Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation
Abstract
1. Introduction
2. HIV: A Cause of PAH
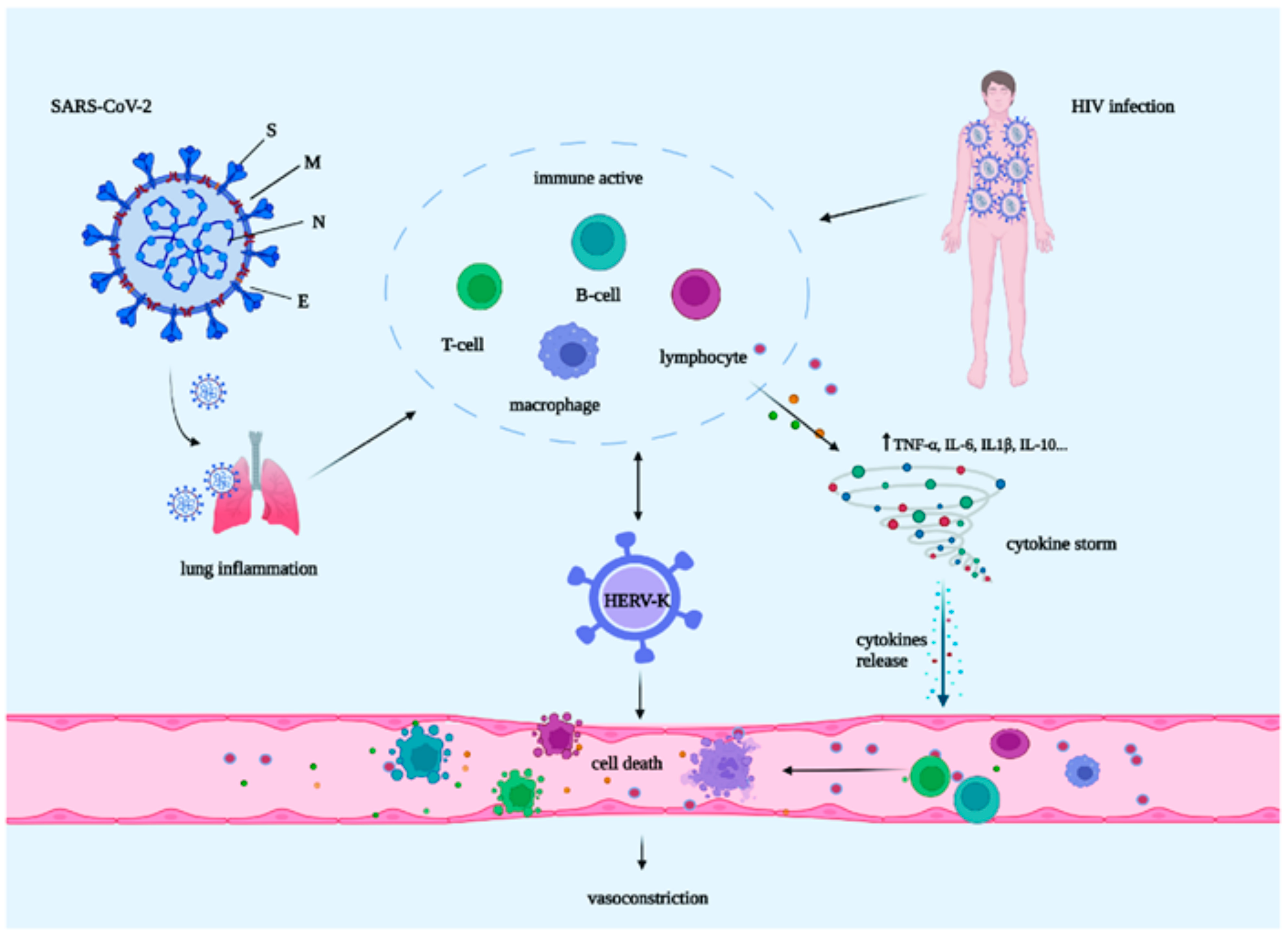
3. Role of SARS-CoV-2
3.1. SARS-CoV-2 and PAH
3.2. SARS-CoV-2 and HIV
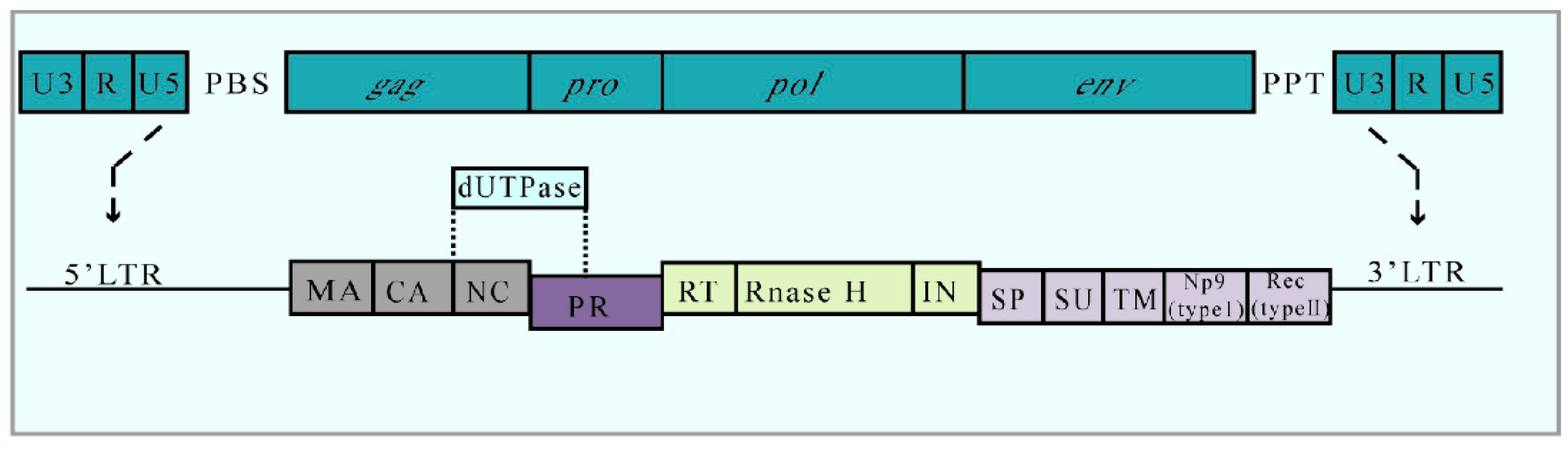
4. HERV-K
4.1. HERV-K and PAH
4.2. SARS-CoV-2 Promotes HERV-K Activation
4.3. The Relationship between HERV-K and HIV
5. The Potential Mechanism of SARS-CoV-2/HERV-K/HIV in PAH
6. Medical Therapy in PAH
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAH | Pulmonary arterial hypertension |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| HERV-K | Human endogenous retrovirus K |
| HIV | Human immunodeficiency virus |
| PAECs | Pulmonary artery endothelial cells |
| PASMCs | Pulmonary artery smooth muscle cells |
| HERVs | Human endogenous retroviruses |
| PH | Pulmonary hypertension |
| SMCs | Smooth muscle cells |
| ECs | Endothelial cells |
| RT | Reverse transcriptase |
| gp120 | Glycoprotein 120 |
| Env | Envelope |
| ART | Antiretroviral therapy |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin 6 |
| BMPR2 | Bone morphogenetic protein receptor type 2 |
| SAM | Sterile alpha motif |
| HD | Histidine–aspartate |
| SAMHD1 | SAM domain and HD domain-containing protein 1 |
| dNTPs | Deoxynucleotide triphosphates |
| gp120 | Glycoprotein 120 |
| ET-1 | Endothelin 1 |
| ORFs | Open reading frames |
| ACE2 | Angiotensin-convertingenzyme2 |
| S | Spike |
| E | Envelope |
| M | Membrane |
| N | Nucleocapsid |
| PVR | Pulmonary vascular resistance |
| PLWH | People living with HIV |
| dUTPase | Deoxyuridine triphosphate nucleoside Hydrolase |
| LTR | Long-terminal repeat |
| cGAS-STING | Cyclic GMP–AMP synthase–stimulator of Interferon genes |
| TM | Transmembrane units |
| MA | Matrix |
| CA | Capsid |
| NC | Nucleocapsid |
| PBMCs | Peripheral blood mononuclear cells |
| PTAP | Pro-Thr-Ala-Pro |
| ESCORT | Endosomal sorting complex required for transport |
| ARDS | Acute respiratory distress syndrome |
| NO | Nitric oxide |
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Heath, D.; Edwards, J.E. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Decrion, A.Z.; Dichamp, I.; Varin, A.; Herbein, G. HIV and inflammation. Curr. HIV Res. 2005, 3, 243–259. [Google Scholar] [CrossRef]
- Masiá, M.; Gutiérrez, F. HIV-related cardiovascular risk factors. Enferm. Infecc. Microbiol. Clin. 2009, 27 (Suppl. 1), 17–23. [Google Scholar] [CrossRef]
- Saito, T.; Miyagawa, K.; Chen, S.Y.; Tamosiuniene, R.; Wang, L.; Sharpe, O.; Samayoa, E.; Harada, D.; Moonen, J.A.J.; Cao, A.; et al. Upregulation of Human Endogenous Retrovirus-K Is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension. Circulation 2017, 136, 1920–1935. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Opravil, M.; Sereni, D. Natural history of HIV-associated pulmonary arterial hypertension: Trends in the HAART era. AIDS 2008, 22 (Suppl. 3), S35–S40. [Google Scholar] [CrossRef]
- Halawa, S.; Pullamsetti, S.S.; Bangham, C.R.M.; Stenmark, K.R.; Dorfmüller, P.; Frid, M.G.; Butrous, G.; Morrell, N.W.; de Jesus Perez, V.A.; Stuart, D.I.; et al. Potential long-term effects of SARS-CoV-2 infection on the pulmonary vasculature: A global perspective. Nat. Rev. Cardiol. 2022, 19, 314–331. [Google Scholar] [CrossRef]
- Terry, S.N.; Manganaro, L.; Cuesta-Dominguez, A.; Brinzevich, D.; Simon, V.; Mulder, L.C.F. Expression of HERV-K108 envelope interferes with HIV-1 production. Virology 2017, 509, 52–59. [Google Scholar] [CrossRef]
- Kitsou, K.; Kotanidou, A.; Paraskevis, D.; Karamitros, T.; Katzourakis, A.; Tedder, R.; Hurst, T.; Sapounas, S.; Kotsinas, A.; Gorgoulis, V.; et al. Upregulation of Human Endogenous Retroviruses in Bronchoalveolar Lavage Fluid of COVID-19 Patients. Microbiol. Spectr. 2021, 9, e0126021. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Blanco, J.L.; Reyes-Urueña, J.M.; Davies, M.A.; Sued, O.; Marcos, M.A.; Martínez, E.; Bertagnolio, S.; Alcamí, J.; Miro, J.M. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV 2021, 8, e294–e305. [Google Scholar] [CrossRef] [PubMed]
- Sulica, R.; Cefali, F.; Motschwiller, C.; Fenton, R.; Barroso, A.; Sterman, D. COVID-19 in Pulmonary Artery Hypertension (PAH) Patients: Observations from a Large PAH Center in New York City. Diagnostics 2021, 11, 128. [Google Scholar] [CrossRef]
- Turner, B.G.; Summers, M.F. Structural biology of HIV. J. Mol. Biol. 1999, 285, 1–32. [Google Scholar] [CrossRef]
- Barbosa, P.; Kneass, Z. Molecular biology of HIV. Clin. Podiatr. Med. Surg. 1998, 15, 189–202. [Google Scholar]
- Lucas, S.; Nelson, A.M. HIV and the spectrum of human disease. J. Pathol. 2015, 235, 229–241. [Google Scholar] [CrossRef]
- Wilen, C.B.; Tilton, J.C.; Doms, R.W. HIV: Cell binding and entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866. [Google Scholar] [CrossRef]
- Varbanov, M.; Espert, L.; Biard-Piechaczyk, M. Mechanisms of CD4 T-cell depletion triggered by HIV-1 viral proteins. Aids Rev. 2006, 8, 221–236. [Google Scholar] [PubMed]
- Moir, S.; Chun, T.W.; Fauci, A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. 2011, 6, 223–248. [Google Scholar] [CrossRef]
- Gallant, J.; Hsue, P.Y.; Shreay, S.; Meyer, N. Comorbidities among US Patients with Prevalent HIV Infection—A Trend Analysis. J. Infect. Dis. 2017, 216, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Worm, S.W.; De Wit, S.; Weber, R.; Sabin, C.A.; Reiss, P.; El-Sadr, W.; Monforte, A.D.; Kirk, O.; Fontas, E.; Dabis, F.; et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study). Circulation 2009, 119, 805–811. [Google Scholar] [CrossRef]
- Humbert, M. Mediators involved in HIV-related pulmonary arterial hypertension. Aids 2008, 22 (Suppl. 3), S41–S47. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E.; Ali, A.M.; Barbaryan, A.; Prueksaritanond, S. Human immunodeficiency virus and pulmonary arterial hypertension. ISRN Cardiol. 2013, 2013, 903454. [Google Scholar] [CrossRef]
- Hofman, F.M.; Wright, A.D.; Dohadwala, M.M.; Wong-Staal, F.; Walker, S.M. Exogenous tat protein activates human endothelial cells. Blood 1993, 82, 2774–2780. [Google Scholar] [CrossRef]
- Almodovar, S.; Swanson, J.; Giavedoni, L.D.; Kanthaswamy, S.; Long, C.S.; Voelkel, N.F.; Edwards, M.G.; Folkvord, J.M.; Connick, E.; Westmoreland, S.V.; et al. Lung Vascular Remodeling, Cardiac Hypertrophy, and Inflammatory Cytokines in SHIVnef-Infected Macaques. Viral Immunol. 2018, 31, 206–222. [Google Scholar] [CrossRef]
- Stumptner-Cuvelette, P.; Morchoisne, S.; Dugast, M.; Le Gall, S.; Raposo, G.; Schwartz, O.; Benaroch, P. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 2001, 98, 12144–12149. [Google Scholar] [CrossRef]
- Ferdin, J.; Goričar, K.; Dolžan, V.; Plemenitaš, A.; Martin, J.N.; Peterlin, B.M.; Deeks, S.G.; Lenassi, M. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS ONE 2018, 13, e0191613. [Google Scholar] [CrossRef]
- Chelvanambi, S.; Bogatcheva, N.V.; Bednorz, M.; Agarwal, S.; Maier, B.; Alves, N.J.; Li, W.; Syed, F.; Saber, M.M.; Dahl, N.; et al. HIV-Nef Protein Persists in the Lungs of Aviremic Patients with HIV and Induces Endothelial Cell Death. Am. J. Respir. Cell Mol. Biol. 2019, 60, 357–366. [Google Scholar] [CrossRef]
- Chelvanambi, S.; Gupta, S.K.; Chen, X.; Ellis, B.W.; Maier, B.F.; Colbert, T.M.; Kuriakose, J.; Zorlutuna, P.; Jolicoeur, P.; Obukhov, A.G.; et al. HIV-Nef Protein Transfer to Endothelial Cells Requires Rac1 Activation and Leads to Endothelial Dysfunction Implications for Statin Treatment in HIV Patients. Circ. Res. 2019, 125, 805–820. [Google Scholar] [CrossRef]
- Wang, T.; Green, L.A.; Gupta, S.K.; Amet, T.; Byrd, D.J.; Yu, Q.; Twigg, H.L., 3rd; Clauss, M. Intracellular Nef detected in peripheral blood mononuclear cells from HIV patients. AIDS Res. Hum. Retrovir. 2015, 31, 217–220. [Google Scholar] [CrossRef]
- Wang, T.; Green, L.A.; Gupta, S.K.; Kim, C.; Wang, L.; Almodovar, S.; Flores, S.C.; Prudovsky, I.A.; Jolicoeur, P.; Liu, Z.; et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS ONE 2014, 9, e91063. [Google Scholar] [CrossRef]
- Dupuis, J. Endothelin: Setting the scene in PAH. Eur. Respir. Rev. 2007, 16, 3–7. [Google Scholar] [CrossRef]
- El Chami, H.; Hassoun, P.M. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog. Cardiovasc. Dis. 2012, 55, 218–228. [Google Scholar] [CrossRef]
- Ehrenreich, H.; Rieckmann, P.; Sinowatz, F.; Weih, K.A.; Arthur, L.O.; Goebel, F.D.; Burd, P.R.; Coligan, J.E.; Clouse, K.A. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J. Immunol. 1993, 150, 4601–4609. [Google Scholar] [CrossRef]
- Kanmogne, G.D.; Primeaux, C.; Grammas, P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem. Biophys. Res. Commun. 2005, 333, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Stelzner, T.J.; O’Brien, R.F.; Yanagisawa, M.; Sakurai, T.; Sato, K.; Webb, S.; Zamora, M.; McMurtry, I.F.; Fisher, J.H. Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am. J. Physiol. 1992, 262, L614–L620. [Google Scholar] [CrossRef]
- Fuji, S.; Matsushita, S.; Hyodo, K.; Osaka, M.; Sakamoto, H.; Tanioka, K.; Miyakawa, K.; Kubota, M.; Hiramatsu, Y.; Tokunaga, C. Association between endothelial function and micro-vascular remodeling measured by synchrotron radiation pulmonary micro-angiography in pulmonary arterial hypertension. Gen. Thorac. Cardiovasc. Surg. 2016, 64, 597–603. [Google Scholar] [CrossRef]
- Shao, D.; Park, J.E.; Wort, S.J. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharm. Res. 2011, 63, 504–511. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Yacoub, M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 2014, 2014, 62–78. [Google Scholar] [CrossRef]
- Parikh, R.V.; Ma, Y.; Scherzer, R.; Heringer, A.S.; Macgregor, J.S.; Martin, J.N.; Deeks, S.G.; Ganz, P.; Hsue, P.Y. Endothelin-1 Predicts Hemodynamically Assessed Pulmonary Arterial Hypertension in HIV Infection. PLoS ONE 2016, 11, e0146355. [Google Scholar] [CrossRef] [PubMed]
- McMillen, M.A.; Huribal, M.; Cunningham, M.E.; Kumar, R.; Sumpio, B.E. Endothelin-1 increases intracellular calcium in human monocytes and causes production of interleukin-6. Crit. Care Med. 1995, 23, 34–40. [Google Scholar] [CrossRef]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef]
- Vattulainen-Collanus, S.; Southwood, M.; Yang, X.D.; Moore, S.; Ghatpande, P.; Morrell, N.W.; Lagna, G.; Hata, A. Bone morphogenetic protein signaling is required for RAD51-mediated maintenance of genome integrity in vascular endothelial cells. Commun. Biol. 2018, 1, 149. [Google Scholar] [CrossRef]
- Tcherakian, C.; Rivaud, E.; Catherinot, E.; Zucman, D.; Metivier, A.C.; Couderc, L.J. Pulmonary arterial hypertension related to HIV: Is inflammation related to IL-6 the cornerstone? Rev. Pneumol. Clin. 2011, 67, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, P.; O’Brien-Ladner, A.; Dhillon, N.K. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: Implications for HIV-related pulmonary arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2585–2595. [Google Scholar] [CrossRef]
- Caldwell, R.L.; Gadipatti, R.; Lane, K.B.; Shepherd, V.L. HIV-1 TAT represses transcription of the bone morphogenic protein receptor-2 in U937 monocytic cells. J. Leukoc. Biol. 2006, 79, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lahouassa, H.; Daddacha, W.; Hofmann, H.; Ayinde, D.; Logue, E.C.; Dragin, L.; Bloch, N.; Maudet, C.; Bertrand, M.; Gramberg, T.; et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 2012, 13, 223–228. [Google Scholar] [CrossRef]
- Jermy, A. Viral infection: SAMHD1 cuts the power to HIV-1. Nat. Rev. Microbiol. 2012, 10, 237. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Holmes, E.C. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell 2020, 181, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tong, Z.; Guan, X.; Du, B.; Qiu, H. Clinical Characteristics of Patients Who Died of Coronavirus Disease 2019 in China. JAMA Netw. Open 2020, 3, e205619. [Google Scholar] [CrossRef]
- Chen, R.; Liang, W.; Jiang, M.; Guan, W.; Zhan, C.; Wang, T.; Tang, C.; Sang, L.; Liu, J.; Ni, Z.; et al. Risk Factors of Fatal Outcome in Hospitalized Subjects with Coronavirus Disease 2019 from a Nationwide Analysis in China. Chest 2020, 158, 97–105. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Milks, M.W.; Sahay, S.; Benza, R.L.; Farber, H.W. Risk assessment in patients with pulmonary arterial hypertension in the era of COVID 19 pandemic and the telehealth revolution: State of the art review. J. Heart Lung Transpl. 2021, 40, 172–182. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vasc. Pharmacol. 2021, 137, 106823. [Google Scholar] [CrossRef]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Farha, S.; Heresi, G.A. COVID-19 and Pulmonary Arterial Hypertension: Early Data and Many Questions. Ann. Am. Thorac. Soc. 2020, 17, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Hinojosa, W.; Cristo-Ropero, M.J.; Cruz-Utrilla, A.; Segura de la Cal, T.; López-Medrano, F.; Salguero-Bodes, R.; Pérez-Olivares, C.; Navarro, B.; Ochoa, N.; Arribas Ynsurriaga, F.; et al. The impact of COVID-19 pandemic on pulmonary hypertension: What have we learned? Pulm. Circ. 2022, 12, e12142. [Google Scholar] [CrossRef]
- Nuche, J.; Pérez-Olivares, C.; Segura de la Cal, T.; Jiménez López-Guarch, C.; Ynsaurriaga, F.A.; Subías, P.E. Clinical course of COVID-19 in pulmonary arterial hypertension patients. Rev. Esp. Cardiol. 2020, 73, 775–778. [Google Scholar] [CrossRef]
- Nuche, J.; Segura de la Cal, T.; Jiménez López Guarch, C.; López-Medrano, F.; Delgado, C.P.; Ynsaurriaga, F.A.; Delgado, J.F.; Ibáñez, B.; Oliver, E.; Subías, P.E. Effect of Coronavirus Disease 2019 in Pulmonary Circulation. The Particular Scenario of Precapillary Pulmonary Hypertension. Diagnostics 2020, 10, 548. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Khan, A.W.; Ullah, I.; Khan, K.S.; Tahir, M.J.; Masyeni, S.; Harapan, H. Pulmonary arterial hypertension post COVID-19: A sequala of SARS-CoV-2 infection? Respir. Med. Case Rep. 2021, 33, 101429. [Google Scholar] [CrossRef]
- Mandler, D.; Lichtblau, M.; Ulrich, S. The course of COVID-19 in a 55-year-old patient diagnosed with severe idiopathic pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894020936659. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef]
- Guzzi, P.H.; Mercatelli, D.; Ceraolo, C.; Giorgi, F.M. Master Regulator Analysis of the SARS-CoV-2/Human Interactome. J. Clin. Med. 2020, 9, 982. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Nikolaienko, S.I.; Shults, N.V.; Gychka, S.G. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med. Hypotheses 2021, 147, 110483. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Papa, S.; D’Alto, M.; Ghio, S.; Agostoni, P.; Ameri, P.; Argiento, P.; Brunetti, N.D.; Casamassima, V.; Casu, G.; et al. The paradox of pulmonary arterial hypertension in Italy in the COVID-19 era: Is risk of disease progression around the corner? Eur. Respir. J. 2022, 60, 2102276. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, I.T.; Giannakoulas, G. Management of COVID-19 in Patients with Pulmonary Arterial Hypertension. Heart Fail. Clin. 2023, 19, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Hozumi, Y.; Liu, G.; Qiu, Y.; Wei, X.; Wei, G.W. Emerging Dominant SARS-CoV-2 Variants. J. Chem. Inf. Model. 2023, 63, 335–342. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Ahmad, B.; Benchoula, K.; Vohra, M.S.; Mea, H.J.; Chong, P.P.; Palanisamy, N.K.; Wong, E.H. Emergence and molecular mechanisms of SARS-CoV-2 and HIV to target host cells and potential therapeutics. Infect. Genet. Evol. 2020, 85, 104583. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Chang, C.C.; Crane, M.; Zhou, J.; Mina, M.; Post, J.J.; Cameron, B.A.; Lloyd, A.R.; Jaworowski, A.; French, M.A.; Lewin, S.R. HIV and co-infections. Immunol. Rev. 2013, 254, 114–142. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Bannert, N.; Kurth, R. The evolutionary dynamics of human endogenous retroviral families. Annu. Rev. Genom. Hum. Genet. 2006, 7, 149–173. [Google Scholar] [CrossRef]
- Vargiu, L.; Rodriguez-Tomé, P.; Sperber, G.O.; Cadeddu, M.; Grandi, N.; Blikstad, V.; Tramontano, E.; Blomberg, J. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology 2016, 13, 7. [Google Scholar] [CrossRef]
- Stoye, J.P. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat. Rev. Microbiol. 2012, 10, 395–406. [Google Scholar] [CrossRef]
- Kraus, B.; Boller, K.; Reuter, A.; Schnierle, B.S. Characterization of the human endogenous retrovirus K Gag protein: Identification of protease cleavage sites. Retrovirology 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Chiu, E.S.; VandeWoude, S. Endogenous Retroviruses Drive Resistance and Promotion of Exogenous Retroviral Homologs. Annu. Rev. Anim. Biosci. 2021, 9, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Sibata, M.; Ikeda, H.; Katumata, K.; Takeuchi, K.; Wakisaka, A.; Yoshoki, T. Human endogenous retroviruses: Expression in various organs in vivo and its regulation in vitro. Leukemia 1997, 11 (Suppl. 3), 145–146. [Google Scholar]
- Katsumata, K.; Ikeda, H.; Sato, M.; Ishizu, A.; Kawarada, Y.; Kato, H.; Wakisaka, A.; Koike, T.; Yoshiki, T. Cytokine regulation of env gene expression of human endogenous retrovirus-R in human vascular endothelial cells. Clin. Immunol. 1999, 93, 75–80. [Google Scholar] [CrossRef]
- Freimanis, G.; Hooley, P.; Ejtehadi, H.D.; Ali, H.A.; Veitch, A.; Rylance, P.B.; Alawi, A.; Axford, J.; Nevill, A.; Murray, P.G.; et al. A role for human endogenous retrovirus-K (HML-2) in rheumatoid arthritis: Investigating mechanisms of pathogenesis. Clin. Exp. Immunol. 2010, 160, 340–347. [Google Scholar] [CrossRef]
- Blomberg, J.; Benachenhou, F.; Blikstad, V.; Sperber, G.; Mayer, J. Classification and nomenclature of endogenous retroviral sequences (ERVs): Problems and recommendations. Gene 2009, 448, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; Doucet-O’Hare, T.; Henderson, L.; Nath, A. Human endogenous retrovirus-K (HML-2): A comprehensive review. Crit. Rev. Microbiol. 2018, 44, 715–738. [Google Scholar] [CrossRef]
- Löwer, R.; Löwer, J.; Kurth, R. The viruses in all of us: Characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl. Acad. Sci. USA 1996, 93, 5177–5184. [Google Scholar] [CrossRef]
- Tönjes, R.R.; Löwer, R.; Boller, K.; Denner, J.; Hasenmaier, B.; Kirsch, H.; König, H.; Korbmacher, C.; Limbach, C.; Lugert, R.; et al. HERV-K: The biologically most active human endogenous retrovirus family. J. Acquir. Immune Defic. Syndr. Hum. Retrovirology 1996, 13 (Suppl. 1), S261–S267. [Google Scholar] [CrossRef]
- Maldonado, J.O.; Martin, J.L.; Mueller, J.D.; Zhang, W.; Mansky, L.M. New insights into retroviral Gag-Gag and Gag-membrane interactions. Front. Microbiol. 2014, 5, 302. [Google Scholar] [CrossRef] [PubMed]
- Bannert, N.; Kurth, R. Retroelements and the human genome: New perspectives on an old relation. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. 2), 14572–14579. [Google Scholar] [CrossRef]
- Liu, M.; Jia, L.; Li, H.; Liu, Y.; Han, J.; Wang, X.; Li, T.; Li, J.; Zhang, B.; Zhai, X.; et al. p53 Binding Sites in Long Terminal Repeat 5Hs (LTR5Hs) of Human Endogenous Retrovirus K Family (HML-2 Subgroup) Play Important Roles in the Regulation of LTR5Hs Transcriptional Activity. Microbiol. Spectr. 2022, 10, e0048522. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Meese, E.U. Presence of dUTPase in the various human endogenous retrovirus K (HERV-K) families. J. Mol. Evol. 2003, 57, 642–649. [Google Scholar] [CrossRef]
- Otsuki, S.; Saito, T.; Taylor, S.; Li, D.; Moonen, J.R.; Marciano, D.P.; Harper, R.L.; Cao, A.; Wang, L.; Ariza, M.E.; et al. Monocyte-released HERV-K dUTPase engages TLR4 and MCAM causing endothelial mesenchymal transition. JCI Insight 2021, 6, e146416. [Google Scholar] [CrossRef] [PubMed]
- Chertow, D.; Stein, S.; Ramelli, S.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.; Dickey, J.; Ylaya, K. SARS-CoV-2 infection and persistence throughout the human body and brain. Biol. Sci. 2021, 612, 758–763. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, C.; Liu, Y.; Li, T.; Li, H.; Han, J.; Jia, L.; Wang, X.; Zhang, B.; Li, J.; et al. High Expression of HERV-K (HML-2) Might Stimulate Interferon in COVID-19 Patients. Viruses 2022, 14, 996. [Google Scholar] [CrossRef]
- Ganser-Pornillos, B.K.; Pornillos, O. Restriction of HIV-1 and other retroviruses by TRIM5. Nat. Rev. Microbiol. 2019, 17, 546–556. [Google Scholar] [CrossRef]
- Hohenadl, C.; Germaier, H.; Walchner, M.; Hagenhofer, M.; Herrmann, M.; Stürzl, M.; Kind, P.; Hehlmann, R.; Erfle, V.; Leib-Mösch, C. Transcriptional activation of endogenous retroviral sequences in human epidermal keratinocytes by UVB irradiation. J. Investig. Derm. 1999, 113, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Radvanyi, L.; Rycaj, K.; Plummer, J.B.; Yan, P.; Sastry, K.J.; Piyathilake, C.J.; Hunt, K.K.; Johanning, G.L. Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer Res. 2008, 68, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.E.; Jones, R.B.; Meiklejohn, D.A.; Anwar, N.; Ndhlovu, L.C.; Chapman, J.M.; Erickson, A.L.; Agrawal, A.; Spotts, G.; Hecht, F.M.; et al. T cell responses to human endogenous retroviruses in HIV-1 infection. PLoS Pathog. 2007, 3, e165. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Garrison, K.E.; Mujib, S.; Mihajlovic, V.; Aidarus, N.; Hunter, D.V.; Martin, E.; John, V.M.; Zhan, W.; Faruk, N.F.; et al. HERV-K-specific T cells eliminate diverse HIV-1/2 and SIV primary isolates. J. Clin. Investig. 2012, 122, 4473–4489. [Google Scholar] [CrossRef] [PubMed]
- SenGupta, D.; Tandon, R.; Vieira, R.G.; Ndhlovu, L.C.; Lown-Hecht, R.; Ormsby, C.E.; Loh, L.; Jones, R.B.; Garrison, K.E.; Martin, J.N.; et al. Strong human endogenous retrovirus-specific T cell responses are associated with control of HIV-1 in chronic infection. J. Virol. 2011, 85, 6977–6985. [Google Scholar] [CrossRef] [PubMed]
- Hurst, T.P.; Magiorkinis, G. Activation of the innate immune response by endogenous retroviruses. J. Gen. Virol. 2015, 96, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, X.; Cui, I.H.; Wu, S.; Dou, C.; Liu, Y.; Sun, Z.; Xue, S.; Geng, T.; Liu, Z.; et al. An endogenous retroviral element exerts an antiviral innate immune function via the derived lncRNA lnc-ALVE1-AS1. Antivir. Res. 2019, 170, 104571. [Google Scholar] [CrossRef]
- Brun-Vézinet, F.; Charpentier, C. Update on the human immunodeficiency virus. Med. Mal. Infect. 2013, 43, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Michaud, H.A.; de Mulder, M.; SenGupta, D.; Deeks, S.G.; Martin, J.N.; Pilcher, C.D.; Hecht, F.M.; Sacha, J.B.; Nixon, D.F. Trans-activation, post-transcriptional maturation, and induction of antibodies to HERV-K (HML-2) envelope transmembrane protein in HIV-1 infection. Retrovirology 2014, 11, 10. [Google Scholar] [CrossRef]
- Petrara, M.R.; Cattelan, A.M.; Zanchetta, M.; Sasset, L.; Freguja, R.; Gianesin, K.; Cecchetto, M.G.; Carmona, F.; De Rossi, A. Epstein-Barr virus load and immune activation in human immunodeficiency virus type 1-infected patients. J. Clin. Virol. 2012, 53, 195–200. [Google Scholar] [CrossRef]
- Sutkowski, N.; Conrad, B.; Thorley-Lawson, D.A.; Huber, B.T. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity 2001, 15, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, M.J.; Swanson, M.D.; Contreras-Galindo, R.; Cookinham, S.; King, S.R.; Noel, R.J., Jr.; Kaplan, M.H.; Markovitz, D.M. Expression of human endogenous retrovirus type K (HML-2) is activated by the Tat protein of HIV-1. J. Virol. 2012, 86, 7790–7805. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Maldarelli, F.; Mellors, J.; Coffin, J.M. HIV-1 infection leads to increased transcription of human endogenous retrovirus HERV-K (HML-2) proviruses in vivo but not to increased virion production. J. Virol. 2014, 88, 11108–11120. [Google Scholar] [CrossRef]
- Boller, K.; Schönfeld, K.; Lischer, S.; Fischer, N.; Hoffmann, A.; Kurth, R.; Tönjes, R.R. Human endogenous retrovirus HERV-K113 is capable of producing intact viral particles. J. Gen. Virol. 2008, 89, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Han, J.; Liu, J.; Zheng, J.; Zhong, D.; Liu, R. ISDTool: A computational model for predicting immunosuppressive domain of HERVs. Comput. Biol. Chem. 2014, 49, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Boller, K.; Frank, H.; Löwer, J.; Löwer, R.; Kurth, R. Structural organization of unique retrovirus-like particles budding from human teratocarcinoma cell lines. J. Gen. Virol. 1983, 64 Pt 12, 2549–2559. [Google Scholar] [CrossRef]
- Balasubramaniam, M.; Freed, E.O. New insights into HIV assembly and trafficking. Physiology 2011, 26, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Monde, K.; Terasawa, H.; Nakano, Y.; Soheilian, F.; Nagashima, K.; Maeda, Y.; Ono, A. Molecular mechanisms by which HERV-K Gag interferes with HIV-1 Gag assembly and particle infectivity. Retrovirology 2017, 14, 27. [Google Scholar] [CrossRef]
- Monde, K.; Contreras-Galindo, R.; Kaplan, M.H.; Markovitz, D.M.; Ono, A. Human endogenous retrovirus K Gag coassembles with HIV-1 Gag and reduces the release efficiency and infectivity of HIV-1. J. Virol. 2012, 86, 11194–11208. [Google Scholar] [CrossRef]
- Lee, Y.N.; Bieniasz, P.D. Reconstitution of an infectious human endogenous retrovirus. PLoS Pathog. 2007, 3, e10. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, C.; Harper, F.; Pierron, G.; Heidmann, T.; Dewannieux, M. The HERV-K human endogenous retrovirus envelope protein antagonizes Tetherin antiviral activity. J. Virol. 2014, 88, 13626–13637. [Google Scholar] [CrossRef]
- Brinzevich, D.; Young, G.R.; Sebra, R.; Ayllon, J.; Maio, S.M.; Deikus, G.; Chen, B.K.; Fernandez-Sesma, A.; Simon, V.; Mulder, L.C. HIV-1 interacts with human endogenous retrovirus K (HML-2) envelopes derived from human primary lymphocytes. J. Virol. 2014, 88, 6213–6223. [Google Scholar] [CrossRef] [PubMed]
- Löving, R.; Wu, S.R.; Sjöberg, M.; Lindqvist, B.; Garoff, H. Maturation cleavage of the murine leukemia virus Env precursor separates the transmembrane subunits to prime it for receptor triggering. Proc. Natl. Acad. Sci. USA 2012, 109, 7735–7740. [Google Scholar] [CrossRef]
- Roberts, L.; Passmore, J.A.; Williamson, C.; Little, F.; Bebell, L.M.; Mlisana, K.; Burgers, W.A.; van Loggerenberg, F.; Walzl, G.; Djoba Siawaya, J.F.; et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. Aids 2010, 24, 819–831. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Price, L.C.; McCabe, C.; Garfield, B.; Wort, S.J. Thrombosis and COVID-19 pneumonia: The clot thickens! Eur. Respir. J. 2020, 56, 2001608. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. COVID-19 and thrombosis: What do we know about the risks and treatment? BMJ 2020, 369, m2058. [Google Scholar] [CrossRef]
- Sharov, K.S. HIV/SARS-CoV-2 co-infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int. J. Infect. Dis. 2021, 102, 163–169. [Google Scholar] [CrossRef]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef]
- Pergola, V.; Caruso, C.; Gnarini, R.; Fazio, S.; Ferraro, S. Efficacy of sildenafil in HIV-related pulmonary arterial hypertension. J. Cardiovasc. Med. 2015, 16 (Suppl. 2), S136–S137. [Google Scholar] [CrossRef]
- Sitbon, O.; Gressin, V.; Speich, R.; Macdonald, P.S.; Opravil, M.; Cooper, D.A.; Fourme, T.; Humbert, M.; Delfraissy, J.F.; Simonneau, G. Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2004, 170, 1212–1217. [Google Scholar] [CrossRef]
- Chinello, P.; Petrosillo, N. Pharmacological treatment of HIV-associated pulmonary hypertension. Expert Rev. Clin. Pharm. 2016, 9, 715–725. [Google Scholar] [CrossRef]
- Chinello, P.; Cicalini, S.; Pichini, S.; Pacifici, R.; Tempestilli, M.; Cicini, M.P.; Pucillo, L.P.; Petrosillo, N. Sildenafil and bosentan plasma concentrations in a human immunodeficiency virus- infected patient with pulmonary arterial hypertension treated with ritonavir-boosted protease inhibitor. Infect. Dis. Rep. 2015, 7, 5822. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Franco, V.; Bradley, E.A.; Badagliacca, R.; Sabanayagam, A.; Rajpal, S.; Lastinger, L.T.; Daniels, C.J.; Smith, J.S.; Benza, R.L. Pulmonary vasodilators: Beyond the bounds of pulmonary arterial hypertension therapy in COVID-19. Pulm. Circ. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Hoffmann, M.; Jin, Y.; Pöhlmann, S. Dalbavancin: Novel candidate for COVID-19 treatment. Cell Res. 2021, 31, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chang, J. Azvudine (FNC): A promising clinical candidate for COVID-19 treatment. Signal Transduct. Target. Ther. 2020, 5, 236. [Google Scholar] [CrossRef]
- Young, B.; Tan, T.T.; Leo, Y.S. The place for remdesivir in COVID-19 treatment. Lancet Infect. Dis. 2021, 21, 20–21. [Google Scholar] [CrossRef]
- Zamanian, R.T.; Pollack, C.V., Jr.; Gentile, M.A.; Rashid, M.; Fox, J.C.; Mahaffey, K.W.; de Jesus Perez, V. Outpatient Inhaled Nitric Oxide in a Patient with Vasoreactive Idiopathic Pulmonary Arterial Hypertension and COVID-19 Infection. Am. J. Respir. Crit. Care Med. 2020, 202, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.A.; Berra, L.; Gladwin, M.T. Home Nitric Oxide Therapy for COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 16–20. [Google Scholar] [CrossRef]
| Virus | Contributes to PAH |
|---|---|
| HIV | Tat + TNF-α → IL-6 → Endothelial permeability |
Tat → BMPR2  PASMC proliferation PASMC proliferation | |
ET-1 → IL-6  BMPR2 → PASMC proliferation BMPR2 → PASMC proliferation | |
| HERV-K | dUTPase → Sensitivity of PAEC to apoptosis |
| SARS-CoV-2 | Depletion of T lymphocyte inflammatory responses  endothelial function endothelial function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Gomes, M.T.; Mo, Y.; Prohaska, C.C.; Zhang, L.; Chelvanambi, S.; Clauss, M.A.; Zhang, D.; Machado, R.F.; Gao, M.; et al. Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation. Int. J. Mol. Sci. 2023, 24, 7472. https://doi.org/10.3390/ijms24087472
Wang D, Gomes MT, Mo Y, Prohaska CC, Zhang L, Chelvanambi S, Clauss MA, Zhang D, Machado RF, Gao M, et al. Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation. International Journal of Molecular Sciences. 2023; 24(8):7472. https://doi.org/10.3390/ijms24087472
Chicago/Turabian StyleWang, Desheng, Marta T. Gomes, Yanfei Mo, Clare C. Prohaska, Lu Zhang, Sarvesh Chelvanambi, Matthias A. Clauss, Dongfang Zhang, Roberto F. Machado, Mingqi Gao, and et al. 2023. "Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation" International Journal of Molecular Sciences 24, no. 8: 7472. https://doi.org/10.3390/ijms24087472
APA StyleWang, D., Gomes, M. T., Mo, Y., Prohaska, C. C., Zhang, L., Chelvanambi, S., Clauss, M. A., Zhang, D., Machado, R. F., Gao, M., & Bai, Y. (2023). Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation. International Journal of Molecular Sciences, 24(8), 7472. https://doi.org/10.3390/ijms24087472







