Abstract
Chronic spontaneous urticaria (CSU) is a common skin disorder characterized by daily or almost daily recurring skin edema and flare with itch and pruritus anywhere on the body for more than 6 weeks. Although basophil- and mast cell-released inflammatory mediators, such as histamine, play important roles in the pathogenesis of CSU, the detailed underlying mechanism is not clear. Since several auto-antibodies, IgGs which recognize IgE or the high-affinity IgE receptor (FcεRI) and IgEs against other self-antigens, are detected in CSU patients, they are considered to activate both mast cells in the skin and basophils circulating in the blood. In addition, we and other groups demonstrated that the coagulation and complement system also contribute to the development of urticaria. Here, we summarized the behaviors, markers and targets of basophils in relation to the coagulation–complement system, and for the treatment of CSU.
1. Pathogenesis of CSU
Urticaria is a common skin disease characterized by repetitive and transient appearance of skin edema and flare, mostly with itch, anywhere on the body [1]. It is classified based on the time from onset; acute urticaria with a course less than 6 weeks, and chronic urticaria lasting for 6 weeks or longer [1]. Lifetime prevalence of chronic urticaria is considered around 4%, with a substantial variation of time point prevalence among regions of the globe from 0.5% in Europe to 1.4% in Asia [2]. Chronic urticaria is further divided into chronic spontaneous urticaria (CSU), also called chronic idiopathic urticaria (CIU), and chronic inducible urticaria (CIndU). CSU is characterized by daily or almost daily recurring skin edema and flare without an apparent trigger for each emergence of eruption, whereas CIndU is characterized by the occurrence of wheals in response to specific stimuli, such as temperature, light and mechanical stress. Considerable evidence highlights the major and critical roles of mast cells in the skin and basophils in the skin or peripheral blood. However, the exact mechanism by which mast cells and basophils are activated in the skin and peripheral blood in patients with CSU is not yet clear. Approximately 40% of patients with CSU have IgG autoantibodies against IgE antibodies and/or the high-affinity IgE receptors (FcεRIs) (type IIb autoimmune) [3,4,5] (Figure 1). Moreover, IgE autoantibodies against several self-molecules, such as Interleukin (IL)-24, thyroid peroxidase (TPO), eosinophil peroxidase (EPO), eosinophilic cationic protein (ECP), double-strand deoxyribonucleic acid (dsDNA) and tissue factor (TF), which induce type I autoimmune CSU, have also been detected in certain populations of patients with CSU (type I autoallergy) [6,7,8,9,10,11] (Figure 1).
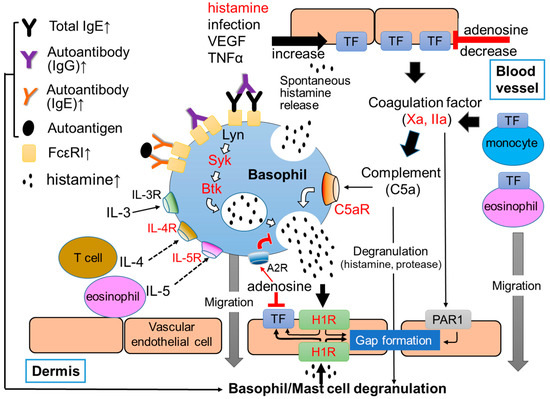
Figure 1.
Summarized image of the role of basophils in CSU. Red characters show targets of current and/or developing for CSU. Upward arrows at the end of molecule names indicate the elevation of their concentration in sera of patients with CSU.
A high efficacy of anti-IgE antibodies, such as omalizumab and ligelizumab, suggests the critical role of IgE antibodies in the serum of the pathogenesis of CSU [5]. In addition, the effectiveness of H1 anti-histamines for the treatment of CSU supports the importance of histamine, which is stored and released from skin mast cells and/or peripheral blood basophils, as well [12,13]. However, histamine release activity of such autoantibodies in type IIb autoimmune and autoantigens via auto-IgE in type I autoallergy CSUs is not very potent and does not change in the body of patients in accordance with their clinical symptoms. Moreover, these autoimmunities are not apparent in all patients with CSU. Therefore, the pathogenesis of CSU by autoantibody-independent mechanisms should also be considered in order to understand the whole picture of CSU. Recently, the increase of various substances and cytokines, such as substance P (SP), C-reactive protein (CRP), tumor necrosis factor (TNF)α, IL-1β, -6, -17, -31, -33, and transforming growth factor (TGF)β, has been reported in the plasma of patients with CSU [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. In addition, coagulation/fibrinolysis factors in the plasma of CSU patients has also been reported, whereas the levels of IL-35 and vitamin D in the plasma of patients with CSU were decreased [16,17]. Moreover, various medications that target cytokines and receptors, such as IL-4, IL-5, IL-13, thymic stromal lymphopoietin (TSLP), complement 5a (C5a), C5a receptor (C5aR), sialic acid-binding immunoglobulin-like lectins 8 (siglec-8) and chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells (CRTH2), are being investigated in clinical trials for the treatment of CSU. These findings suggest that the pathogenesis of CSU is much more complicated than previously understood [17,18,19,20].
2. Characteristics of Basophils and Mast Cells
It is well known that basophils and mast cells are important cells which contribute to the induction of inflammatory and/or allergic responses, especially type I allergy. Both human basophils and mast cells develop from hematopoietic stem cells into precursor cells of each lineage in the bone marrow. They share several characteristics, such as the presence of secretory granules showing metachromasia and the expression of FcεRI [21,22]. Cross-linkage of IgE antibodies bound to FcεRIs by a multivalent antigen, also called allergen, induce the phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM) domain of FcεRI, resulting in the activation of tyrosine kinase, such as Lyn, spleen tyrosine kinase (Syk) and Bruton’s tyrosine Kinase (Btk), in both basophils and mast cells (Figure 1). Activation of these molecules then activate the functions of multiple intracellular molecules, such as protein lipase C (PLC), phosphoinositide 3-kinase (PI3K), mitogen-activated protein (MAP) kinases, protein kinase C (PKC), and increase Ca2+ concentration in cytoplasm, resulting in the release of preformed chemical mediators, such as histamine, protease and platelet-activating factor (PAF), stored in secretory granules. Basophils and mast cells also release newly synthesized inflammatory lipid mediators, including arachidonic acid metabolites, such as leukotriene (LT) and prostaglandin (PG), and inflammatory cytokines, such as IL-4 and IL-13 [21,22,23].
However, the process for maturation of basophils and mast cells are largely different. Precursor cells of basophil become mature basophils in response to IL-3 in the bone marrow and then circulate in the blood, accounting for only less than 1% of peripheral blood leukocytes [24,25]. Basophils may migrate into tissues with inflammation out of blood vessels. On the other hand, precursor cells of mast cells, that develop in the bone marrow, migrate into tissues, and mature in response to stem cell factor (SCF) under the influence of surrounding tissues. Human mast cells are divided into two groups, MCTC and MCT, according to the expression of proteases, tryptase and chymase [26]. MCTC, expressing tryptase and chymase, resides in connective tissues, such as skin, and peritoneal cavity. MCT, only expressing tryptase, resides in mucosal tissues. Although MCTC expresses C5aR on their surface, MCT do not have C5aR [26]. The increase of serum tryptase can be taken as a marker of mast cell activation, because basophils do not release tryptase [21,22]. In addition, the lifetime of circulating basophils is only a few days (around 3 days), whereas it tends to be a few months or longer for tissue-resident mast cells [21,22].
Since basophils are a major source of IL-4, as well as in peripheral blood mononuclear cells, basophils are considered to play critical roles in type 2 inflammations and IgE antibody-associated allergic disorders (type I allergy), such as urticaria, asthma, pollen allergy, food allergy, anaphylactic shock and atopic dermatitis (AD) [21,22]. In addition, basophils express the IL-3 receptor (IL-3R) on the surface of cells, which play important roles for the enhancement of antigen-IgE antibody interaction-induced histamine release, as well as the development and survival of basophils [27].
Human skin mast cells, but not peripheral basophils, express mas-related G-protein coupled receptor member X2 (MRGPRX2), which is activated by various molecules, such as neuropeptides, including SP [28]. However, a recent study reported that basophils also quickly express MRGPRX2 on the surface of cells after FcεRI-dependent activation [29]. As inhibitory molecules of basophils and mast cells, we found adenosine, a metabolite of adenosine triphosphate (ATP), suppressed histamine release from human peripheral blood basophils and skin mast cells via A2a and A2b adenosine receptors, respectively [30]. Therefore, complement factors, proteases, neuropeptides and adenosine may also be critical regulators of peripheral basophils and skin mast cells in the pathogenesis of CSU.
3. Basophil-Related Molecules in Plasma and on Cell Surface of Patients with CSU
Several basophil characteristics and their behavior in CSU patients are summarized in Table 1. In a group of patients, the concentration of histamine in the plasma of patients with CSU was significantly higher than that of healthy controls (Table 1) [31]. Moreover, spontaneous histamine release from basophils without stimulation in patients with CSU was increased, compared to that in healthy donors (Table 1) [31,32]. Nevertheless, the degree of spontaneous release of histamine was not correlated with total amount of histamine in the whole blood of patients with CSU [31], suggesting that the percent release of histamine from individual basophils of patients with CSU is higher than that of healthy control. Levels of IgE antibodies in serum are also increased in patients with CSU (Table 1) [32,33]. Moreover, IgE levels in serum are proportional to the levels of FcεRI expression on the surface of human peripheral basophils [33] (Table 1). We previously reported that a high concentration of IgE antibodies (>1 μM) activate IgE-depleted human peripheral basophils, resulting in the release of histamine [33]. As described above, the lifetime of basophils is only a few days. Therefore, a substantial population of basophils may be differentiated on a daily basis in the bone marrow and exposed to a high concentration of IgE antibodies in the peripheral blood and/or the bone marrow. That may result in the continuous release of histamine from newly developed peripheral basophils every day, and therefore, the elevation of histamine concentration in peripheral blood. Moreover, IgE-induced basophil activation and histamine release are enhanced in the presence of IL-3 [33]. In addition, the depletion of IgE antibodies in serum by anti-IgE antibodies, such as omalizumab and ligelizumab, decreases FcεRIs expressed on the surface of basophils by 78% in 10 days, together with a decrease in the severity of CSU [34]. Therefore, a high IgE concentration and high levels of FcεRI expression on the surface of basophils could be good markers of CSU. Paradoxically, a population of patients with CSU express a low level of FcεRI on basophils and is refractory to omalizumab [5]. Further studies on the relationship between high-IgE antibody concentrations in plasma and the expression level of FcεRI on the surface of peripheral basophils may unveil the detailed role of basophils in the pathogenesis of CSU, and enable us to develop more useful drugs for refractory CSU.
In line with previous reports, the ratio of basophils in leukocytes and the number of basophils in the peripheral blood of patients with CSU is also significantly low compared to healthy donors and patients with AD (Table 1) [31,33,34,35]. Several reports demonstrated that basophils migrate and accumulate in urticarial lesions [36,37]. Migration of activated basophils to the dermis may decrease the number of basophils in peripheral blood.
We reported that the expression levels of CD63 and CD69, degranulation markers, and CD203c, an activation marker on the surface of peripheral basophils of patients with CSU, were not significantly increased (Table 1) [31]. However, Vasagar et al. reported that the elevation of CD63, but not CD69 and CD203c, on the surface of basophils [38]. Mizuno et al. also reported that the expression of CD63 and CD203c on the surface of basophils are elevated compared to healthy donors [39]. Expression levels of degranulation/activation markers of basophils may change during cell preparation and timing of blood collection due to the migration of activated basophils to the dermis.
The ratio of non-/low-responders, whose basophils in peripheral blood are not activated by IgE-FcεRI-dependent signals, tends to increase in patients with CSU compared to healthy donors and those with other allergic diseases, such as AD (Table 1) [31,34]. However, the reason why the number of no- or low- responsive basophils are increased in the patients with CSU remains unclear. Moreover, how basophils in patients of low- and non-responders release histamine is uncertain. We recently demonstrated that basophils of low- and non-responder CSU patients, whose basophils release low or no amounts of histamine in response to anti-IgE (FcεRI-dependent activation), maintain the capacity to release histamine in response to IgE-independent stimuli, such as C5a and N-Formylmethionyl-leucyl-phenylalanine (fMLP) [31,40]. Moreover, histamine release in response to C5a from basophils of patients with CSU was similar to or even higher than that of healthy donors (Table 1). Of note, a degree of histamine release from basophils in response to anti-IgE and, that to C5a in CSU responders, whose basophils can release normal amount of histamine in response to anti-IgE, tended to be negatively correlated, whereas those in healthy donors showed a tendency of positive correlation [31]. Therefore, IgE-FcεRI-independent signal transduction, such as C5a-C5aR interaction induced activation, may be a main pathway instead of IgE-dependent activation in the low/non-responders. Although basophils express C3aR on their surface, C3a did not induce histamine release from basophils unexpectedly [40]. C3a-C3aR interaction may contribute to the migration of basophils to lesions of urticaria.

Table 1.
Changes of behavior of peripheral basophils in CSU.
Table 1.
Changes of behavior of peripheral basophils in CSU.
| Basophil-Related Conditions | Changes in CSU Patients |
|---|---|
| Plasma histamine level | Increase [31] |
| Spontaneous histamine release | Increase [31,32] |
| Plasma IgE level | Increase [33,34] |
| Expression level of FcεRI | Increase [33,34,39] |
| Reaction to anti-IgE antibody medication (omalizumab) | Quick (few days) [34,41,42] |
| Ratio of basophils in leukocytes | Decrease [31,33,34,35] |
| Activation marker (CD203c, CD69 and CD63) | No change or increase [33,38,39] |
| Migration to dermis | Increase [36,37] |
| Non- or low-responder | Tend to increase [31,34] |
| Reaction to C5a and fMLP | No change or increase [31,43] |
4. Coagulation System, Protease Receptors and Complement System
Two major pathways, the intrinsic and the extrinsic coagulation pathways, are involved in the physiological blood coagulation. The intrinsic coagulation pathway is activated by the exposure of coagulation factors in the blood to collagen because of the abrasion of vascular endothelial cells from collagen. Coagulation factor XII is firstly activated and the coagulation cascade is proceeded, resulting in the formation of a coagulation clot. On the other hand, the extrinsic coagulation pathway is activated by the exposure of coagulation factors in the blood to TF, which is expressed on tissues out of blood vessel. Recently, the activation of the extrinsic coagulation cascade-producing active forms of the coagulation factors has been revealed in patients with CSU. Figure 2 shows a part of the extrinsic coagulation pathway. When a small amount of VIIa, the active form VII, binds to TF on vascular endothelial cells in the presence of calcium ion (Ca2+) and phosphatidylserine (PS), the extrinsic coagulation cascade is activated and produces active form of coagulation factors, such as coagulation factor Xa and thrombin (Factor IIa). Since activated basophils express PS on their surface, basophils may contribute to this step. The IIa then converts fibrinogen (I) to fibrin (Ia) which form fibrin polymers as a blood clot. Of note, heparin, which activates anti-thrombin, improved symptoms in cases of CSU [44]. Moreover, warfarin which inhibits the production of coagulation factors, such as coagulation factor VII, X, IX and II, has also been suggested to be effective for the treatment of CSU [44]. These findings imply that the activation of the extrinsic coagulation cascade and subsequently produced active form of coagulation factors play important roles as the trigger of CSU. PF1+2 is generated when factor Xa changes prothrombin (II) to factor IIa, followed by the generation of Ia polymers (coagulation clot) from factor I. D-dimers and fibrin/fibrinogen degradation products (FDPs) are produced in the process of fibrinolysis: the cleavage of fibrin polymers by plasmin (Figure 2). Several groups have reported that levels of PF1+2, FDP and D-dimer in plasma of patients with CSU were elevated in association with the severities of clinical symptoms [45,46]. Moreover, the potential of thrombin-producing activity in response to TF exposure in plasma of patients with CSU is elevated, compared to that in the plasma of healthy donors [47]. Active forms of coagulation/fibrinolysis factors not only activate descending factors in the coagulation/fibrinolysis pathway, but may also cleave the extracellular domain of protease-activated receptors (PARs) by serine-specific protease activities. PARs are expressed on the surface of several types of cells, including vascular endothelial cells, monocytes, platelets, monocytes, mast cells, basophils, and neuron, coupled with G-proteins, and mediate information to target cells [48]. To date, four types of mammalian PARs (PAR-1/2/3/4) have been reported. Among coagulation factors, VIIa, Xa and IIa may activate PAR-2, PAP-1,2,3 and PAR-1,3,4, respectively. The complement system is composed of several molecules including complement 1 (C1)-C9. As the coagulation system, most complement components are normally inactive, but sequentially activated via enzymatic cascade in response to the recognition of molecular components of microorganism or non-self-antigens via immunoglobulins. It is classified into three pathways; the classical pathway, the alternative pathway and the lectin pathway [49]. As shown in Figure 2, the classical pathway is triggered by the activation of C1 bound to the immune complex of IgG. The alternative pathway commences with the cleaving of C3 into C3b and C3a (anaphylatoxin). C3b cleaves C5 into C5b and C5a (anaphylatoxin). C5b creates a complex with C6–C9 to make a pore of plasma membrane of microorganisms (host defense). On the other hand, C3a and C5a activate macrophages, neutrophils, basophils and mast cells via C3aR and C5aR, respectively, resulting in allergic reactions. Recently, thrombin (IIa) and plasmin have been reported to contribute to nontraditional complement activation, even in the absence of C3b and C4 [50,51,52]. Thus, the activation of the coagulation cascade may play critical roles for the production of the C3a and C5a by the complement system-independent manner. The other report suggested that C5a levels in plasma is increased in patients with CSU [53].
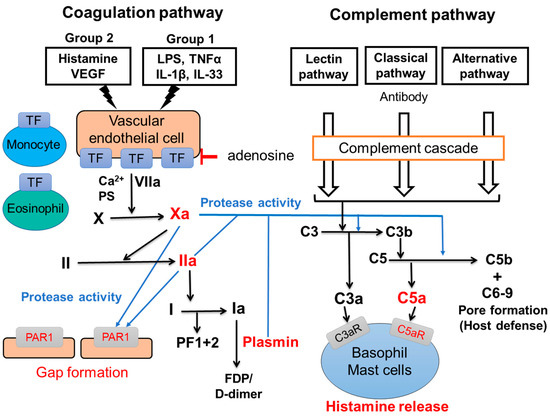
Figure 2.
Interactions of coagulation and complement pathway. Blue lines show direct activation of PAR1 and complement pathway-independent production of anaphylatoxins (C3a and C5a) by active forms of coagulation factors. Red characters indicate potential new targets for the treatment for CSU.
5. The Role of Vascular Endothelial Cells, Eosinophils and Monocytes for the Activation of Coagulation Pathway
As described above, the extrinsic coagulation pathway is usually activated when a blood vessel is damaged and coagulation factors are exposed to TF expressed out of a blood vessel. However, we and other groups have reported that human umbilical vein endothelial cells (HUVECs) and human dermal microvascular endothelial cells (HMVECs) express a large amount of TF on the surface of cells in response to the combination of several molecules (TF-inducers), such as histamine, VEGF, LPS, TNFα, IL-33 and IL-1β, without damage of the blood vessel [54,55]. We divided TF-inducers into two groups according to their signaling pathways, as shown in Figure 2; Group 1 (LPS, TNF-α, IL-33, IL-β) and Group 2 (histamine, VEGF). Factors in Group 1 activate the nuclear factor-kappa B (NF-kB)-related signaling pathway, whereas histamine and VEGF in Group 2 activate the phospholipase C-linked pathway. A high expression of TF on vascular endothelial cells was induced by the co-stimulation of different types of molecules; one in Group 1 and another in Group 2 TF-inducers. Moreover, the highly expressed TF on HUVECs induced the activation of the extrinsic coagulation pathways and produced active forms of the coagulation factor, and then induced the gap-formation of vascular endothelial cells via PAR1, followed by a leakage of plasma from blood vessels (Figure 2) [54,55]. TF expression on vascular endothelial cells induced by TF inducers was suppressed by adenosine: a metabolite of ATP [54]. Of note, adenosine does not only prevent a newly expression of TF, but also decreases TF which is already expressed on endothelial cells on a dose-dependent manner, suggesting that adenosine-related molecules might be effective therapeutically for CSU [54]. As other sources of TF in blood vessel, we found that TF expression levels on human peripheral blood monocytes are significantly increased in CSU patients compared with healthy donors. Moreover, we clarified that stimulation via TLR, -1, -2, -4, or -5 enhanced TF expression levels on the surface of human peripheral monocytes [56]. Moreover, Asero et al. reported that eosinophils express TF on their surface and migrate into the dermis in response to stimulation via the low-affinity IgE receptor, FcɛRII (CD23) [57]. These findings suggest that monocytes and eosinophils also activate the extrinsic coagulation cascade in blood vessels, together with TF expressed on vascular endothelial cells. However, clinical evidence on the roles of TF expressed on vascular endothelial cells, monocytes and eosinophils in patients with CSU is still limited. Further studies on the functions of them, especially in relation to disease severity and reactivities to currently available medications are warranted to find useful drugs and treatments for severe and refractory CSU patients.
6. The Role of Basophils as Triggers of CSU
The role of basophils and mast cells for the development of urticaria through the extrinsic coagulation cascade, protease receptors and complement cascade may be explained by the following model. As described above, several autoantibodies against IgE antibody and/or FcεRI (IgG antibody) and self-molecules, such as IL-24, dsDNA and TPO (IgE antibody), contribute to the basophils and mast cells activation, resulting in the release of chemical mediators, such as histamine, followed by the development of urticaria (autoimmune CSU). In addition, vascular endothelial cells and the extrinsic coagulation pathway may also contribute to the activation of peripheral blood basophils and skin mast cells by the autoimmune system independent pathway. A combination of several exacerbating factors of CSU (TF inducers), such as histamine, VEGF, TNF, IL-1β, IL-33 and LPS, induces high levels of TF expression on vascular endothelial cells, and triggers the extrinsic coagulation pathway [54,55]. Since the combination of histamine and LPS/TNF potently increases the TF expression of vascular endothelial cells, the spontaneous release of histamine from basophils may contribute to the high expression of TF on the surface of vascular endothelial cells. When basophils are activated, PS is also expressed out of cells. This phenomena may accelerate the activation of the extrinsic coagulation cascade. Highly expressed TF on the surface of vascular endothelial cells then activates the extrinsic coagulation pathway together with PS and Ca2+ and produces active forms of coagulation factors, such as coagulation factors, Xa and IIa, and the fibrinolysis factor, plasmin. In addition, the high expression of TF was reported on the surface of eosinophils in the lesion of urticaria and monocytes in the peripheral blood of patients with CSU. TF expressed by these cells may also contribute to the activation of the coagulation cascade (Figure 2). Then, Xa and IIa bind to PAR1 on the surface of vascular endothelial cells and induce gap formation and the leakage of plasma from blood vessels. Consequentially, Xa, IIa, and plasmin produces C5a (anaphylatoxin) from the C5 complement by the complement cascade-independent manner, and induces histamine release from basophils and skin mast cells via the C5aR, resulting in the release of a large amount of histamine and the development of urticaria (Figure 1 and Figure 2) [44,54]. It is noteworthy that C5aRs are selectively expressed on the surface of both peripheral basophils and skin mast cells, but not of other kinds of mast cells in humans. Since the release of histamine from human peripheral basophils and skin mast cells in response to C3a is low compared to C5a, C3a may regulate other functions, such as the migration and accumulation of basophils and mast cells to the lesion of urticaria via C3aR [44,54].
7. Treatment Targeting Basophil-Related Molecules in the Pathogenesis of CSU
In many patients with CSU, peripheral blood basophils and skin mast cells may be activated in response to several types of auto-antibodies (IgG and IgE). Treatment with the anti-IgE antibody quickly decreases serum IgE, and then decreases the expression levels of FcεRI on the surface of peripheral blood basophils followed by those on skin mast cells. Since, FcεRI dependent activation is transduced via several tyrosine kinase, such as Lyn, Syk and Btk, in mast cells and basophils, inhibitors for these molecules should also be useful in the treatment of CSU [58,59,60]. In addition, the involvement of the coagulation-complement cascade in the vascular hyper permeability via Xa- and IIa-PAR1 interaction, and the activation of basophils and mast cells by C5a-C5aR interaction, suggests the efficacy of inhibitors against the coagulation cascade, such as warfarin and heparin, antagonists or antibodies for PAR1, and those against C5a and C5aR for CSU refractory to currently used medications. Moreover, since adenosine suppresses the gap formation of vascular endothelial cells and antigen-IgE-related histamine release from peripheral blood basophils and skin mast cells activation via adenosine receptors (A2a and A2b), adenosine and adenosine analogs (including agonist for A2a and A2b) could also be effective for treating CSU. On the other hand, crucial involvements of IL-4 and IL-5 in the pathogenesis of CSU were suggested via the favorable results of clinical trials of the anti-IL-4/13 receptor antibody, dupilumab [61], anti-IL-5 (mepolizumab, reslizumab) [62,63] and anti-IL-5R (benralizumab) antibody [64]. Moreover, levels of IL-4 in plasma and numbers of IL-4 expressing cells in the skin of patients with CSU are higher than those of health controls [65,66]. IL-5 may also contribute to the pathogenesis of CSU by the activation of eosinophils and basophils [60]. Of note, basophils and eosinophils are major sources of IL-4 and IL-5, respectively, and their decrease in peripheral circulation is a character of sever and type 2b-autoimmune CSU, which is refractory to antihistamines and omalizumab [67].
8. Conclusions
In this review, we focused on the role of basophils and introduced details of the behavioral changes of basophils in CSU. These observations show that not only basophil-associated biomarkers, such as plasma histamine and total IgE levels, but also cytomarkers, such as basophil numbers in peripheral blood, the ratio in leukocytes, responsiveness to stimuli, expression of receptors and location may also be useful information to determine the status of patients with CSU. Moreover, we introduced potential roles of basophil- and mast cell- receptors, PARs, adenosine receptors and C5aR. These findings suggest that agonists, antagonists or antibodies against these molecules could be explored as useful drugs for patients with CSU. Further studies on the detailed roles of basophils and the role of their receptors in CSU are warranted to clarify the detailed pathogenesis and discover more useful markers and potential treatments of CSU.
Author Contributions
Y.Y., D.M., S.T., A.T., K.O. and M.H. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The Study was partially funded by grants to Y.Y. from Takeda Science Foundation, Japanese Society of Allergology Clinical Research Support Program, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, Grant-in-Aid for Scientific Research (C), JST-CREST Japan (Grant Number JPMJCR2016) and to M.H. from Grant-in-Aid for Scientific Research (C).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented by this article are available from the corresponding author up on reasonable requests.
Acknowledgments
We wish to thank Faiz Kermani for his manuscript review. All authors have approved the content of this article and publication.
Conflicts of Interest
M.H. has received lecture and/or consultation fees from Kaken, Kyorin-Pharmaceutical, Kyowa-Kirin, Mitsubishi Tanabe, Novartis, Sanofi, Sanofi and TAIHO, Teikoku Seiyaku, and Uriach. The other authors declare no conflict of interest.
References
- Hide, M.; Takahagi, S.; Hiragun, T. Urticaria and Angioedema. In Fitzpatrick’s Dermatology, 9e; Kang, S., Amagai, M., Bruckner, A., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- Ricke, J.; Ávila, G.; Keller, T.; Weller, K.; Lau, S.; Maurer, M.; Zuberbier, T.; Keil, T. Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy 2020, 75, 423–432. [Google Scholar]
- Hide, M.; Francis, D.M.; Grattan, C.E.; Hakimi, J.; Kochan, J.P.; Greaves, M.W. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 1993, 328, 1599–1604. [Google Scholar] [CrossRef]
- Maurer, M.; Rosén, K.; Hsieh, H.J.; Saini, S.; Grattan, C.; Gimenéz-Arnau, A.; Agarwal, S.; Doyle, R.; Canvin, J.; Kaplan, A.; et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N. Engl. J. Med. 2013, 368, 924–935. [Google Scholar] [CrossRef]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.K.; Maurer, M. Autoimmune chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef]
- Sánchez, J.; Sánchez, A.; Cardona, R. Clinical Characterization of Patients with Chronic Spontaneous Urticaria according to Anti-TPO IgE Levels. J. Immunol. Res. 2019, 2019, 4202145. [Google Scholar] [CrossRef] [PubMed]
- Hatada, Y.; Kashiwakura, J.; Hayama, K.; Fujisawa, D.; Sasaki-Sakamoto, T.; Terui, T.; Ra, C.; Okayama, Y. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int. Arch. Allergy Immunol. 2013, 161, 154–158. [Google Scholar]
- Schmetzer, O.; Lakin, E.; Topal, F.A.; Preusse, P.; Freier, D.; Church, M.K.; Maurer, M. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2018, 142, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Marzano, A.V.; Ferrucci, S.; Lorini, M.; Carbonelli, V.; Cugno, M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin. Exp. Immunol. 2020, 200, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Sánchez, A.; Munera, M.; Garcia, E.; Lopez, J.F.; Velásquez-Lopera, M.; Cardona, R. Presence of IgE Autoantibodies Against Eosinophil Peroxidase and Eosinophil Cationic Protein in Severe Chronic Spontaneous Urticaria and Atopic Dermatitis. Allergy Asthma Immunol. Res. 2021, 13, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Maronese, C.A.; Ferrucci, S.M.; Moltrasio, C.; Lorini, M.; Carbonelli, V.; Asero, R.; Marzano, A.V.; Cugno, M. IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab. J. Clin. Med. 2023, 12, 378. [Google Scholar] [CrossRef]
- Plan, A.P.; Greaves, M. Pathogenesis of chronic urticaria. Clin. Exp. Allergy 2009, 39, 777–787. [Google Scholar]
- Termeer, C.; Staubach, P.; Kurzen, H.; Strömer, K.; Ostendorf, R.; Maurer, M. Chronic spontaneous urticaria—A management pathway for patients with chronic spontaneous urticaria. J. Dtsch. Dermatol. Ges. 2015, 13, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; André, F.; Church, M.K.; Maurer, M.; Metz, M. Potential blood biomarkers in chronic spontaneous urticaria. Clin. Exp. Allergy 2017, 47, 19–36. [Google Scholar] [CrossRef]
- Yanase, Y.; Takahagi, S.; Hide, M. Chronic spontaneous urticaria and the extrinsic coagulation system. Allergol. Int. 2018, 67, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, J.; Liu, R.; Zhu, L.; Peng, C. The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria. Front. Immunol. 2022, 13, 879754. [Google Scholar] [CrossRef]
- Chandrashekar, L.; Rajappa, M.; Munisamy, M.; Ananthanarayanan, P.H.; Thappa, D.M.; Arumugam, B. 25-Hydroxy vitamin D levels in chronic urticaria and its correlation with disease severity from a tertiary care centre in South India. Clin. Chem. Lab. Med. 2014, 52, e115–e118. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B. Novel biologics for treatment of chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2022, 150, 1256–1259. [Google Scholar] [CrossRef]
- Alizadeh Aghdam, M.; van den Elzen, M.; van Os-Medendorp, H.; van Dijk, M.R.; Knol, E.F.; Knulst, A.C.; Röckmann, H.; Otten, H.G. Systemic and local evidence for complement involvement in chronic spontaneous urticaria. Clin. Transl. Allergy 2021, 11, e12011. [Google Scholar] [CrossRef]
- Yan, S.; Chen, W.; Wen, S.; Zhu, W.; Guo, A.; Chen, X.; Zhang, C.; Chen, M.; Zhang, J.; Su, J.; et al. Influence of component 5a receptor 1 (C5AR1)–1330T/G polymorphism on nonsedating H1-antihistamines therapy in Chinese patients with chronic spontaneous urticaria. J. Dermatol. Sci. 2014, 76, 240–245. [Google Scholar] [CrossRef]
- Galli, S.J. Mast cells and basophils. Curr. Opin. Hematol. 2000, 7, 32–39. [Google Scholar] [CrossRef]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human mast cells and basophils—How are they similar how are they different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Yanase, Y.; Hide, I.; Mihara, S.; Shirai, Y.; Saito, N.; Nakata, Y.; Hide, M.; Sakai, N. A critical role of conventional protein kinase C in morphological changes of rodent mast cells. Immunol. Cell. Biol. 2011, 89, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M. Mast cells and basophils in allergic inflammation. Curr. Opin. Immunol. 2018, 28, 74–79. [Google Scholar] [CrossRef]
- Sasaki, H.; Kurotaki, D.; Tamura, T. Regulation of basophil and mast cell development by transcription factors. Allergol. Int. 2016, 65, 127–134. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Zhao, W.; Min, H.K.; Xia, H.Z.; Pozez, A.; Kiev, J.; Schwartz, L.B. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005, 115, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Morita, Y.; Misaki, Y.; Ohta, K.; Takaishi, T.; Suzuki, S.; Motoyoshi, K.; Miyamoto, T. Modulation of human basophil histamine release by hemopoietic growth factors. J. Immunol. 1988, 141, 3958–3964. [Google Scholar] [CrossRef]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633. [Google Scholar] [CrossRef]
- Toscano, A.; Elst, J.; Van Gasse, A.L.; Beyens, M.; van der Poorten, M.L.; Bridts, C.H.; Mertens, C.; Van Houdt, M.; Hagendorens, M.M.; Van Remoortel, S.; et al. Mas-related G protein-coupled receptor MRGPRX2 in human basophils: Expression and functional studies. Front. Immunol. 2023, 13, 1026304. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Yanase, Y.; Irifuku, R.; Ishii, K.; Kawaguchi, T.; Takahagi, S.; Hide, I.; Hide, M. The role of adenosine for IgE receptor-dependent degranulation of human peripheral basophils and skin mast cells. Allergol. Int. 2018, 67, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, D.; Yanase, Y.; Ishii, K.; Takahagi, S.; Tanaka, A.; Ozawa, K.; Hide, M. Basophils activation of patients with chronic spontaneous urticaria in response to C5a despite failure to respond to IgE-mediated stimuli. Front. Immunol. 2022, 13, 994823. [Google Scholar] [CrossRef]
- Luquin, E.; Kaplan, A.P.; Ferrer, M. Increased responsiveness of basophils of patients with chronic urticaria to sera but hypo-responsiveness to other stimuli. Clin. Exp. Allergy 2005, 35, 456–460. [Google Scholar] [CrossRef]
- Yanase, Y.; Matsuo, Y.; Kawaguchi, T.; Ishii, K.; Tanaka, A.; Iwamoto, K.; Takahagi, S.; Hide, M. Activation of Human Peripheral Basophils in Response to High IgE Antibody Concentrations without Antigens. Int. J. Mol. Sci. 2018, 20, 45. [Google Scholar] [CrossRef]
- Kaplan, A.P.; Giménez-Arnau, A.M.; Saini, S.S. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 2017, 72, 519–533. [Google Scholar] [CrossRef]
- Kishimoto, I.; Kambe, N.; Ly, N.T.M.; Nguyen, C.T.H.; Okamoto, H. Basophil count is a sensitive marker for clinical progression in a chronic spontaneous urticaria patient treated with omalizumab. Allergol. Int. 2019, 68, 388–390. [Google Scholar] [CrossRef]
- Ito, Y.; Satoh, T.; Takayama, K.; Miyagishi, C.; Walls, A.F.; Yokozeki, H. Basophil recruitment and activation in inflammatory skin diseases. Allergy 2011, 66, 1107–1113. [Google Scholar] [CrossRef]
- Kishimoto, I.; Ma, N.; Takimoto-Ito, R.; Nakashima, C.; Otsuka, A.; Walls, A.F.; Tanizaki, H.; Kambe, N. Decreased peripheral basophil counts in urticaria and mouse model of oxazolone-induced hypersensitivity, the latter suggesting basopenia reflecting migration to skin. Front. Immunol. 2022, 13, 1014924. [Google Scholar] [CrossRef] [PubMed]
- Vasagar, K.; Vonakis, B.M.; Gober, L.M.; Viksman, A.; Gibbons, S.P., Jr.; Saini, S.S. Evidence of in vivo basophil activation in chronic idiopathic urticaria. Clin. Exp. Allergy 2006, 36, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Oda, Y.; Imamura, S.; Washio, K.; Fukumoto, T.; Fukunaga, A. IgE receptor responsiveness of basophils in chronic inducible urticaria. Front. Immunol. 2022, 13, 995596. [Google Scholar] [CrossRef]
- Yanase, Y.; Matsuo, Y.; Takahagi, S.; Kawaguchi, T.; Uchida, K.; Ishii, K.; Tanaka, A.; Matsubara, D.; Ozawa, K.; Hide, M. Coagulation factors induce human skin mast cell and basophil degranulation via activation of complement 5 and the C5a receptor. J. Allergy Clin. Immunol. 2021, 147, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, M.; Košnik, M.; Koren, A.; Kopač, P.; Šelb, J.; Vantur, R.; Kogovšek, Ž.; Bizjak, M.; Bajrović, N.; Zidarn, M.; et al. A very low number of circulating basophils is predictive of a poor response to omalizumab in chronic spontaneous urticaria. Allergy 2021, 76, 1254–1257. [Google Scholar] [CrossRef]
- Oda, Y.; Fukunaga, A.; Washio, K.; Imamura, S.; Mizuno, M.; Hatakeyama, M.; Ogura, K.; Nishigori, C. Improved FcεRI-Mediated CD203c Basophil Responsiveness Reflects Rapid Responses to Omalizumab in Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. Pract. 2021, 9, 1166–1176. [Google Scholar] [CrossRef]
- Alizadeh Aghdam, M.; Knol, E.F.; van den Elzen, M.; den Hartog Jager, C.; van Os-Medendorp, H.; Knulst, A.C.; Otten, H.G.; Röckmann, H. Response of FcεRI-bearing leucocytes to omalizumab in chronic spontaneous urticaria. Clin. Exp. Allergy 2020, 50, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Takahagi, S.; Ozawa, K.; Hide, M. The Role of Coagulation and Complement Factors for Mast Cell Activation in the Pathogenesis of Chronic Spontaneous Urticaria. Cells 2021, 10, 1759. [Google Scholar] [CrossRef]
- Asero, R.; Tedeschi, A.; Riboldi, P.; Cugno, M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J. Allergy Clin. Immunol. 2006, 117, 1113–1117. [Google Scholar] [CrossRef]
- Takahagi, S.; Mihara, S.; Iwamoto, K.; Morioke, S.; Okabe, T.; Kameyoshi, Y.; Hide, M. Coagulation/fibrinolysis and inflammation markers are associated with disease activity in patients with chronic urticaria. Allergy 2010, 65, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Morioke, S.; Takeda, T.; Takahagi, S.; Hide, M.; Shima, M. Increased thrombin generation potential in patients with chronic spontaneous urticaria. Allergol. Int. 2015, 64, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J. Tissue factor, blood coagulation, and beyond: An overview. Int. J. Inflam. 2011, 2011, 367284. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S.; Rittirsch, D.; Neff, T.A.; McGuire, S.R.; Lambris, J.D.; Warner, R.L.; Flierl, M.A.; Hoesel, L.M.; et al. Generation of C5a in the absence of C3: A new complement activation pathway. Nat. Med. 2006, 12, 682–687. [Google Scholar] [CrossRef]
- Clark, A.; Weymann, A.; Hartman, E.; Turmelle, Y.; Carroll, M.; Thurman, J.M.; Holers, V.M.; Hourcade, D.E.; Rudnick, D.A. Evidence for non-traditional activation of complement factor C3 during murine liver regeneration. Mol. Immunol. 2008, 45, 3125–3132. [Google Scholar] [CrossRef]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Brückner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, B.; Li, R.; Li, J.; Lin, L.; Ma, S.; Wang, J. Activation of coagulation, anti-coagulation, fibrinolysis and the complement system in patients with urticaria. Asian Pac. J. Allergy Immunol. 2013, 31, 43–50. [Google Scholar]
- Yanase, Y.; Morioke, S.; Iwamoto, K.; Takahagi, S.; Uchida, K.; Kawaguchi, T.; Ishii, K.; Hide, I.; Hide, M. Histamine and Toll-like receptor ligands synergistically induce endothelial cell gap formation by the extrinsic coagulating pathway. J. Allergy Clin. Immunol. 2018, 141, 1115–1118.e7. [Google Scholar] [CrossRef]
- Kamegashira, A.; Yanase, Y.; Takahagi, S.; Saito, R.; Uchida, K.; Kawaguchi, T.; Ishii, K.; Tanaka, A.; Ozawa, K.; Hide, M. Histamine- or vascular endothelial growth factor-induced tissue factor expression and gap formation between vascular endothelial cells are synergistically enhanced by lipopolysaccharide, tumor necrosis factor-α, interleukin (IL)-33 or IL-1β. J. Dermatol. 2020, 47, 1293–1300. [Google Scholar] [CrossRef]
- Saito, R.; Yanase, Y.; Kamegashira, A.; Takahagi, S.; Tanaka, A.; Uchida, K.; Kawaguchi, T.; Hide, M. Increase of tissue factor expression on the surface of peripheral monocytes of patients with chronic spontaneous urticaria. Allergy 2020, 75, 971–974. [Google Scholar] [CrossRef]
- Cugno, M.; Marzano, A.V.; Tedeschi, A.; Fanoni, D.; Venegoni, L.; Asero, R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int. Arch. Allergy Immunol. 2009, 148, 170–174. [Google Scholar] [CrossRef]
- Kolkhir, P.; Giménez-Arnau, A.M.; Kulthanan, K.; Peter, J.; Metz, M.; Maurer, M. Urticaria. Nat. Rev. Dis. Prim. 2022, 8, 61. [Google Scholar] [CrossRef]
- Giménez-Arnau, A.M.; DeMontojoye, L.; Asero, R.; Cugno, M.; Kulthanan, K.; Yanase, Y.; Hide, M.; Kaplan, A.P. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J. Allergy Clin. Immunol. Pract. 2021, 9, 2195–2208. [Google Scholar] [CrossRef]
- Maurer, M.; Khan, D.A.; Elieh Ali Komi, D.; Kaplan, A.P. Biologics for the Use in Chronic Spontaneous Urticaria: When and Which. J. Allergy Clin. Immunol. Pract. 2021, 9, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Simpson, R.S. Dupilumab as a novel therapy for difficult to treat chronic spontaneous urticaria. J. Allergy Clin. Immunol. Pract. 2019, 7, 1659–1661. [Google Scholar] [CrossRef]
- Maurer, M.; Altrichter, S.; Metz, M.; Zuberbier, T.; Church, M.K.; Bergmann, K.C. Benefit from reslizumab treatment in a patient with chronic spontaneous urticaria and cold urticaria. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e112–e113. [Google Scholar] [CrossRef]
- Magerl, M.; Terhorst, D.; Metz, M.; Altrichter, S.; Zuberbier, T.; Maurer, M.; Bergmann, K.C. Benefit of mepolizumab treatment in a patient with chronic spontaneous urticaria. J. Dtsch. Dermatol. Ges. 2018, 16, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Magen, E.; Komarova, I.; Magen, I.; Phirtskhalava, S. Case of benralizumab-induced exacerbations of chronic spontaneous urticaria. Clin. Case Rep. 2022, 10, e05930. [Google Scholar] [CrossRef]
- Ying, S.; Kikuchi, Y.; Meng, Q.; Kay, A.B.; Kaplan, A.P. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: Comparison with the allergen-induced late-phase cutaneous reaction. J. Allergy Clin. Immunol. 2002, 109, 694–700. [Google Scholar] [CrossRef]
- Caproni, M.; Cardinali, C.; Giomi, B.; Antiga, E.; D’Agata, A.; Walter, S.; Fabbri, P. Serological detection of eotaxin, IL-4, IL-13, IFN-gamma, MIP-1alpha, TARC and IP-10 in chronic autoimmune urticaria and chronic idiopathic urticaria. J. Dermatol. Sci. 2004, 36, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Church, M.K.; Altrichter, S.; Skov, P.S.; Hawro, T.; Frischbutter, S.; Metz, M.; Maurer, M. Eosinopenia, in Chronic Spontaneous Urticaria, Is Associated with High Disease Activity, Autoimmunity, and Poor Response to Treatment. J. Allergy Clin. Immunol. Pract. 2020, 8, 318–325. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).