of Different Gelatins in Solutions and in Films An Analysis of Circular Dichroism (CD) Measurements. Colloid Polym. Sci. 1987, 265, 1036–1045. [Google Scholar] [CrossRef]
- Wustneck, R.; Wetzel, R.; Buder, E.; Hermel, H. The Modification of the Triple Helical Structure of Gelatin in Aqueous Solution. I. The Influence of Anionic Surfactants, PH-Value, and Temperature. Colloid Polym. Sci. 1988, 266, 1061–1067. [Google Scholar] [CrossRef]
- Tiffany, M.L.; Krimm, S. Circular Dichroism of the “Random” Polypeptide Chain. Biopolymers 1969, 8, 347–359. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin Methacryloyl and Its Hydrogels with an Exceptional Degree of Controllability and Batch-to-Batch Consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef]
- Azilawati, M.I.; Hashim, D.M.; Jamilah, B.; Amin, I. RP-HPLC Method Using 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate Incorporated with Normalization Technique in Principal Component Analysis to Differentiate the Bovine, Porcine and Fish Gelatins. Food Chem. 2015, 172, 368–376. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Badii, F.; MacNaughtan, W.; Mitchell, J.R.; Farhat, I.A. The Effect of Drying Temperature on Physical Properties of Thin Gelatin Films. Dry. Technol. 2014, 32, 30–38. [Google Scholar] [CrossRef]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. A Sensitive Method for Free Amino Acids Analysis by Liquid Chromatography with Ultraviolet and Mass Spectrometric Detection Using Precolumn Derivatization with Diethyl Ethoxymethylenemalonate: Application to the Honey Analysis. Anal. Chim. Acta 2010, 672, 79–84. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Harding, S.E. The Intrinsic Viscosity of Biological Macromolecules. Progress in Measurement, Interpretation and Application to Structure in Dilute Solution. Prog. Biophys Mo.l Biol. 1997, 68, 207–262. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Liu, B.; Lei, Y.T.; Yang, S.L. Structure Analysis of a Highly Hydrophilic Recombinant Human-Source Gelatin. Chem. Sci. Trans. 2012, 1, 347–354. [Google Scholar] [CrossRef]

| Amino Acid | Content [mmol/g] | % From Total Amino Acids | ||
|---|---|---|---|---|
| SGel | PGel | SGel | PGel | |
| Glycine | 2.40 ± 0.02 | 2.60 ± 0.03 | 25.07 ± 0.24 | 28.33 ± 0.18 |
| Proline | 0.57 ± 0.01 | 0.88 ± 0.01 | 9.08 ± 0.01 | 14.79 ± 0.01 |
| Hydroxyproline | 0.45 ± 0.02 | 0.76 ± 0.01 | 8.19 ± 0.27 | 14.62 ± 0.19 |
| Lysine | 0.19 ± 0.01 | 0.18 ± 0.01 | 3.78 ± 0.06 | 3.95 ± 0.09 |
| Sample | DF [%] by OPA | DF [%] by 1H-NMR | [η] [mL/g] | Mw [kDa] | IEP at 25 °C [mV] |
|---|---|---|---|---|---|
| SGel | - | - | 34.17 ± 0.25 | 73.40 ± 0.73 | 9.2 |
| PGel | - | - | 38.70 ± 0.69 | 86.87 ± 2.09 | 9.4 |
| SGelMA | 96.3 ± 0.3 | 91.9 | 14.61 ± 0.13 | 23.28 ± 0.27 | 4.9 |
| PGelMA | 96.5 ± 0.1 | 83.2 | 13.44 ± 0.03 | 20.81 ± 0.06 | 4.8 |
| Sample | Tgel pH 4.8 [°C] | Tgel pH 3.6 [°C] | Tgel [°C] |
|---|---|---|---|
| SGel | 7.0 ± 0.2 | 4.5 ± 0.2 | 2.5 |
| PGel | 25.3 ± 0.1 | 24.2 ± 0.2 | 1.1 |
| SGelMA | 2.4 ± 0.3 | −0.4 ± 0.6 | 2.8 |
| PGelMA | 18.5 ± 0.3 | 17.2 ± 0.2 | 1.3 |
| Sample | 10% w/v | 20% w/v | ||
|---|---|---|---|---|
| Normalized ΔHgel [J/g] | Tonset [°C] | Normalized ΔHgel [J/g] | Tonset [°C] | |
| SGel pH 4.8 | 4.9 ± 0.9 | 10.2 ± 0.5 | 6.5 ± 0.3 | 10.6 ± 0.1 |
| SGel pH 3.6 | 2.9 ± 2.0 | 7.2 ± 0.7 | 5.7 ± 1.1 | 7.6 ± 0.4 |
| SGelMA pH 3.6 | 2.5 ± 0.4 | 7.0 ± 0.6 | 3.7 ± 0.7 | 8.6 ± 2.2 |
| PGel pH 4.8 | 2.8 ± 1.1 | 22.4 ± 1.4 | 9.2 ± 4.1 | 27.3 ± 1.4 |
| PGel pH 3.6 | 3.9 ± 0.6 | 20.8 ± 1.8 | 6.4 ± 0.6 | 22.6 ± 0.4 |
| PGelMA pH 3.6 | 2.0 ± 0.1 | 18.8 ± 1.3 | 2.7 ± 0.9 | 20.8 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla, C.; Quero, F.; Pępczyńska, M.; Díaz-Calderon, P.; Acevedo, J.P.; Byres, N.; Blaker, J.J.; MacNaughtan, W.; Williams, H.E.L.; Enrione, J. Understanding the Molecular Conformation and Viscoelasticity of Low Sol-Gel Transition Temperature Gelatin Methacryloyl Suspensions. Int. J. Mol. Sci. 2023, 24, 7489. https://doi.org/10.3390/ijms24087489
Padilla C, Quero F, Pępczyńska M, Díaz-Calderon P, Acevedo JP, Byres N, Blaker JJ, MacNaughtan W, Williams HEL, Enrione J. Understanding the Molecular Conformation and Viscoelasticity of Low Sol-Gel Transition Temperature Gelatin Methacryloyl Suspensions. International Journal of Molecular Sciences. 2023; 24(8):7489. https://doi.org/10.3390/ijms24087489
Chicago/Turabian StylePadilla, Cristina, Franck Quero, Marzena Pępczyńska, Paulo Díaz-Calderon, Juan Pablo Acevedo, Nicholas Byres, Jonny J. Blaker, William MacNaughtan, Huw E. L. Williams, and Javier Enrione. 2023. "Understanding the Molecular Conformation and Viscoelasticity of Low Sol-Gel Transition Temperature Gelatin Methacryloyl Suspensions" International Journal of Molecular Sciences 24, no. 8: 7489. https://doi.org/10.3390/ijms24087489
APA StylePadilla, C., Quero, F., Pępczyńska, M., Díaz-Calderon, P., Acevedo, J. P., Byres, N., Blaker, J. J., MacNaughtan, W., Williams, H. E. L., & Enrione, J. (2023). Understanding the Molecular Conformation and Viscoelasticity of Low Sol-Gel Transition Temperature Gelatin Methacryloyl Suspensions. International Journal of Molecular Sciences, 24(8), 7489. https://doi.org/10.3390/ijms24087489






