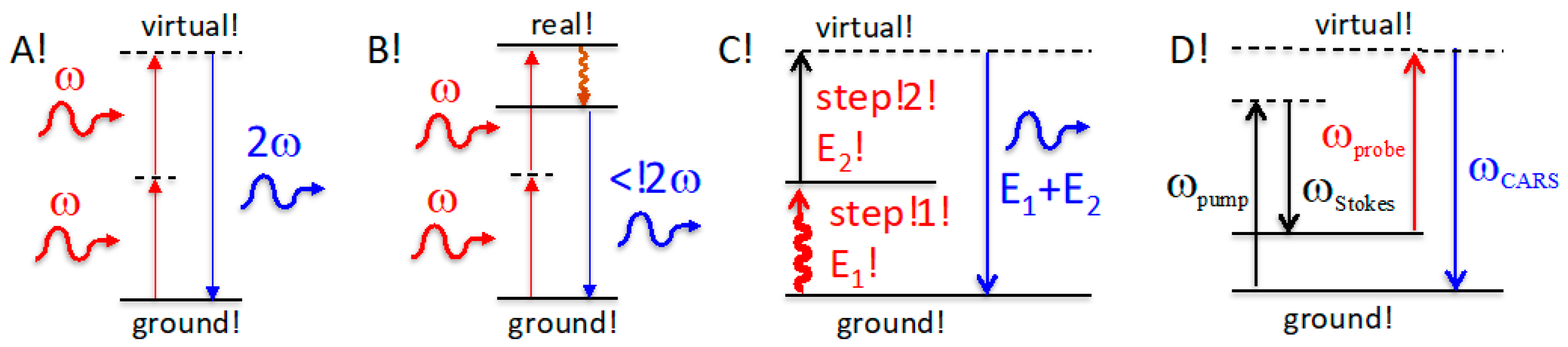
2.1. Temperature Independent Supracence Excitation and Emission
Most anti-Stokes fluorescence reports provide neither validation of the pseudo-first-order to photo-excitation power (
IEX) nor heat energy or temperature (
T) dependence. Others validate the pseudo-first-order to light power but did not confirm how heat or temperature is involved. Some reports plotted the anti-Stokes intensity versus temperature, but they did not consider that extinction coefficient and quantum yield, which are instrumental to emission brightness, also depend on temperature. Because of these, the literature on anti-Stokes fluorescence has been in a state of confusion currently, not entirely certain whether to treat it as second order or somewhere in between first and second order.
In light of our own work on supracence, we came across two reports [
12,
21] that claimed (1) anti-Stokes fluorescence was used for laser cooling and (2) rhodamine 101 in
acidified ethanol satisfied a nonlinear process and its anti-Stokes fluorescence intensity increased as a function of both excitation light (
IEX) and temperature (
T). Among all anti-Stokes fluorescence reports, these were the best description of the excitation process involving two steps and two variables, thus second order. Their putative theory of anti-Stokes intensity as a function of both excitation power and temperature is summarized in Equation (1), thus a nonlinear process, because two variables (
IEX and
T) were assumed to contribute to the excitation, where
v = 0, 1, 2, …N, and
k is Boltzmann constant,
T is temperature, and ε
i is the i-th energy level. Immediately after their publication, Mungan and Gosnell commented and concluded that anti-Stokes laser cooling was impossible under their experimental conditions [
22]. Meanwhile, our supracence work raises the objection of whether the observed effect was indeed anti-Stokes fluorescence. Not only was the temperature-dependence of quantum yield and extinction coefficient not considered in anti-Stokes laser cooling, but the
acidification of ethanol will also alter the equilibrium significantly between rhodamine spirolactone and its ring-open form. It is well-known that acid will catalyze reversible ring-opening and ring-closure in rhodamine dyes [
23]. Therefore, the temperature-dependence of equilibrium shift will significantly change the concentration of the ring-open rhodamine emitter, hence yielding a temperature-dependent emission intensity. Without proper calibration of temperature-dependence of quantum yields and extinction coefficients and, more importantly, consideration of acid-catalyzed ring-opening and ring-closure that affect absorption and emission, the asserted observation of anti-Stokes fluorescence is unmeaningful and misleading.
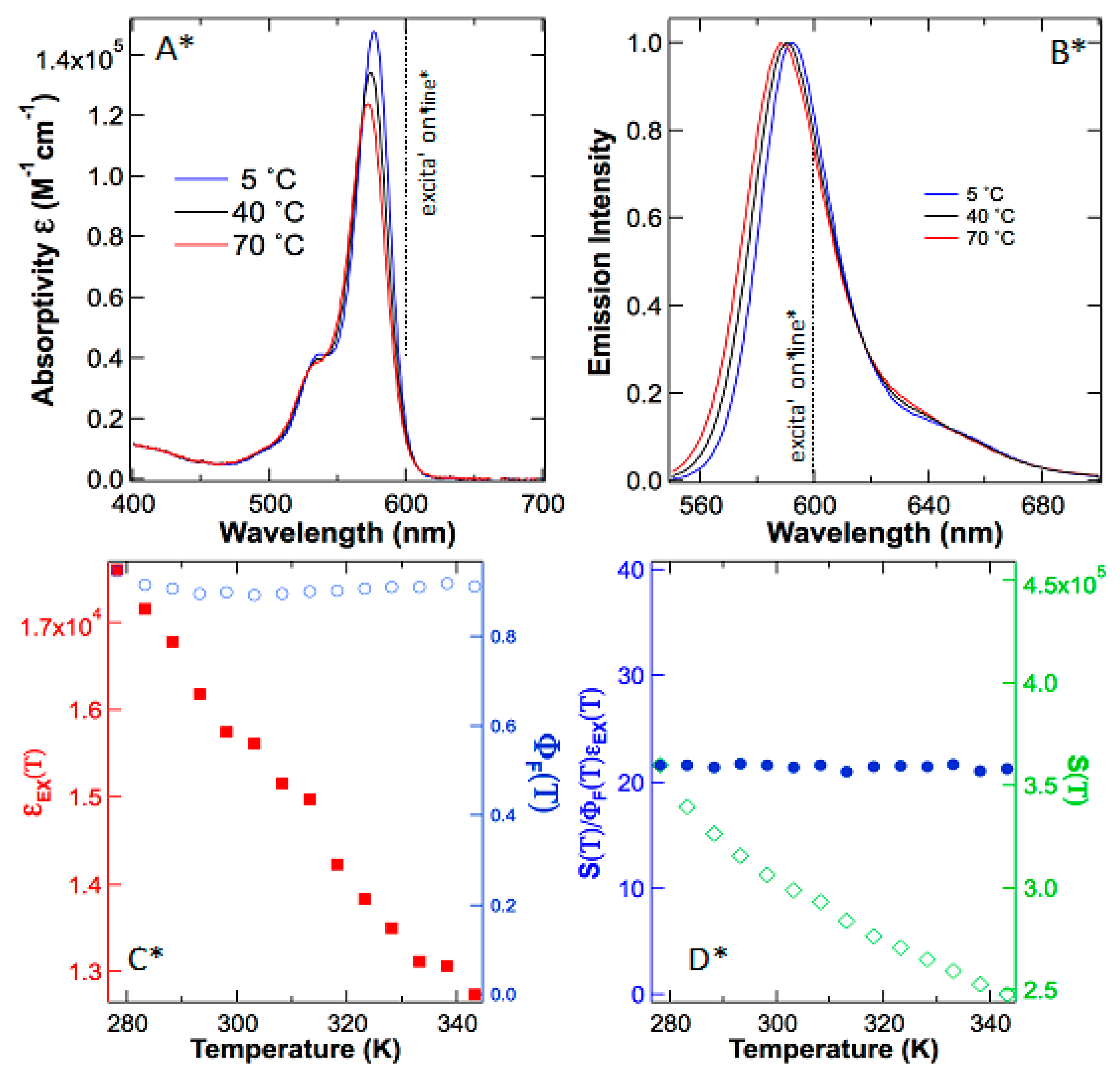
To find the truth, we repeat the previously reported “anti-Stokes fluorescence” using sulforhodamine 101 in ethanol without acidification. As depicted in
Figure 3A,B, both absorption and emission spectra of sulforhodamine 101 have subtle changes as the temperature rises. These results reveal that extinction coefficient and quantum yield are both a function of temperature and any variable temperature experiments must be calibrated using these parameters before reaching further conclusions about temperature effects on emission intensity.
Next, we plotted the extinction coefficient ε(T) change against temperature and use these data to calculate the quantum yields Φ(T) at various temperatures of sulforhodamine 101 (
Figure 3C), which are in good agreement with what was reported earlier [
24]. Because both quantum yield and extinction coefficient are temperature dependent, true anti-Stokes fluorescence must be calibrated against these influences by improving the superficial model described by Equation (1) to the adequate Equation (2). Only after removing the influences by temperature-dependent quantum yields and temperature-dependent extinction coefficients, can the temperature-promoted, vibrationally excited population in |g
1〉 state be investigated. Here, the ground state wavefunction is |g
0〉 ≈ |φ
g〉|0〉 and the vibrationally excited but electronic ground state is |g
1〉 ≈ |φ
g〉|1〉. The lowest excited state wavefunction is |e
0〉 ≈ |φ
e〉|0〉; |φ
g〉 and |φ
e〉 are electronic ground and excited wavefunctions, respectively, and |0〉 is the lowest vibrational wavefunction of the atoms while |1〉 is the first vibrationally excited state. Accordingly, the appearance and growth in |g
1〉 population should contribute
IAS in a manner like a concentration increase, which is distinct from influences by extinction coefficient and quantum yield that physically alter transition probabilities.
Finally, to determine the supracence peak maximum intensity at 588–592 nm, we excite sulforhodamine 101 in ethanol at 600 nm. Contrary to what was reported in rhodamine 101, our results on sulforhodamine 101 illustrated an opposite trend; supracence
S(
T) peak intensity plummets as the temperature rises (
Figure 3D). The major reason for the peak maximum to abate is that molar extinction coefficient ε(T) is diminishing due to the hypsochromic peak leaning in the absorption spectrum (
Figure 3A). After calibration to the temperature effects of extinction coefficient and quantum yield, the emission peak intensity reveals a zero-order dependence on temperature (
Figure 3D). In other words, anti-Stokes fluorescence did not occur in sulforhodamine 101. What was observed is supracence that obeys Equation (3), independent of temperature (n = 0).
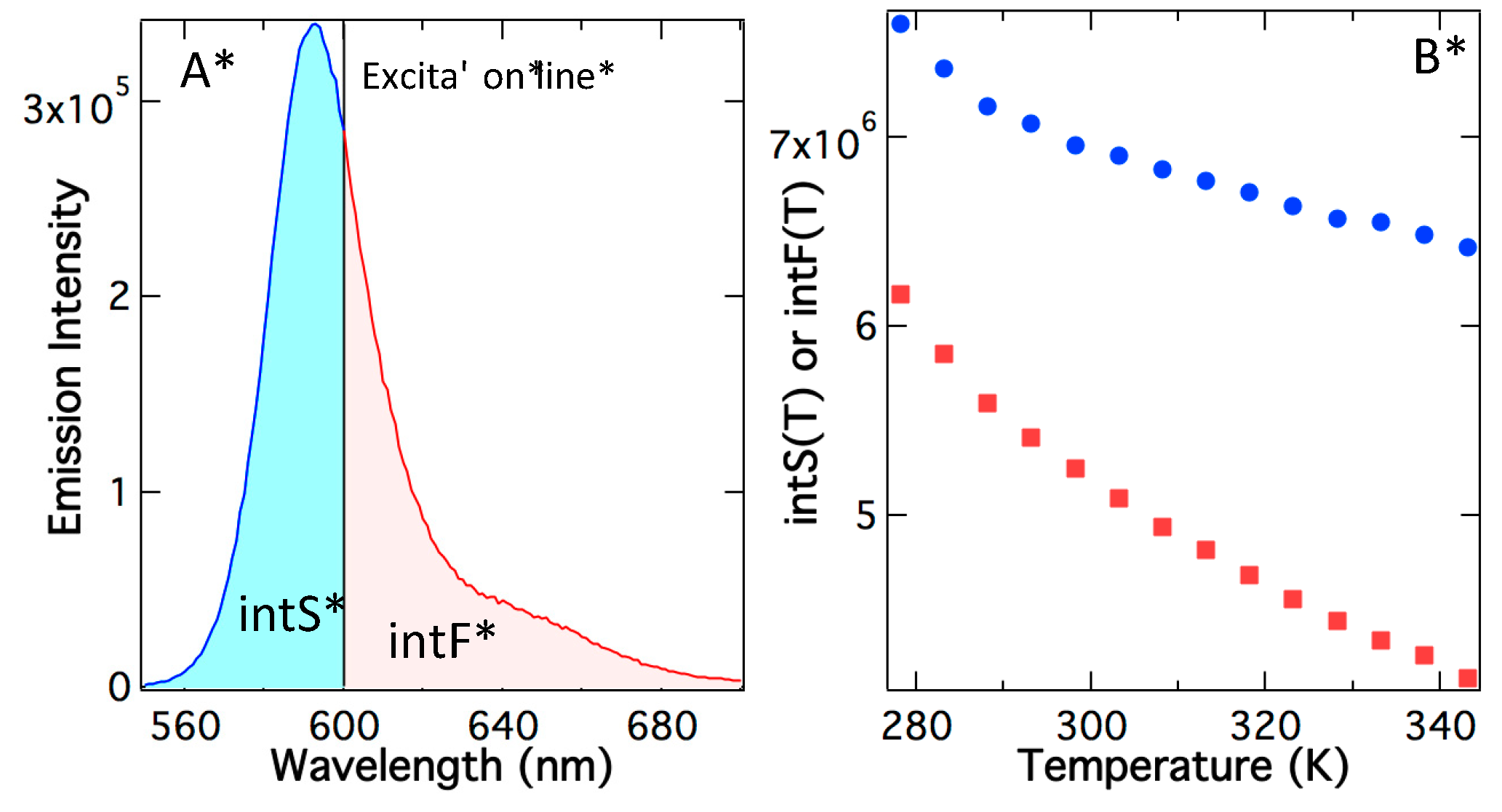
The above analysis uses peak maximum, but a more detailed analysis should use the integrated photons under the emission curve. In
Figure 4A, the excitation line divides the total emission into integrated supracence area (intS) and integrated fluorescence area (intF). As temperature rises, both the fluorescence area and supracence area decrease, although at different rates (
Figure 4B). The main reason for the slow reduction in the supracence area is hypsochromic shifting in the emission spectrum (
Figure 3B). As a result, at a higher temperature, supracence gains more share of the total emitted photons. This prediction is validated in
Figure 4C, in which quantum yields as a function of temperature are depicted. The top curve is the total quantum yields at various temperatures determined using the total area under the emission curve. The area-determined quantum yields agree with the peak-maximum quantum yields (
Figure 3C); both show little change via temperature. The total quantum yield (Φ
T) is the sum of fluorescence quantum yield (Φ
F) and supracence quantum yield (Φ
S), Φ
T = Φ
F + Φ
S. It is apparent that supracence quantum yields are increasing as the temperature rises, but fluorescence quantum yields are diminishing as the temperature rises. The reason is that at the fixed excitation line, the total emission spectrum is blue shifting, slicing a larger and larger share for supracence at the expense of fluorescence.
If anti-Stokes fluorescence were the underpinning mechanism, the supracence area after calibrating quantum yield and extinction coefficient would rise as the temperature rose, obeying Equation (2). If supracence is the underpinning mechanism, the supracence area after calibrating quantum yield and extinction coefficient will be constant or independent of temperature, obeying Equation (3). The experimental results in
Figure 4D reveal that the calibrated supracence area has zero-order to temperature (n = 0). In conclusion, after removing temperature effects by quantum yields and extinction coefficients, both supracence peak maximum and supracence emission area are independent of temperature, demonstrating that heat plays no role in supracence excitation.
Figure 4D also shows that the fluorescence area after calibration of quantum yields and extinction coefficients also remains constant as temperature varies. Supracence is a new phenomenon and less is known about its excitation order, but fluorescence has been well known and its first-order photoexcitation does not depend on temperature. The fact that fluorescence and supracence behave the same way toward temperature variation states that both excitations have no heat involvement. Thus, both supracence and fluorescence are independent of, and zero-order to, temperature.
The crucial merit in determining linear supracence or nonlinear anti-Stokes fluorescence is that first-order phenomena are much more sensitive and intense than second-order phenomena, typically orders of magnitude greater (compare Equation (2) to Equation (3)). Thus, supracence is expected to have broader impacts in technological applications than that of second-order anti-Stokes fluorescence because the absolute intensity and sensitivity of supracence will be orders of magnitude higher than those of a nonlinear, two-step process.
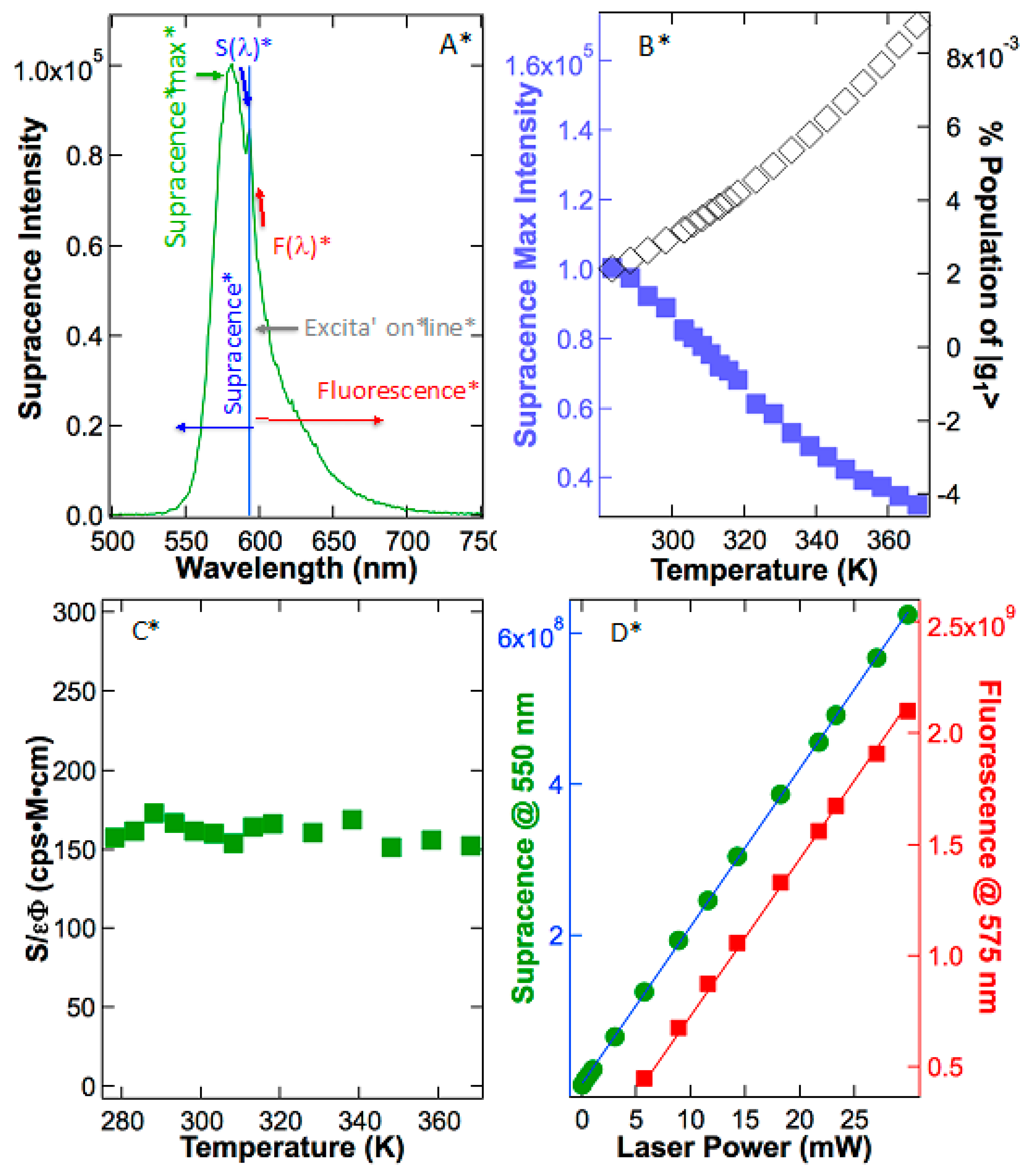
The above analysis of anti-Stokes fluorescence provides the framework to test hypotheses 1 and 2 again and again. In
Figure 5A, rhodamine B was excited at 595 nm, and strong emission with a peak at 581 nm, a higher frequency than that of excitation, is observed. Again, do the emitted photons with higher frequency than that of excitation photons belong to anti-Stokes fluorescence or supracence? If the above-excitation emission were anti-Stokes fluorescence due to absorbing heat energy, the observed intensity would increase as the supplied heat energy increased. As demonstrated in
Figure 5B, supracence intensity did not increase when the solution temperature was increased. Rather, the supracence intensity plummeted as the temperature rose primarily because temperature-induced quenching reduced the dye’s quantum yield. In sulforhodamine 101, the extinction coefficients decrease as the temperature rises mainly causes the reduction in the supracence peak. In rhodamine B, however, quantum yields play the major role in the abatement of supracence peak intensity at elevated temperatures (
Figure 5B).
Following the anti-Stokes mechanism, molecules must
slowly absorb heat obeying Boltzmann distribution. This will not keep up with ultrafast photon excitation, but still offers the molecule a putative way to hot band |g
1〉 once in a long while. For typical rhodamines, the experimental measured vibrational level difference between |g
0〉 and |g
1〉 in an electronic ground state is Δ = 0.15 eV. According to Boltzmann distribution, anti-Stokes fluorescence should curve upward, mirroring the population or concentration increase in |g
1〉 in
Figure 5B (black diamonds) as described in Equation (2), where Δ = ε
1 − ε
0 = 0.15 eV. Had the molecules recovered slowly via Boltzmann equilibrium, the calibrated anti-Stokes fluorescence intensity would have steadily increased from 283 to 368 K by ~4.2 times because the concentration of the “hot molecules” were more than quadrupled. In reality, the emission response does not change at all after calibrating to quantum yield and extinction coefficient changes (
Figure 5C; green squares). Therefore, Boltzmann distribution analyses also confirm that higher energy emission does not originate from the hot band |g
1〉 or anti-Stokes fluorescence. However, temperature independence does support supracence, as illustrated in hypothesis 2 (n = 0).
Specifically, hypothesis 2 does not consider heat as an excitation step; it only considers a single photon excitation as described by Equation (3).
Figure 3D,
Figure 4D and
Figure 5C proved zero-order dependence on temperature, justifying no involvement of temperature during excitation.
Figure 5D reveals that there is a linear relationship between the supracence intensity at 550 nm versus the excitation power at 561 nm (m = 1). This linear relationship is crucially important because it proves that only a single photon is involved in the excitation. In fact, the behavior of the supracence resembles very much like the manner of fluorescence in
Figure 5D, which is also a single-photon-excitation phenomenon. These data prove that supracence is zero-order to temperature (n = 0) and first-order to photoexcitation (m = 1), fitting the supracence mechanism described by Equation (3), and disputing the anti-Stokes mechanism.
2.2. Phantom Theoretical Absorption Peak-Shape
Thus far, all reports on anti-Stokes fluorescence follow the same belief: molecules somehow absorb heat and become vibrationally excited, which are then undergoing below-gap-energy absorption. Specifically, all anti-Stokes fluorescence requires a 1→0 vibronic excitation or absorption, i.e., an electro-optical transition from |g
1〉 to |e
o〉, in which |g
1〉 is a “hot” state because of vibrationally absorbing heat. For dyes such as rhodamines, it is slightly challenging to identify where these vibronic bands are, but it is abundantly obvious for fused-ring aromatic compounds such as perylene diimide [
25,
26,
27].
Figure 6A illustrates the absorption spectrum of perylene diimide, and in particular, the emphasis is that the vibronic positions are clearly identified, with 0→0 at 527 nm, 0→1 at 491 nm and 0→2 at 460 nm. According to the anti-Stokes fluorescence mechanism, it must have an absorption peak at the 1→0 position, which is at 569 nm because such absorption is pivotal to downstream anti-Stokes fluorescence. However, experimental data reveal that there is no peak at the 1→0 position of 569 nm, nor at the 2→0 position of 618 nm. The residue absorbance at the 1→0 position is due to the spillover of 0→0 absorption, a tailing effect. These results conclude that all anti-Stokes fluorescence approaches are building on an imaginary absorption—a mirage that was never identified experimentally. The absorption band, which is responsible for all the anti-Stokes fluorescence, simply does not exist.
The above results demonstrated that there is no absorption resonance at the 1→0 position in absorption spectroscopy. Is there an emission resonance at the 1→0 position using emission spectroscopy?
Figure 6B illustrates the tunable laser exciting fluorescence maximum at 534 nm; as the laser excitation wavelength approaches the 0→0 position (527 nm), the fluorescence maximum intensifies. Similarly, yet in the opposite direction,
Figure 6C illustrates the supracence peak maximum at 534 nm. When the laser excitation line approaches the 0→0 position of 527 nm, the supracence maximum strengthens. Along the path to the 0→0 position, the supracence laser excitation line passes the 1→0 position at 569 nm. According to the anti-Stokes fluorescence theory, there must be a resonance at the 1→0 position because it is the resonance position for anti-Stokes fluorescence and the whole anti-Stokes intensity depends on it. In reality, as shown in
Figure 6D, there is no emission resonance when excitation arrives at the 1→0 position of 569 nm, either.
In fact, when both fluorescence and supracence are converging to the 0→0 position from opposite directions, the emission responds with maximizing intensity (
Figure 6D). Corollary to these observations is that both supracence and fluorescence are exciting into the same 0→0 transition as proved earlier using the full quantum model [
19,
20], in which electrons and atoms are treated as two sub-quantum systems or dual quantum systems. Nevertheless, how does low-energy light excite into the higher 0→0 electro-optical transition to create high-energy supracence?
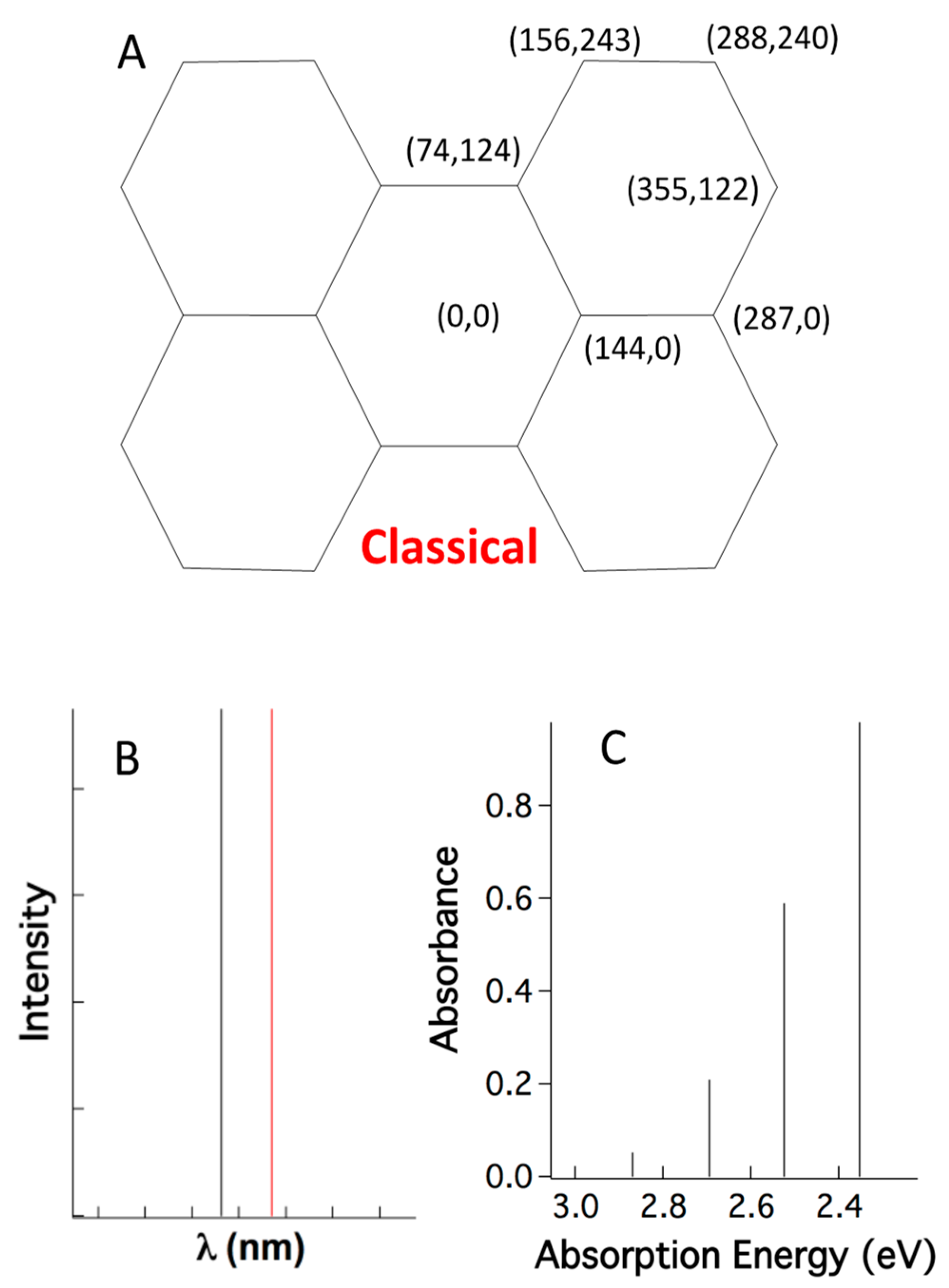
2.3. A Single Classical Structure versus Myriad Quantum Structures
The enigma is that, as yet, no known linear optical processes create output photons with more energy than that of input photons. The reason that current understanding cannot rationalize supracence is that it drops the dual quantum realism and treats atoms in molecules as non-quantum particles. The quantum treatment of vibrating atoms stands alone and does not interact and integrate with the electronic quantum results. This way, quantum chemistry typically fixes the coordinates of atoms (
Figure 7A) and determines the quantum behavior of the electrons. Thus, the atoms with fixed positions are treated as classical Newtonian particles, while the electrons are treated as quantum particles. In other words, the vibrational wavefunction of atoms |υ〉 is reduced to a pair of classical coordinates (x, y), thus ignoring quantum structural dynamics. This classical-quantum hybrid treatment yields bizarre stick spectra for electronic absorption (
Figure 7B, black stick) and emission (
Figure 7B, red stick). After calculating vibrational levels and electronic levels separately, and then simply adding them together, the “coupled” vibronic absorption spectrum follows the Franck–Condon progression [
28], but still comprises sticks, unlike experimentally broad and smooth peaks (
Figure 7C).
Thus far, the hybrid treatment (Newtonian atoms mixed with quantum electrons) of molecules or a single-quantum system has typically been applied in explaining experimentally observed peaks in electronic spectra using many congested rotational levels associated with electronic transition [
29]. However, dyes in a solid state do not rotate and their absorptions are still not sticks [
30,
31]. This puzzling disparity leads to another explanation of sample inhomogeneity [
32,
33], but the unique peak shape must originate from a matching inhomogeneous sub-species population, yet there is no such evidence. Importantly, one single molecule has no inhomogeneity and, thus, single-molecule emission has no inhomogeneous issues; however, single-molecule spectra still uniquely shape into broad peaks, not sticks [
34,
35,
36,
37].
Experimental data prove hypothesis 2 is correct, but what is the underpinning foundation for this full (electron and atoms) quantum dynamic model? In fact, an atom trapped by chemical bonds is a quantum particle in an ellipsoid potential well and must be treated quantum mechanically so that vibrational quantum levels emerge. Mixing Newtonian mechanics and the Schrodinger equation is physically meaningless. In full quantum treatment, the atoms in their molecule no longer have fixed classical positions, rather they are moving in ellipsoidal quantum orbitals (
Figure 8A and
Movie S1). For example, atoms with symmetric bonds (center ring of perylene) have quantum orbitals that are nearly spherical, resembling much of the electronic s-orbital. Atoms without symmetric bonds, such as periphery carbons of perylene, have orbitals that are ellipsoidal due to asymmetry (
Figure 8A). These are quantum orbitals describing the positional probability of the atoms and should not be confused with conventional “atomic orbitals” that describe electrons.
Applying full quantum mechanics to both electrons and atoms results in two sub-quantum systems for electrons φ
g and atoms υ, respectively, but these two sub-quantum or dual quantum systems are not independent; rather they (φ
g and υ) are entangled [
38]. In any molecule, lighter quantum electrons, φ
g, are entangled, coupled, and integrated with the heavier quantum atoms, υ, resulting in convoluted phenomena and, hence, intricate optical spectra. In such full quantum systems, the energy levels of the heavy quantum particles or atoms remain moderately discrete and fluctuate due to potential limitations, but lighter quantum particles or electrons constantly change their energy levels within a narrow distribution to satisfy the quantum dynamics of the atoms and molecular potential energy restricting the atoms. Such dynamic electronic energy levels predict that absorption and emission must disperse from a single line into uniquely structured peaks (not sticks) according to the quantum effects of the atoms (
Figure 8B). This means both ground states and excited states have energy level distribution rather than a single discrete level, thus rationalizing both fluorescence and supracence will occur.
The quantum interaction slates that the probability of vibrational transitions of the heavier quantum atoms is convoluted with the broad, intricate electronic absorption of the lighter quantum electrons (
Figure 8B), thus yielding realistic vibronic absorption and emission spectra (
Figure 8C: circles) in excellent agreement with experimental observation (
Figure 8C: line). Applying Fermi’s Golden rule to both electron wavefunction, φ
g, and atom wavefunctions υ results in the observed vibrational transition between different vibrational states, which is governed by Franck–Condon factors (
Figure 8C) with characteristics of the progressive envelope, i.e., |〈0|0〉|
2 > |〈1|0〉|
2 > |〈2|0〉|
2, etc. [
28,
39]. The validation of intricate peak shape enlighteningly endorses that full quantum treatment fundamentally underpins molecular absorption, emission, and supracence.
Of particular importance is the quantum interaction energy caused by the dynamics of the dual sub-quantum systems of atoms and electrons. Here, we will write the general Hamiltonian as a sum of three parts (Equation (4)), where
is the Hamiltonian for atoms,
is the Hamiltonian for electrons, and
is the interaction Hamiltonian between atoms and electrons [
40,
41]. To compute the quantum interaction energy, we use the Born–Oppenheimer or adiabatic ansatz |φ
g(t)〉|υ(t)〉 and plug into the Born rule. The conclusion explicitly reveals that the quantum interaction energy,
is dynamically evolving as a function of time, even if a non-entangled state, |φ
g(t)〉|υ(t)〉, is used [
38]. This energy exchange between atoms and electrons is depicted in
Figure 2C and
Figure 9.
The full-quantum treatment of molecules requires that Schrodinger equations for
both atoms and electrons within a molecule be solved
simultaneously, either entangled or non-entangled [
19]. Precisely, the lighter quantum particles and their electronic transitions are correlated, concatenated, and entangled to the positions of the heavier quantum particles. The single-quantum system of molecules only solves the electron Schrodinger equation using Newtonian coordinates to describe the atoms, while ignoring entanglement and quantum interaction, thus leaving some physical properties, e.g., absorption intricate peak shape, emission tails, and supracence, dangling in no-man’s land. Considering a full-quantum system for molecules, however, divulges both realistic molecular absorption and emission properties and predicts the existence of novel supracence spectroscopy and microscopy. Because the positions of quantum atoms vary continuously and smoothly in a Gaussian-like fashion, the optical absorption and emission are also shaped into smooth Gaussian-like peaks accordingly.
The full quantum treatment of molecular systems using adiabatic ansatz can be explicitly solved [
42]. A simple harmonic potential well could be used for the molecular ground state and Morse potential could be used for an excited state potential well, both work well because they produce emission and absorption peaks matching experimental spectra. One important conclusion replaces the Newtonian coordinates with an atom probability map, such as in
Figure 8A. Another important conclusion is that the quantum energy of the atoms and the quantum energy of the electrons are constantly exchanging while time elapses, as evidenced in Equation (5). Molecular potential energy exchange atom quantum energy, which in turn exchange with electronic energy. These energy flows dominate within the molecule. Because optical absorption and emission are ultrafast; at typical emission rates, heat transfer is insufficient to keep up with the above energy flows. As such, ultrafast absorption and emission and full quantum theory simply rule out anti-Stokes fluorescence or heat involvement during a repeated optical transition.
In the full quantum model, using Born–Oppenheimer approximation, and further isolating each atom of an
N-atom molecule in its potential well, there are
N sub-Schrodinger equations for atoms, but there are only
3N–6 independent vibrational energies/frequencies for a non-linear molecule, because when all
N atoms are moving in synchrony, the whole molecule is translating or rotating along an axis, the quantum effect disappears and the molecule simply follows Newtonian mechanics, drifting, diffusing, or spinning. These
irreducible 3
N–6 vibration frequencies correspond to the conventional normal modes but are here regarded as molecular phonons because it is more logical considering their energy exchange within the molecular system. Each phonon has a characteristic vibration frequency (
), which may be either infrared or Raman active, but it also stores or releases internal potential energy (
P) by varying the barriers of limiting potential wells, which manifests as the amplitude of atomic displacements (x, y, or z):
Pk = ½(
kxx
2 +
kyy
2 +
kzz
2), i.e., the changes in bond length during quantum dynamics (
Figure 2C and
vide infra).
Precisely, the full quantum treatment of molecules manifests as the lighter quantum system of electrons riding on the heavier quantum system of atoms. The parallel example in Newtonian mechanics is a motorcycle traveling on top of a moving train. One would reach surreal conclusions about the motorcycle had the motion of the train been ignored. Because experimentally atoms for the most part retain their identity in molecules, full quantum theory describes molecules more realistically than dual quantum sub-systems using Born–Oppenheimer approximation, which is still more realistic than single-quantum models that do not update the origins of electron orbitals according to the position of the quantum atom, such as describing the motorcycle speed without considering the train. As such, the electronic energy levels are determined by not only the electrons but also by the atoms, whose quantum movement cannot be ignored. For example, Newtonian–quantum hybrid treatment of a dye fixes the positions of all conjugate p-orbitals, thus placing supracence in no-man’s land. However, the full quantum principle recognizes that each p-orbital is riding on a quantum atom and constantly “dancing” according to the probability produced by the orbital of the atom, υ (see
Movie S2). Note, atoms are treated quantum mechanically, not nuclei. The electron orbitals (for example, p orbitals above) are convoluted by the orbital of atoms unlike single-quantum models, in which the electron orbital is not convoluted. In such concatenated quantum systems, absorption and emission automatically shape into intricate peaks and supracence emerges naturally.
Proof of the quantum behavior of atoms comes from the infrared and Raman vibrational spectroscopy experimentally. Had atoms in a molecule behaved according to classical Newtonian mechanics, infrared and Raman spectra would not exist, because classical particles do not have quantized energy levels, hence absorbing and emitting light. Another proof of quantum atoms originates from experimental vibronic bands in absorption (
Figure 6 and
Figure 8C). Electronic absorption having fine vibronic structures also concludes that atoms in molecules behave according to dual sub-quantum systems, rather than Newtonian particles plus a single electronic quantum system.
2.4. Full Quantum (FQ) Molecules Make All Pieces Fall into Place
Because the lighter quantum system of electrons and the heavier quantum system of atoms, namely, molecular phonons, are entangled, coupled, concatenated, and bonded, together they produce vibronic bands due to strong electron–phonon coupling. This coupling goes beyond simply adding the energy levels that are solved separately, but rather each move of the atoms or phonons creates a different transition energy for the electrons. While this is under-appreciated, other correlations due to the dual interacting quantum systems have not broadly been studied thus far. FQ theory predicts that many properties may be correlated in a predictable way. The two properties concerning supracence, for example, are bond elongation (structural variation) produced by the quantum effect of atoms and transition energy in absorption or emission spectra determined by the electronic states. Here, we show that the bond elongation and transition gap are correlated or entangled, such that a change in one will result in a predictable trend in the other.
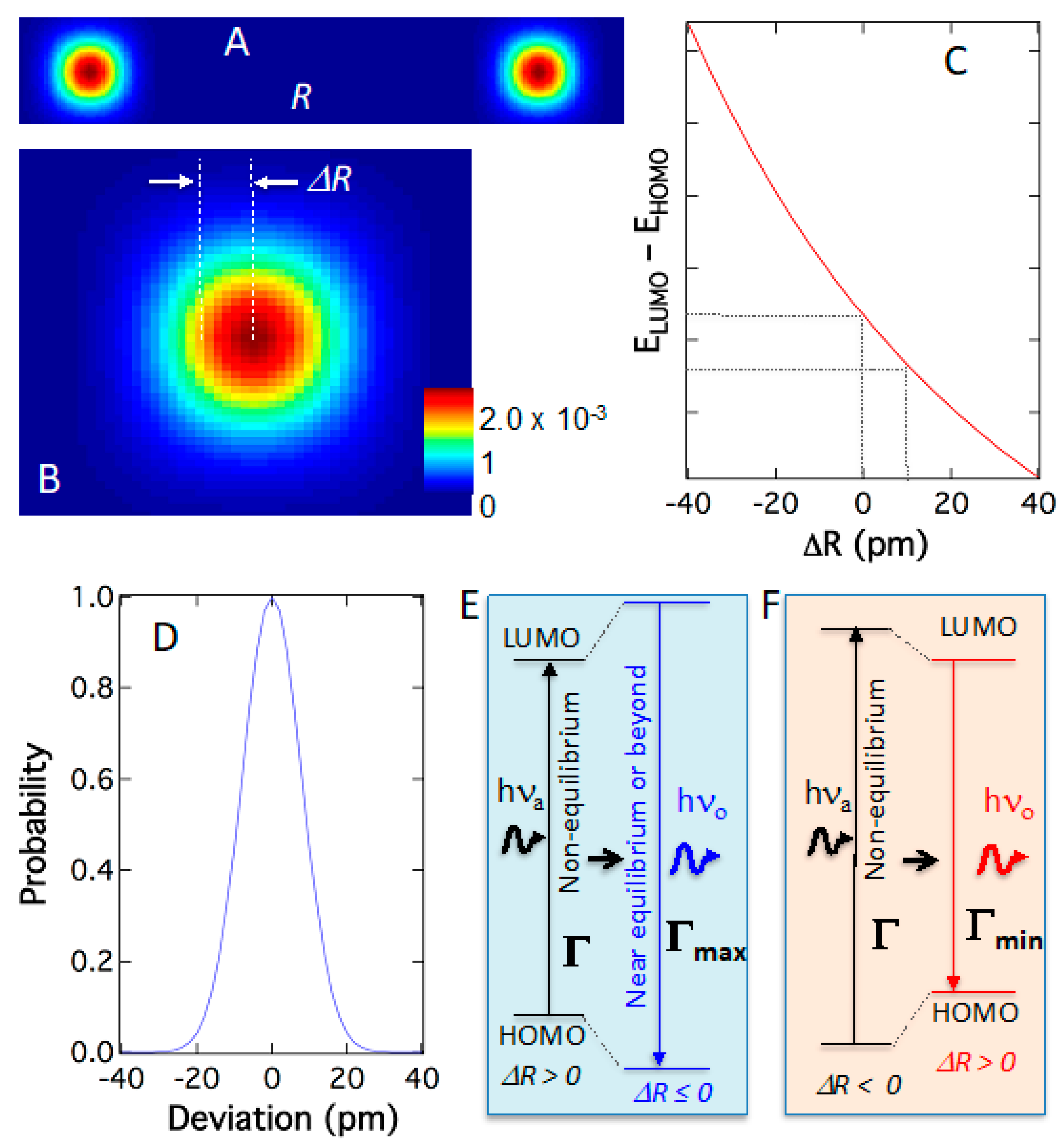
Figure 9A depicts the simplest model of two-atom in simplified full quantum treatment, where each atom distributes according to its wavefunction squared (probability), not classical coordinates. The reason for the simplest model is that current computation power is not adequate for calculating a single quantum system and consequently full quantum computations for dyes are beyond current computation capacity. Nonetheless, the full quantum model leads us in the correct directions. When the quantum atom elongates the bond or Δ
R > 0 according to its quantum probability (
Figure 9B), the electronic energy levels change; accordingly, particularly the absorption-or-emission gap or transition energy
ELUMO–EHOMO diminishes (
Figure 9C). Thus, as the quantum atoms dance in their orbitals following pre-determined quantum probability [υ]
2, the transition energy disperses into a peak-shaped distribution around their most probable positions as shown in
Figure 9D. These phenomena originate from quantum–quantum effects intrinsically and are independent of temperature or other perturbations. Such a correlated relationship reveals that the atomic position and electronic transition are strongly correlated. Therefore, bond elongation correlates to the shrinkage of electronic transition energy for absorption or emission and
vice versa. This demonstrates that the quantum dynamics of the atoms will result in spectral dispersion in absorption and emission. Importantly, this means that there are multiple energies for absorption or emission, all within a narrow distribution of energy. Some absorb more energy and emit less, while others absorb less and emit more energy. For example, absorbing at elongated bonds and emitting at shortened bonds will result in supracence (
Figure 9E), and conversely, fluorescence (
Figure 9F).
Relevant to our discussions are three forms of energies within a molecule: total electronic bonding energy (), total phonon vibrational energy () from 3N-6 molecular phonons, and the total internal potential energy (P) stored within all chemical bonds and their molecular structure that forms the potential wells for holding the atoms in place. Energy conservation or the thermodynamic first law requires their sum or total energy to be constant in adiabatic conditions without photo-absorption or emission (E + A + P = constant). In full quantum systems, the quantum movements of the heavier system are dictating the system, intrinsic and inalienable, maintaining a constant energy (A); thus, the first FQ law is that the total electron-bonding energy (E) of the lighter quantum system must fluctuate due to such motion of heavier quantum particles. Experimentally, such fluctuation is the origin of the absorption or emission intricate peak shape instead of a single-quantum stick. The fluctuating electronic total energy seminally advances our understanding of current static energy minimum that yields a fixed molecular structure. Thus, the single-quantum system yields a single classical molecular structure, but the electron–atom dual-quantum systems predict not a single molecular structure, rather a series of quantum dynamic structures with a smooth distribution.
In view of FQ molecules, energies (E, A, or P) can freely exchange. Potential energy (P) from a molecular structure and bonds set up the potential wells for the atom to behave quantum mechanically and determine its vibrational energy (A). Moreover, the quantum movement of the atoms is constantly changing the energy landscape for the electrons (E) and regulates the energies of electronic orbitals. Naturally and significantly, potential energy (P) and quantum energies (A and E) flow back and forth like current.
Because the total energy of the molecule is constant, the second enlightening FQ principle reveals that the electronic quantum energy must exchange with the molecular internal potential energy in the bonds to maintain the total energy constant, i.e., high E with low P and vice versa. Because phonon energy A can be considered as a constant in each state for the molecule before or after photoexcitations, the sum of electron energy E and potential energy P must be constant (E + P = constant), too. During photoexcitation, phonon energy A is facilitating energy exchange from E and P or vice versa. The fluctuation of electron energy E requires the potential energy P to mirror its fluctuation and change accordingly. Otherwise, molecules cannot exist. In a vacuum, for example, a molecule must exchange its total electronic bonding energy with internal energy, because no surrounding functioning as an energy reservoir for it to deposit energy to or to recover energy from. Thus, the molecule must have a mechanism to store energy internally, in the form of internal potential energy, P. Therefore, the correlation between bond elongation and electronic transition gap shrinkage or bond shortening and transition gap expansion is facilitated by the potential energy stored in chemical bonds and molecular structures.
In short, molecular structures or bonds function as an energy reservoir during photoexcitation. Such energy exchange, which originates from the electron and atom dual quantum entanglement, is unique for molecules, and it disappears completely in atomic absorption or emission that is purely a single quantum system. Thus, molecules enable supracence, but single atoms or ions without bonds cannot produce supracence.
When photoexcitation occurs in bond-stretched structures (Δ
R > 0; far from equilibrium and lower transition energy) and subsequent relaxation brings the molecule to near-equilibrium structures for emission (Δ
R ≈ 0 and higher transition energy), the molecule will emit a photon with more energy than that absorbed (
Figure 9E). This is supracence [
19,
20]. Considering the photon and the molecule as a thermodynamic system before absorption (
EA,
AA,
PA) or after emission (
EE,
AE,
PE) leads to energy conservation, i.e.,
EA +
AA +
PA +
hνA =
EE +
AE +
PE +
hνE, where ν
A and ν
E are absorbed and emitted photon frequencies, respectively. When the molecule returns to the same quantum state (equilibrium structures for both atoms and electrons) after emission, all quantum energies are canceled due to the same quantum state (
EA =
EE &
AA =
AE). A conclusion expressed in Equation (6) thus emerges, where Δ
ν =
νE −
νA. Supracence hence occurs when Δ
P =
PA −
PE > 0 and fluorescence occurs when Δ
P < 0 (
Figure 9F). A stretched quantum bond is analogous to a stretched mechanical bow, both building up potential energy in it. The stretched bow releases its potential energy to accelerate an arrow and likewise, the stretched quantum bond releases its strain, through quantum-potential energy exchange (
vide supra), to emit a high-energy photon or a quantum “arrow” with a different color in the case of supracence. Thus, a quantum–quantum interaction between electrons and atoms unfolds as molecular quantum archery that imparts supracence.
One clarification worth mentioning is where does the energy of supracence come from. This question becomes clear when considering typical excitations in which a molecule absorbs multiple photons (M). Some photons result in fluorescence yielding negative Δ
P, while other photons impart supracence producing positive Δ
P. Yet, still, non-radiative processes end up negative Δ
P. Therefore, the sum of all Δ
P is approximately zero, retaining the molecule in its original ground state (Equation (7)). The molecule functions as an energy reservoir. In summary, supracence gains energy probably at the expense of fluorescence and other energy-related processes.
2.5. Super-Spectral Resolution of Supracence Spectroscopy and Imaging—Experimental Validation
The value of a theory resides in its ability to guide experiments. Many theories fail to guide experiments and remain as an after-facts tool to rationalize what was observed. The electron–atom quantum entanglement predicts that the supracence peak will be near-Gaussian-like and narrow, which was validated experimentally in supracence imaging below.
In the electron–atom quantum interaction, the electronic transitions, both absorption and emission, are restricted within the maximum (Γ
max) and minimum (Γ
min) gaps between HOMO (E
HOMO) and LUMO (E
LUMO) (
Figure 2C and
Figure 9E,F). The maximum (Γ
max) and minimum (Γ
min) transition energies are the results of energy exchange with quantum atoms restricted by the molecular internal potential energies. As demonstrated in
Figure 8A, the quantum movements of the atoms are severely limited by molecular potentials, and the energy-gain range available for supracence is rather limited, δΓ = Γ
max − Γ
min. This narrow fluctuation of electronic transition energy (δΓ) originated from the quantum effect of the atoms is compensated by the molecular potential energy (
P), which fundamentally results in a super-spectral resolution in supracence spectroscopy and imaging.
Only when excitation photons (Γ) have energies in-between maximum (Γ
max) and minimum (Γ
min) transition energies, i.e., Γ
min < Γ < Γ
max (
Figure 2C and
Figure 9E,F), supracence can occur, because there is no absorption when Γ < Γ
min, and there is no supracence when Γ > Γ
max as the energy gap cannot expand anymore. Anti-Stokes fluorescence would have excited far lower than Γ
min using the phantom hot band, while fluorescence will continue to excite the molecule above excitation energy above Γ
max. Both fluorescence and anti-Stokes fluorescence have no excitation specificity, thus producing broad excitation and emission bands. Supracence, however, has a narrow excitation band and a narrow emission band between Γ
max and Γ
min.
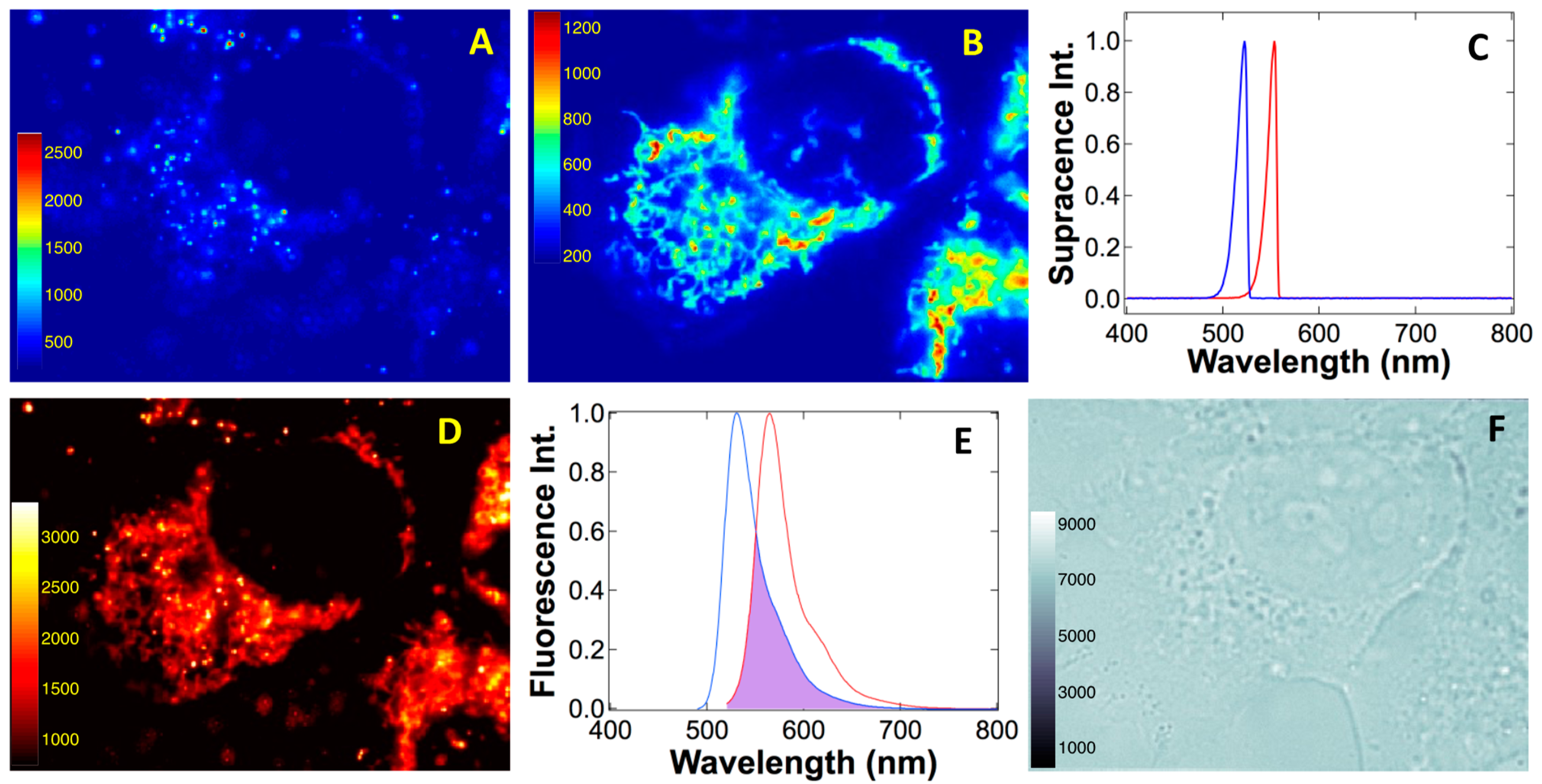
To demonstrate super-spectral resolution created by electron–atom quantum interaction and entanglement,
Figure 10 contrasts supracence spectra and imaging to those of fluorescence. Rhodamine 123 (Rh123) and rhodamine B (RhB) have peak-maximum separation of only 32 nm, but their supracence windows δΓ
Rh123 and δΓ
Rh123 practically do not overlap. As a result, we selectively excited RhB within nanoparticles (561 nm) used to label endosomes and lysosomes in living cells [
43], but Rh123 supracence in mitochondria was not excited (
Figure 10A). Conversely, when we excited Rh123 supracence in mitochondria using 532 nm, no RhB supracence was observed (
Figure 10B). Supracence spectroscopy reveals the super-spectral resolution in
Figure 10C. Because of the mitochondria worm-like patterns [
44,
45,
46], endosome/lysosome sharp spots [
47,
48,
49,
50] can be easily recognized, and they were chosen to corroborate the super spectral resolution.
Supracence imaging of mitochondria has no measurable crosstalk from endosomes/lysosomes. Moreover, endosomes/lysosomes imaging has no mitochondrial encroachment. Fluorescence imaging at 532 nm, however, reveals both RhB sharp spots of endosomes/lysosomes and Rh123 worm-like patterns of mitochondria (
Figure 10D). Not only is fluorescence excitation not selective, but, also, broad fluorescence emissions have a strong overlap in a large spectral region (
Figure 10E). These results demonstrate the superiority of supracence at wavelength multiplexing and dyes with absorption or emission peaks separated by only 32 nm that can be effectively resolved during the imaging of a living cell (
Figure 10F).